Panderodus from the Waukesha Lagerstätte of Wisconsin, USA: a primitive macrophagous vertebrate predator
Abstract
Conodonts are an extinct group of early vertebrates. Articulated fossils of their feeding apparatus (‘natural assemblages’) are rare, and preserved soft tissues vanishingly so. Here, a primitive conodont with preserved soft tissues is redescribed from the Waukesha Lagerstätte of Wisconsin, USA. Although the feeding apparatus of derived prioniodontid conodonts is well understood, together with the homologies between taxa, the same is not true of more primitive conodonts that have apparatuses composed entirely of coniform elements. The new data provide insights into the long-term problem of determining homology across different types of conodont feeding apparatus. The Waukesha Panderodus preserves an almost complete apparatus, consisting of two parallel rows of elements that occluded across the sagittal plane. A pair of M elements lies at the rostral end of the apparatus, with four pairs of S elements located immediately caudal to them. Three pairs of P elements are identified at the caudal end of the apparatus, for the first time in a primitive conodont with coniform elements. A symmetrical S0 element is located on the midline between the M–S and P suites and provides the key for establishing homology with more derived ramiform–pectiniform apparatuses. The exceptional preservation reveals cartilaginous supports for the elements that inserted into their basal cavities. The trunk of the animal is poorly preserved but was dorsoventrally flattened in life with transverse myomeres containing muscle fibrils. Overall, the specimen shows that Panderodus was a macrophagous feeder and provides an insight into the functional anatomy of early vertebrate predation.
The fossil record of vertebrates currently extends back to the early Cambrian and the initial diversification of bilaterian animal groups. The oldest widely acknowledged vertebrate is Myllokunmingia fengjiao and its probable synonym, Haikouichthys ercaicunensis (Hou et al. 2017), from the Chengjiang Lagerstätte of Yunnan Province, China (Shu et al. 1999, 2003; Hou et al. 2002), which dates to 518 Ma (Cambrian, Series 2, Stage 3; Yang et al. 2018). These animals lack the feeding apparatuses consistent with a predatory lifestyle and are presumed to have been nektonic filter feeders. The same is true of the only vertebrate recorded from the Burgess Shale, Metaspriggina walcotti, which has bipartite branchial arches with processes on the ventral bars that are anatomically consistent with microphagous feeding (Conway Morris & Caron 2014). The Burgess Shale contains agnostid arthropods consistent with a late Wuliuan age (505–507 Ma) (Moysiuk & Caron 2019), but by 504 Ma the first evidence of vertebrate predation appears in the fossil record in the form of paraconodont elements. Gapparodus bisulcatus is the oldest paraconodont and appears low in the Ptychagnostus atavus trilobite biozone of the Drumian stage (Dong & Bergström 2001; Dong & Zhang 2017). Conodonts are retrieved as vertebrates in phylogenetic analyses (Donoghue et al. 2000; Donoghue & Smith 2001; Donoghue & Keating 2014), but the evolutionary relationship of paraconodonts to euconodonts (and other vertebrates) was unclear for a long time, although a close phylogenetic link had long been mooted (Bengtson 1976). Detailed histological investigation has now confirmed that paraconodonts have growth patterns consistent with them being plesiomorphic euconodonts (Murdock et al. 2013a), thereby indicating that the oldest evidence for macrophagous predation by vertebrates is the first appearance of paraconodonts.
Both paraconodonts and euconodonts are mainly represented in the fossil record by the phosphatic elements that made up the feeding apparatus. A small number of exceptionally preserved specimens provide information on the soft tissues of euconodonts, but these are almost entirely confined to highly derived prioniodontids (Fig. 1). These include the ozarkodinin Clydagnathus from the Granton Shrimp Bed of Edinburgh, Scotland (Aldridge et al. 1986, 1993) and the balognathid Promissum from the late Ordovician Soom Shale of South Africa (Gabbott et al. 1995), using the phylogenetic classification of Donoghue et al. (2008). To date, only a single example of the more primitive euconodonts, the [Protopanderodontida + Panderodontida] of Sweet & Donoghue (2001), which have only coniform elements within the feeding apparatus, has been preserved with soft tissues. There are no known paraconodont soft tissues preserved. The soft tissues of Panderodus unicostatus (Branson & Mehl, 1933) from the Waukesha Lagerstätte of Wisconsin, USA (Smith et al. 1987), therefore represent the only available data on the soft tissue anatomy of the most primitive vertebrate macrophagous predators, and the single specimen also contains one of the best preserved and most anatomically informative feeding apparatuses known from primitive conodonts (Sansom et al. 1994; Smith et al. 2005). The Waukesha Panderodus was documented using light microscopy and back-scattered electron microscopy in the 1980s (Smith et al. 1987) but, given the importance of this specimen, it was decided to re-examine it using techniques that were not available for the original description.
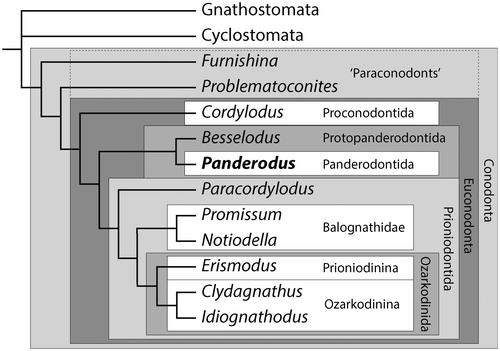
While present in low diversity through most of the Cambrian, conodonts exploded in diversity as part of the Great Ordovician Biodiversity Event. Paraconodonts became extinct in the Tremadocian (Wills 1993) but after a first appearance in the latest Jiangshanian (latest Cambrian, 490 Ma; Dong & Zhang 2017), euconodonts diversified to achieve a global species diversity of at least 100 by the Floian (Early Ordovician; Sweet 1988) and remained the dominant vertebrate group throughout the early Palaeozoic. The most successful conodont group from the mid-Ordovician and throughout the Silurian was the Prioniodontida, consisting of the Ozarkodinina, Prioniodinina and a paraphyletic array of more primitive ‘prioniodontids’ (Donoghue et al. 2008). These conodonts had feeding apparatuses consisting of elements with denticulate processes attached to the main cusp and, normally, a high level of functional differentiation between the array of grasping M and S elements situated rostrally and the pairs of P elements situated caudally for food processing (Aldridge et al. 1995; Purnell & Donoghue 1997, 1998, 1999; Donoghue & Purnell 1999). However, from the late Cambrian to the Silurian, protopanderodontid and panderodontid conodonts with feeding apparatuses of more subtly differentiated, adenticulate coniform elements formed a large part of the fauna. These groups remain poorly understood phylogenetically (Sweet 1988; Sweet & Donoghue 2001), and also in terms of the detailed anatomy of the feeding apparatus for all but a few taxa (Aldridge 1982; Smith et al. 1987; Sansom et al. 1994; Leslie 1997). Nevertheless, they represented a dominant component of the zooplankton in Cambrian–Silurian oceans (Fortey 1997, pp 132–133).
The soft tissues of derived ozarkodinin conodonts in the Granton Lagerstätte show that the animals were 4–5 cm long, bilaterally flattened and anguilliform with an asymmetrical tail fin, well-developed myomeres and large eyes, and possessed a bilaterally operative feeding apparatus (Briggs et al. 1983; Aldridge et al. 1986, 1993). The single specimen with preserved soft tissues from the Soom Shale adds little additional anatomical information but is much larger, with an estimated length of 40 cm (Gabbott et al. 1995). Phylogenetic analysis resolves these conodonts as crown-vertebrates and the sister group to ‘ostracoderms’ and jawed vertebrates, and as more derived than hagfishes and lampreys (Donoghue et al. 2000; Donoghue & Smith 2001), or in a polytomy with cyclostomes and gnathostomes (Donoghue & Keating 2014). The Waukesha specimen shows that at least some primitive conodonts had a very different body anatomy despite having histologically and structurally homologous feeding apparatuses.
Material and method
The exceptionally preserved specimen of Panderodus unicostatus (Branson & Mehl) is deposited in the University of Wisconsin Geology Museum, Madison, WI, USA (specimen number UW4001/7a+b). Scanning electron and back-scattered electron microscopy, together with element mapping, was carried out on an FEI Quanta 650 electron microscope fitted with an Oxford Instruments energy-dispersive spectroscopy (EDS) system in the Department of Earth Sciences, University of Oxford, using an accelerating voltage of 20 kV at a working distance of 10 mm and at a low vacuum of 50 Pa. Elemental maps were collected and analysed using Oxford Instruments Aztec software, and subsequently composited using Adobe Photoshop. Computed tomography (CT) scanning was undertaken on a Nikon XT H 225 ST x-ray tomography scanner in the Faculty of Science, University of Bristol. Volumetric data were acquired at a voxel size of 6.035 µm, over 1800 projections with an exposure time of 1415 ms. Slice data were analysed and manipulated using the CT software Amira (Thermo Fisher Scientific) versions 2019.1 to 2019.4.
The Waukesha Lagerstätte
The specimen of Panderodus unicostatus with preserved soft tissues was collected from dolostones of the Brandon Bridge Formation of Waukesha, Wisconsin, USA (Mikulic et al. 1985a, b; Smith et al. 1987; Wendruff et al. 2020). The fossils of the Lagerstätte are mainly preserved in mm-scale laminated dolostones with silt-grade grains and more organic-rich, argillaceous, dolostones (Wendruff et al. 2020); the Panderodus specimen is preserved in the former lithology. The formation lies within a tidally influenced, transgressive systems tract above an unconformity with palaeokarstic relief, and the evidence suggests that dolomitization is penecontemporaneous (Wendruff et al. 2020). The biota currently consists of 50 genera that include trilobites, non-trilobitic arthropods, lobopodians, conulariids, annelids, brachiopods, cephalopods, graptolites, and rare echinoderms (Mikulic et al. 1985b; Wendruff et al. 2020). Chordate trunks with preserved myomeres and axial lines are documented (Wendruff et al. 2020), and may represent headless conodonts based on their anatomy. The exceptional preservation was attributed by Wendruff et al. (2020) to the transport of carcasses into the area, followed by bacterial sealing beneath rapidly growing microbial mats, perhaps with associated hypersalinity and anoxia.
Biostratigraphically, the Waukesha Lagerstätte lies within the Pterospathodus eopennatus conodont superzone (Kleffner et al. 2018), corresponding to a mid-Telychian age (Llandovery, Silurian; 437 Ma, Ogg et al. 2016).
The anatomy of Panderodus
Description
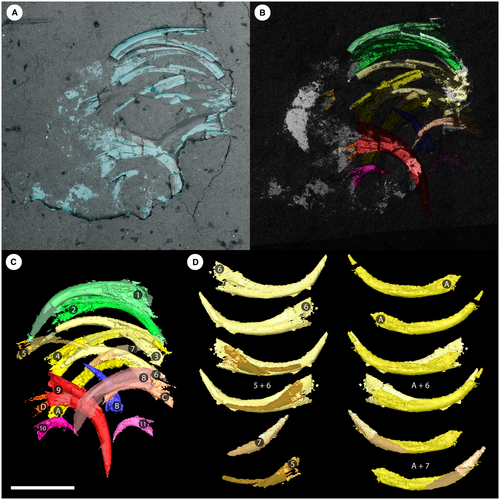
In visible light, the Waukesha Panderodus consists of a body trace that is truncated by the end of the collected slab. A well-preserved feeding apparatus denotes the rostral end of the specimen and is preserved on both part and counterpart (Fig. 2); the apparatus is a maximum of 1.2 mm across the transverse axis, and a maximum of 1.3 mm along the rostral–caudal axis. The elements of the feeding apparatus are symmetrically arranged on either side of the rostral–caudal axis and element pairs occlude across this midline (Figs 2-5).

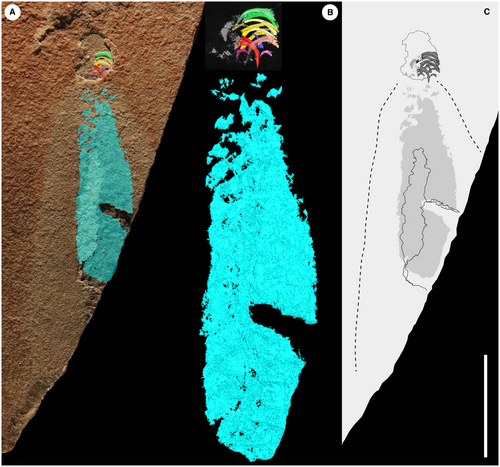
A patch of white mineral lies caudad (i.e. in a caudal direction) to the feeding apparatus on the midline, on both part and counterpart, and shows subtle segmentation. This sheet of mineral is seen to continue underneath the matrix on the part (Fig. 2A). Both the feeding apparatus and the sheet of mineral are surrounded by a halo of beige sediment that contrasts in colour with the red–orange colour of the remainder of the matrix. Within the halo a dark grey area of slightly raised relief extends from the sheet of white mineral almost to the apparatus, and the sheet extends beneath but not beyond this dark grey area.
X-ray CT scanning, which was not available for the original description of Smith et al. (1987), shows that the sheet of white mineral forms a symmetrical structure about the midline that extends almost as far rostrad (i.e. in a rostral direction) as the feeding apparatus (Fig. 3), and which matches the dark grey raised structure in position. It shows clear transverse segmentation, particularly at the rostral end immediately behind the apparatus, with individual segments having rostrocaudal thicknesses up to 0.5 mm. The body has a maximum preserved length of 13.4 mm from the apparatus to the caudal limit of preservation and a maximum preserved width of 2.7 mm, but extrapolation of the truncated edge of the slab suggests a maximum width of at least 3.6 mm for the preserved trunk.
Energy-dispersive x-ray spectroscopy showed that this white mineral is composed predominantly of calcium and phosphorus, and this elemental composition (Fig. 6A; Murdock & Smith 2021) is consistent with the mineral being fluorapatite. Back-scattered electron microscopy showed the presence of fibrils within the calcium phosphate that are up to 3.3 µm in width and up to 50 µm in preserved length (Fig. 6B). The sheet of calcium phosphate does not extend across the full width of the beige halo but occupies the central 40% of the halo structure.
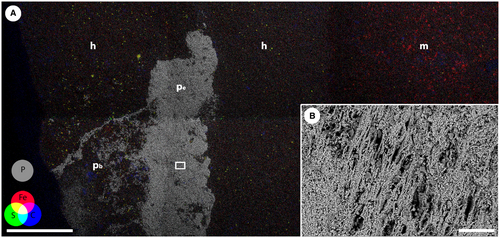
Surrounding the elements of the feeding apparatus are small patches of calcium phosphate, with a morphology and preservation that is clearly distinct from the elements. The mineralization is very thin, so much so that the patches are predominantly only visible in the CT data, but where they are visible on optical microscopy they are dull, opaque and whitish-blue in colour, in distinct contrast to the translucent, amber-coloured elements. The thin patches of calcium phosphate are also unlike the preserved trunk, in both surface texture and morphology, and entirely separate from it. Rather, the calcium phosphate patches are, in most cases, coincident with the bases of the elements, and flare out as a triangle extending beyond the base of each element (Fig. 4A, B) and/or form a sheet connecting the bases of the elements.
Interpretation
The orientation of the elements in the feeding apparatus, with cusps opposed across the long axis of the animal and a plane of bilateral symmetry coincident with that midline, is consistent with the dorsoventral collapse of the apparatus during decay (Smith et al. 1987) and indicates the location of the sagittal plane of the animal, which is perpendicular to the midline of the preserved tissues. Comparison with the feeding apparatus location in other exceptionally preserved conodonts (Aldridge et al. 1986, 1993) means that the apparatus can be assumed with confidence to be rostral.
It has been proposed that paired arrays of coniform elements would need basal support, either in soft tissue or cartilage, in order to function and occlude effectively (Smith et al. 1987). We interpret the newly discovered phosphatic structures in the head region of the Waukesha specimen as the remains of cartilaginous cones that were attached in the basal cavity of the elements, with some evidence for a one-to-one relationship of these cones to individual elements (e.g. elements D, 9 and 10 in Fig. 4A, B). It is possible that these were fused together to form a basal cartilage that linked the elements together in a similar manner to the basal plate of hagfishes (Clark & Summers 2007), but the preservation is too incomplete to be conclusive about this.
The preserved trunk is truncated by the edge of the collected slab so only the rostral part of the animal is present. The trunk tapers towards the apparatus and the anterior of the animal, as well as thinning dorsoventrally. The trunk is preserved as a series of arcuate transverse segments symmetrically opposed across a midline that is parallel and coincident with the rostrocaudal axis of the apparatus, thus we interpret a dorsoventral collapse orientation of the body. The chemical composition of the preserved body tissues, the morphology of the transverse segmentation as paired symmetrical arcuate structures, and the presence of fibrils are all consistent with the sheet of calcium phosphate being preserved muscle tissue. Similar preservation of muscle fibrils is seen in the specimen of the conodont Promissum described from the Soom Shale (Gabbott et al. 1995), as well as fish muscles from the Santana Formation, Brazil (Martill 1990), and in experiments attempting to replicate the phosphatization of muscle tissues (Briggs et al. 1993).
The transverse segmentation is interpreted as myomeric, but is different to the v-shaped myomeres of the Granton animals. However, the presence of the dorsoventral collapse of the apparatus may indicate that the specimen was also dorsoventrally flattened in life and came to rest on a stable body surface. The transverse myomeres would be consistent with this anatomy, in contrast to the acute W-shaped myomeres that would be expected from the dorsoventral collapse of an animal with a laterally compressed body as seen, for example, in Metaspriggina (Conway Morris & Caron 2014, extended data fig. 5). It is not possible to determine the polarity of the dorsal and ventral surfaces of the tissues preserved on the bedding plane.
Although incomplete, the available evidence suggests that the complete animal was of a similar length to the Granton specimens, which are typically around 40–55 mm long (Briggs et al. 1983; Aldridge et al. 1986, 1993), but was significantly smaller than the Promissum specimen, which has a preserved length of 10.9 cm and an estimated total length in life of 40 cm (Gabbott et al. 1995).
Some anatomical features are conspicuous by their absence from the Waukesha specimen, in comparison with other conodont soft tissues. Eyes are a feature of both the Granton and Soom material but are absent here. There is also no evidence of structure within the axial region of the body, no preserved organs or tissues beyond the myomeres, and nothing preserved within the region immediately posterior of the apparatus where pharyngeal structures might be expected. These absences carry implications for the degree of decay present in the specimen (see Taphonomy).
Element composition and architecture of the feeding apparatus
The current apparatus architecture for Panderodus (Sansom et al. 1994; Murdock et al. 2013b) is based predominantly on the Waukesha feeding apparatus, together with a small number of diagenetically fused element clusters of partial apparatuses (An et al. 1983; Balogh & Kozur 1985; Dzik & Drygant 1986). In the available model, the Panderodus apparatus involved 17 elements in eight pairs with cusps occluding across the midline, plus one unpaired aequaliform element (ae; notation of Sansom et al. 1994), which was interpreted as being situated on the midline at the caudal end of the apparatus. The most rostral six pairs of elements constituted the ‘costate suite’ of Sansom et al. (1994), with a rostral arcuatiform (qa) pair and two sets of two graciliform (qg) pairs on both the rostral and caudal side of a pair of truncatiform (qt) elements. Caudad of the costate suite in this interpretation is a pair of falciform (pf) elements and a pair of tortiform (pt) elements that together form the ‘compressed suite’. These elements were further subdivided into three functional units, each having elements with cutting and grasping functions, by Murdock et al. (2013b). The new CT data have provided new anatomical information that enable a significant revision of the architectural model of Sansom et al. (1994) and thus permit a better understanding of the functional anatomy of early vertebrate predation.
The elements of the apparatus are preserved in a near perfect dorsoventral collapse pattern, with two half apparatuses clearly distinguishable on either side of the midline (Fig. 4C). The element pairs are closely spaced in a rostrocaudal sense, and a component of collapse and shortening along this axis cannot be ruled out. The midline of the apparatus is coincident with the axial line of the soft tissue, and there can be confidence that the feeding apparatus is at the rostral end of the body. The rostrocaudal sequence of the elements within the apparatus during life can be ascertained by first identifying the element pairs, and then determining the ‘stacking order’ of the elements that are lying in direct superposition on other elements. In the absence of a defined dorsoventral axis, it is not possible to identify which elements were dextral versus sinistral in life without making a priori assumptions.
The costate element suite
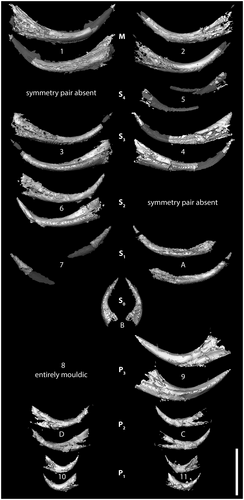
Elements 1 and 2 (Figs 4, 5) are confirmed as a rostral arcuatiform pair, with the furrowed face of element 2 occluded against the unfurrowed face of element 1. Caudal to the arcuatiform elements is a pair of graciliform elements, elements 3 and 4, that are morphologically similar to each other, with a carina on both the furrowed and unfurrowed faces and a subsymmetrical cusp cross-section (Fig. 5; Murdock & Smith 2021).
Elements 6 and A appear to form a pair in terms of disposition, but they are of dissimilar morphology (Fig. 4D), with element A having a lower base and a more proclined cusp. Instead, element A pairs with the incomplete element 7, which has similar cusp curvature and proclination, and perfectly occludes the tip of A (Fig. 4D).
The identification of these pairs leaves two preserved elements, 5 and 6, which are morphologically dissimilar (Figs 4, 5). Element 5 is preserved mostly as a mould and its base is displaced relative to the rest of the apparatus. The element morphology of this proclined element can be reconstructed from the mould, although its size (particularly the length of the cusp) is consequently underrepresented in the 3D model (Fig. 4C).
Element 6 is broadly similar to elements 3 and 4 in morphology, but has a shorter, higher base; a more erect cusp; and an asymmetrical cross-section (Fig. 5; Murdock & Smith 2021). The cusp curvature and proclination of elements 5 and 6 are significantly different (Fig. 4D). Furthermore, in the CT scans, elements 5 and 6 are separated by element pair 3 + 4 and, when the collapse due to decay is restored by rotation of the elements, they sit on either side of 3 + 4. Elements 5 and 6 do not, therefore, form a natural pair and both are interpreted as graciliform elements that have lost the other half of the element pair. Element 5 was partially displaced from the apparatus during collapse and has lost its counterpart, but with the exception of this taphonomically displaced element, the preserved bases of costate elements on the sinistral (2, 4, A) and dextral (1, 3, 6) sides of the apparatus are markedly parallel.
Collectively, elements 1–7 and A correspond to the suite of elements described by Sansom et al. (1994) as the costate suite. Caudad to this suite is a single unpaired element, B, that lies close to the midline of the apparatus and which has a cusp that is interleaved between elements A and C. Its cusp is directed subparallel (rather than near-perpendicular) to the rostrocaudal axis (Fig. 4), and the element is symmetrical in cross-section (Fig. 5; Murdock & Smith 2021). It is best interpreted as the unpaired aequaliform element and is located at the caudal end of the costate suite, not at the caudal end of the apparatus as proposed by Sansom et al. (1994).
The compressed element suite
At the caudal end of the apparatus are three element pairs with a consistent orientation that is well-defined on the CT scans (Fig. 4). Collectively they are equivalent to the compressed suite of Sansom et al. (1994) but the CT scans reveal an unexpected range of morphology. Using the same element rotations to uncollapse both the caudal compressed suite and the rostral costate suite (cf. animation in Murdock & Smith 2021), the most rostral of these three pairs of elements is 8 + 9. Element 9 is well-preserved but element 8 is entirely mouldic, and together they constitute a typical falciform pair, as identified by Smith et al. (1987) and Sansom et al. (1994).
Elements C and D are almost entirely obscured by matrix at the surface and were not identified in previous studies (Smith et al. 1987; Sansom et al. 1994). They form a symmetrical pair opposed across the midline with cusps not quite touching and with lateral faces juxtaposed against the unfurrowed faces of element pair 8 + 9. Comparison of the morphology of these elements (Figs 4, 5; Murdock & Smith 2021) with isolated element collections of P. unicostatus, and with topotype material, shows that they most closely resemble tortiform elements (Armstrong 1990, pl. 17, fig. 5; Sansom 1992, pl. 20, figs 17, 18), with a relatively high base and a short, proclined cusp (cf. also the tortiform element of P. acostatus (Branson & Branson, 1947) in Sansom et al. 1994, text-fig. 2C, F).
At the caudal extreme of the feeding apparatus are elements 10 and 11, which are again only partially visible at the surface. The CT data demonstrate that they form a pair of elements with short, robust bases perpendicular to the midline and very short, erect cusps bearing prominent costae directed caudally. This pair of elements does not occlude but is instead widely spaced across the midline. In comparison with isolated element collections, they have a morphology consistent with truncatiform elements as defined by Sansom et al. (1994, cf. text-fig. 2C, F) (see also the topotype material of Sansom 1992, pl. 20, figs 13, 14). The truncatiform elements were previously reconstructed as part of the rostral costate suite of elements based on their association with graciliform elements in both the Nekézseny and Podolia clusters (Sansom et al. 1994). The lack of consistency between the Waukesha animal and the clusters suggests a significant degree of faecal re-arrangement in the latter.
In summary, from rostral to caudal the feeding apparatus of the Waukesha Panderodus has a costate element suite consisting of arcuatiform (1 + 2), graciliform (5), graciliform (3 + 4), graciliform (6), and graciliform (A + 7) element pairs. Allowing for missing elements, this predicts four pairs of graciliform elements in the animal during life. Caudal to the costate suite is a compressed suite consisting of falciform (8 + 9), tortiform (C + D) and truncatiform (10 + 11) element pairs from rostral to caudal. The unpaired aequaliform element sits on the midline between the costate suite and the compressed suite, not at the caudal end of the apparatus as suggested by Sansom et al. (1994). The apparatus therefore comprised 17 elements during life.
Four morphotypes of graciliform elements were also consistently recognized in isolated collections of Panderodus species by Sansom et al. (1994, text-fig. 7), involving high- and low-based subsymmetrical forms and high- and low-based asymmetrical forms, with the symmetry morphotypes corresponding, respectively, to the similiform and asimiliform elements of Sweet (1979). The distinction between high- and low-based element types is also reflected in the relative length of the upper edge of the base, with high-based elements having a relatively short upper edge to the base, and low-based elements having a correspondingly long upper edge. In the CT scans of the Waukesha specimen, elements 3 + 4 clearly have subsymmetrical cross-sections (with a carina on either side of the cusp and dorsally directed costa) and are long-based. In contrast, element 6 is asymmetrical (one carina) and has a relatively short upper edge to the base. Elements A + 7 are poorly preserved, but A is long-based and asymmetrical, which would leave the incompletely preserved element 5 as a short-based subsymmetrical element, given the consistent presence of this morphotype in isolated collections that would otherwise be absent from the Waukesha apparatus. Although the data are imperfect, the preservation of multiple graciliform morphotypes hints at an architecture in which the two subsymmetrical element pairs lie rostrad of the asymmetrical pairs, and alternate base height and length (i.e. short-based, long-based, short-based, long-based) from rostral to caudal.
Element homology between Panderodus and prioniodontid conodonts
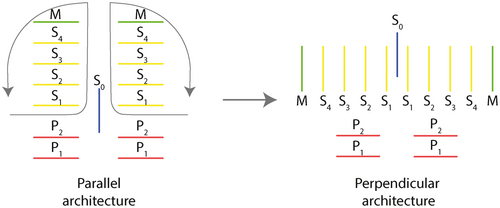
The topological differences between derived, prioniodontid conodont feeding apparatuses and more primitive proconodontid, protopanderodontid and panderodontid ones have made it difficult to identify the individual element homology between the two types (Smith 1990; Smith et al. 2005). Prioniodontid apparatuses have M and S elements with denticulated processes that lie parallel to the sagittal plane, and at right angles to the P array that operates bilaterally across the sagittal plane; this has been termed a ‘perpendicular architecture’ (Smith 1990). In contrast, proconodontid, protopanderodontid and panderodontid apparatuses have coniform elements, without processes, that are paired on either side of the sagittal plane and which occlude across it, in a configuration that has been termed a ‘parallel architecture’ (Smith 1990; Smith et al. 2005). It is relatively easy to identify homologies between elements of the rostral P arrays, which are positionally similar, but this is far more difficult for the M–S array (Smith et al. 2005). The new data relating to the Waukesha specimen provide insights into this long-term problem in determining homology within conodont feeding apparatuses, and facilitate the identification of components of apparatus architecture that are conserved across multiple conodont clades.
The feeding apparatus of the Waukesha Panderodus is characteristic of a parallel architecture that has been subject to dorsoventral collapse during decay (possibly with a degree of rostrocaudal collapse). The newly identified location of the symmetrical aequaliform element (caudal to the costate suite, but rostral to the compressed suite) is consistent with the position of the S0 element on the midline of a prioniodontid apparatus (using the positional terminology of Purnell et al. 2000). Similarly, the division into costate and compressed element suites is equivalent to the division between a rostral ramiform M–S array and caudal pectiniform P elements. The four pairs of costate graciliform elements are interpreted as S elements. The position of the S0, on the midline immediately caudal of the costate paired elements, is decisive for determining the alternative patterns of transformation of the parallel architecture seen in primitive conodonts relative to the perpendicular apparatus of more derived taxa (Smith et al. 2005). The medial to lateral order of elements in ramiform element architectures (S0 > S1 > S2 > S3 > S4 > M) is thus homologous to a caudal to rostral axis in coniform conodonts, via an outward rotation of the dextral and sinistral paired elements about the unpaired S0 (Fig. 7; cf. Smith et al. 2005). The most caudal graciliform element pair of the costate suite (elements A + 7) is therefore homologous with S1, and the subsequent graciliform pairs (6 + ?, 3 + 4, 5 + ?) with S2, S3 and S4, respectively. The arcuatiform element pair lies adjacent to the S4 elements at the rostral end of the feeding apparatus, and these elements are morphologically distinct from the S elements. They also lie in a position that in other taxa, such as Besselodus (Aldridge 1982), is occupied by geniculate coniform (‘oistodiform’) elements that are very similar to M elements in some prioniodontid taxa. The arcuatiform elements in Panderodus are therefore interpreted as being homologous with M elements.
Although several prioniodontid taxa have been described with three or more pairs of P elements (Mannik & Aldridge 1989; Armstrong 1990; Miller & Aldridge 1993; Aldridge et al. 1995, 2013; Aldridge 2002; Hou et al. 2002), rather than the more typical two pairs, this is the first time that three pairs of P elements have been recorded in a primitive conodont with an apparatus of coniform elements. This caudal compressed suite consists of a P1, truncatiform, element pair (elements 10 + 11); a P2, tortiform pair (elements C + D) and a P3, falciform pair (elements 8 + 9) (Figs 4, 5). The anatomical notation P1–P3 is here applied topologically based on the relative position of the elements along the rostrocaudal axis, with P1 rostral, following nomenclatural convention (Purnell et al. 2000) and in line with prioniodontid apparatus reconstructions with more than two P element pairs (Aldridge et al. 1995, 2013). We recognize that such comparisons represent a direct topological comparison rather than a definitive statement of homology, and that other hypotheses are tenable in relation to homologies with apparatuses containing two pairs of P elements.
Apparatus function
The recognition of a new arrangement of elements within the feeding apparatus of Panderodus necessitates a re-appraisal of the functional model of Murdock et al. (2013b). Within this model arcuatiform, truncatiform and, particularly, falciform elements were recognized as having a piercing or cutting role, whereas the graciliform, aequaliform and tortiform elements were better suited to prey capture and restraint. The interpretation of individual element function is unaffected by the new order of elements in the apparatus architecture, given that the analyses were undertaken on isolated collections of Panderodus elements. However, the integration of the aequaliform element into the M–S array, and recognition of the caudal position of the truncatiform elements, alters the functional units in the model of Murdock et al. (2013b). The costate suite can be reconstructed as a single functional unit with a pair of rostral arcuatiform elements performing a piercing/cutting role, with the four pairs of graciliform elements and an unpaired aequaliform element constituting an array of grasping elements caudal to them. Murdock et al. (2013b) did not compare the different graciliform morphotypes, and the effect on function of the differentiation with the S elements remains to be tested. In contrast, the compressed suite consists of three pairs of morphologically, and functionally, distinct elements: a rostral pair of large, cutting, falciform elements; a pair of grasping tortiform elements; and a caudal pair of much smaller, cutting/piercing, truncatiform elements (see Murdock & Smith 2021, fig. S4). This suggests a greater degree of complexity of functional roles within the compressed suite, but a closer equivalence of the architectural suites with functional units (contra Murdock et al. 2013b).
Taphonomy
The elements of the feeding apparatus are preserved with the original calcium phosphate crowns intact and/or as external moulds, in a sediment matrix of dolomite that otherwise has a very low concentrations of phosphorus, and in which most of the calcium is associated with magnesium as dolomite.
The trunk of the Waukesha specimen is preserved as three distinct regions: the thin layer of calcium phosphate that preserves the muscle tissue, the dark grey rock matrix that overlies it, and the pale buff reduction halo. The EDS and CT data together confirm that the white mineral is calcium phosphate, and probably fluorapatite (Smith et al. 1987). The calcium phosphate extends below the exposed surface, coincident with the raised area of dark grey matrix, which represents the buried extent of the body. Other than the feeding elements, no fluorapatite is present outside this region. The pale grey region surrounding the body outline is texturally indistinguishable from the matrix, but EDS analysis shows that iron in this region is associated with sulphur (Fig. 6A). The iron and sulphur are concentrated in mineral phases that have crystal morphologies consistent with pyrite. In the CT data there is an even greater concentration of the same mineral phase associated with the soft tissues themselves, visible as electron-dense clusters that fill the cavities around the phosphate. The remaining matrix has very little visible pyrite but there is abundant iron that appears associated with clays and as iron oxides. This suggests the localized reduction of iron to form pyrite in and immediately surrounding the carcass, to produce a reduction halo that is also indicative of decay of the carcass prior to mineralization of the tissues.
In addition to the reduction halo, the combination of morphological characters preserved in the Waukesha Panderodus is consistent with a vertebrate carcass in an advanced state of decay, with disrupted myomeres (containing preserved fibrils) and the cartilages associated with feeding structures (e.g. the lingual cartilage of hagfish) being among the most decay-resistant structures (Sansom et al. 2010, 2011, 2013). By comparison, the Granton Clydagnathus (Aldridge et al. 1993) and the Soom Shale Promissum (Gabbott et al. 1995) preserve more labile structures in the head region (such as sclerotic cartilages, otic capsules and extrinsic eye musculature) and in the trunk (putative nerve cord and gut relic), consistent with a lower degree of decay before the stabilization of tissues during preservation. Furthermore, a greater fidelity of preservation is evident in other exceptionally preserved chordates, which is indicative of preservation at an even earlier stage of decay, such as the pharyngeal structures in the Chengjiang vertebrate Myllokunmingia, or the visceral organs in the Mazon Creek cyclostome Mayomyzon (Sansom et al. 2011), both of which are absent from all known conodont soft tissues.
However, the anatomy present in the Waukesha specimen cannot be explained entirely by preservation at a late stage of decay, most conspicuously the absence of the large pair of lobate structures interpreted as eyes that are so clearly associated with better preserved conodont taxa (Briggs et al. 1983; Gabbott et al. 1995), and some natural assemblages of feeding apparatuses in which no other soft tissues are present (Aldridge et al. 2013). In both the Granton and Soom Lagerstätten these structures are preserved as outer ring-shaped lobes interpreted as sclerotic cartilages (Aldridge & Theron 1993; Aldridge et al. 1993), with fibrous extrinsic muscles also preserved in one Soom specimen (Gabbott et al. 1995). Darker areas in the rings are described in several specimens (Briggs et al. 1983; Aldridge & Theron 1993; Aldridge et al. 1993, 2013; Gabbott et al. 1995), consistent with carbon-rich preservation of eyes seen in several other vertebrate taxa (e.g. Gabbott et al. 2016). Despite the preservation of soft tissues via carbonaceous compression being known in other taxa, including chordates, from the Waukesha Lagerstätte (Wendruff et al. 2020), we find no evidence for any carbon associated with the soft tissues in Panderodus, and it is present only in the remains of microbial structures across the surface of the slab. The absence of eyes in the Waukesha specimen, therefore, might be explained by an interaction between the loss of more decay-prone structures associated with the eyes, and the absence of a taphonomic pathway to preserve the more recalcitrant pigmented structures of the eyes themselves (Purnell et al. 2018). It seems unlikely that a free-swimming predator such as Panderodus lacked the eyes seen in other conodont taxa.
The axial lines present in a number of fossil vertebrates, including conodonts, have been interpreted as remains of either the notochord or the dorsal nerve chord. In decay experiments, both structures are among the most long-lasting during decay (Sansom et al. 2010, 2011), and the notochord sheath is particularly decay resistant in cephalochordates, cyclostomes and chondrichthyans (Sansom et al. 2013). The Waukesha specimen does not display direct evidence of axial structures, which are seen in the other chordates from Waukesha (Wendruff et al. 2020) and in the Granton Clydagnathus (Aldridge et al. 1993), nor is there a clear gap between the myomeres indicative of their position, as is observed in Promissum (Gabbott et al. 1995). However, the Waukesha specimen differs from these other specimens in two important ways. First, it is preserved in dorsoventral (rather than predominantly lateral) collapse, and second, it is much more heavily phosphatized, both of which could contribute to obscuring the axial structures. The CT data, in tandem with EDS analysis, do, however, show pyrite filling the voids surrounding and between the myomeres. These are particularly concentrated at the lateral margins of the body trace where the remains of arcuate myomeres begin to diverge, and along the midline of the body where the opposing myomeres meet; the latter may represent the filling of voids left by the decay of axial structures.
Discussion
Implications for homology within conodont feeding apparatuses
The availability of preserved feeding apparatuses of Panderodus, in the form of the Waukesha animal and diagenetically fused clusters of elements (Kozur 1984; Dzik & Drygant 1986; Sansom et al. 1994), means that this genus has the best constrained architecture of any primitive conodont with an apparatus composed of coniform elements. The most common apparatus architecture in derived, prioniodontid, conodonts contains one pair of M elements, four pairs of S elements, an unpaired symmetrical S0 element, and two pairs of P elements (Purnell & Donoghue 1997; Sweet & Donoghue 2001; Aldridge et al. 2013). The identification of a buried S0 element in the Waukesha Panderodus (Fig. 4) sheds light on the previously intractable question (Smith et al. 2005) of positional homology between the S elements of primitive conodonts with parallel architecture and those in the prioniodontid conodonts with perpendicular architecture (Purnell et al. 2000). The Panderodus S0 element lies at the caudal end of the S array (Fig. 4), and a pair of morphologically distinct arcuatiform elements, homologous with M elements (Sansom et al. 1994), lies at the rostral end. Together, these observations provide the topological reference points to determine that the S1 lies caudally, adjacent to the S0, and that the S4 lies adjacent to the arcuatiform elements, with the S2 and S3 elements positioned sequentially between them.
Among the few other coniform taxa represented by fused clusters that have sufficient elements to provide robust constraints on the apparatus architecture is Besselodus (a protopanderodontid). A fused cluster of Besselodus from the Late Ordovician of Greenland consists of a geniculate element and an array of six, isomorphous, laterally costate elements (Aldridge 1982). The geniculate (‘oistodiform’) element is best interpreted as an M homologue by comparison with other conodont apparatuses, and lies in a comparable position to the arcuatiform elements of Panderodus. The six costate elements are interpreted as a morphologically undifferentiated series of S and P elements. The presence of seven elements in this half-apparatus does suggest that it might be complete, and representative of one side of a 15-element parallel architecture. The evidence from Panderodus and Besselodus suggests that an 11-element M–S array appears to be remarkably conserved across conodonts, irrespective of whether they have a parallel or a perpendicular architecture. By contrast, there is a convergent tendency for P positions to be duplicated, and this is now known to occur in the Panderodontida and (repeatedly) within the Balognathidae (prioniodontids), in which both 3 and 4 pairs of P elements are recorded (cf. Aldridge et al. 2013, for summary).
A fused cluster of Cordylodus lindstromi (a proconodontid) from the Tremadocian (Ordovician) of Nevada, USA, preserves a partial half-apparatus (Smith et al. 2005). It consists of two compressed elements that are homologous with the P1 and P2 positions in the compressed suite of Panderodus, together with an S element with a rounded cusp cross-section. The identification of M–S–P element homology in the Proconodontida (Cordylodus), the Protopanderodontida (Besselodus) and the Panderondontida (Panderodus) provides a secure template for element homology between the three most speciose groups of primitive conodonts and the more morphologically complex apparatuses of derived prioniodontid conodonts. This will permit a re-evaluation of apparatus composition, taxonomy and phylogeny in primitive conodonts and allow a 15- or 17-element template to be used in apparatus reconstructions. Although it is likely that there is architectural disparity in primitive conodonts, the identification of M–S–P architecture in the Proconodontida, the Protopanderodontida and the Panderodontida also indicates that it is shared, and probably plesiomorphic, across these groups. In turn this suggests that proposals for bi- and trimembrate coniform apparatuses (e.g. Barnes et al. 1979) are, at least in part, a consequence of partial reconstructions, as was suggested for the Early Ordovician genus Ulrichodina by Landing (in Landing & Westrop 2006). In addition, when apparatuses of primitive conodonts with low numbers of element morphotypes do occur, it may be due to limited morphological differentiation in an otherwise standard M–S–P architecture, as in Bessolodus.
Implications for the evolution of a macrophagous predatory lifestyle in vertebrates
The presence of laterally symmetrical muscle blocks and a feeding apparatus that has been subject to almost pure dorsoventral collapse during decay both lead to the conclusion that Panderodus had a dorsoventrally flattened body during life. In the absence of other primitive taxa with preserved soft tissues it is not clear whether this is a character shared across proconodontids, protopanderodontids and panderodontids, or whether it is linked to the cosmopolitan distribution and presumed pelagic habitat of Panderodus (Barnes & Fåhræus 1975; Sweet & Bergström 1984). Either way, it does demonstrate the presence in conodonts of a contrasting body plan to that of the laterally compressed, anguilliform prioniodontids as evidenced by the soft tissues of Clydagnathus and Promissum.
Wear patterns on conodont elements are consistent with their use for predatory feeding (Purnell 1995; Purnell & Jones 2012), including those indicative of shearing on a coniform element of the Ordovician taxon Drepanoistodus (Purnell 1995; Fig. 2D). The function of coniform elements for prey capture and food processing is also corroborated by functional analyses describing their ability to accommodate the stress and strain (Murdock et al. 2014) and differentiation of piercing and grasping functions based on morphology alone (Murdock et al. 2013b). Furthermore, calcium isotopes have provided independent support for the interpretation of conodonts as macrophagous zooplanktivores within oceanic food webs (Balter et al. 2019). As a result, primitive conodonts with feeding apparatuses composed of coniform elements, such as Panderodus, can be considered to be the earliest vertebrate predators, with a fossil record that extends back to the late Cambrian and which significantly pre-dates the earliest jawed fishes (Janvier 1996; Smith et al. 2002). Conodonts went on to become considerably more diverse than other Ordovician–Silurian vertebrates, with a global species diversity of up to 100 and a standing generic diversity of 30–40 in the Ordovician, which decreased to 10–15 in the Silurian (Sweet 1988; Smith et al. 2002). Furthermore, the environmental and biogeographic distribution of Ordovician–Silurian conodont taxa suggests that these early vertebrate predators were wide-ranging in oceanic ecosystems, with a variety of nektobenthic and vertically tiered pelagic niches (Seddon & Sweet 1971; Barnes & Fåhræus 1975; Zhen & Percival 2003) that have been confirmed by palaeotemperatures derived from oxygen isotopes in conodont apatite (Wheeley et al. 2018).
Conclusion
The exceptionally preserved specimen from the Waukesha Lagerstätte demonstrates that Panderodus had a dorsoventrally flattened body with transverse myomeres that preserve muscle fibrils. The rostral feeding apparatus is composed of 17 coniform elements, with eight dextral–sinistral pairs in opposition across the sagittal plane of the animal and an additional symmetrical S0 element that sat on the midline. The elements of the feeding apparatus are divided morphologically into a rostrad costate suite of paired M–S elements and a caudad compressed suite with three pairs of P elements. The identification of an aequaliform S0 element using CT scanning allows homologies to be identified with the feeding apparatuses of more derived, and better understood, prioniodontid conodonts that have feeding apparatuses of contrasting perpendicular, rather than parallel, architecture. The costate suite has a pair of M elements with arcuatiform morphology, which lies rostral to four pairs of S4–S1 elements, with the S4 pair adjacent to the M elements. The four pairs of S elements are of graciliform morphology. Three pairs of P elements are identified here for the first time in a primitive conodont with coniform elements. The P1 elements at the caudal end of the apparatus are short-cusped, erect truncatiform elements with tortiform P2 elements situated immediately rostrad. The most rostral elements of the compressed P suite are a pair of falciform P3 elements. Cartilaginous supports for the feeding apparatus that inserted into the basal cavities of the apatitic elements are preserved as thin films of calcium phosphate. Together, the CT scanning and x-ray mapping provide insights into the anatomy of the body and the feeding apparatus of primitive conodonts. The improved understanding of the apparatus architecture of Panderodus also provides a much-needed template for the reconstruction of apparatuses from isolated conodont elements in acid residues, and a guide to homology across the entire clade. The soft tissues of Panderodus confirm a diversity of body forms in conodonts, and the Waukesha specimen highlights the important role of conodonts as macrophagous vertebrate predators in a wide range of Palaeozoic oceanic environments.
Acknowledgements
Carrie Eaton (University of Wisconsin Geology Museum) is thanked for her assistance in locating and loaning the Waukesha specimen, and Dr Thomas Davies (University of Bristol) for assistance with XTM scanning. Ivan Sansom (University of Birmingham) and Emilia Jarochowska (FAU Erlangen-Nuremberg) provided perceptive and detailed reviews that greatly improved the clarity and accuracy of the manuscript.
Author contributions
MPS conceived the study; DJEM carried out the investigation and provided the figures; and DJEM and MPS wrote and edited the paper, and approved the final version.
Open Research
Data archiving statement
Scan data for this study are available in the MorphoSource repository: https://www.morphosource.org/projects/000346877; https://doi.org/10.17602/M2/M347013; https://doi.org/10.17602/M2/M350281; https://doi.org/10.17602/M2/M353486; https://doi.org/10.17602/M2/M353491; https://doi.org/10.17602/M2/M353496; https://doi.org/10.17602/M2/M353499. Additional material is available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.0p2ngf20z.




