Cu2WS4-PEG Nanozyme as Multifunctional Sensitizers for Enhancing Immuno-Radiotherapy by Inducing Ferroptosis
Abstract
Unavoidable damage to normal tissues and tumor microenvironment (TME) resistance make it challenging to eradicate breast carcinoma through radiotherapy. Therefore, it is urgent to develop radiotherapy sensitizers that can effectively reduce radiation doses and reverse the suppressive TME. Here, a novel biomimetic PEGylated Cu2WS4 nanozyme (CWP) with multiple enzymatic activities is synthesized by the sacrificing template method to have physical radiosensitization and biocatalyzer-responsive effects on the TME. Experiment results show that CWP can improve the damage efficiency of radiotherapy on breast cancer cell 4T1 through its large X-ray attenuation coefficient of tungsten and nucleus-penetrating capacity. CWP also exhibit strong Fenton-like reactions that produced abundant ROS and GSH oxidase-like activity decreasing GSH. This destruction of redox balance further promotes the effectiveness of radiotherapy. Transcriptome sequencing reveals that CWP induced ferroptosis by regulating the KEAP1/NRF2/HMOX1/GPX4 molecules. Therefore, owing to its multiple enzymatic activities, high-atomic W elements, nucleus-penetrating, and ferroptosis-inducing capacities, CWP effectively improves the efficiency of radiotherapy for breast carcinoma in vitro and in vivo. Furthermore, CWP-mediated radiosensitization can trigger immunogenic cell death (ICD) to improve the anti-PD-L1 treatments to inhibit the growth of primary and distant tumors effectively. These results indicate that CWP is a multifunctional nano-sensitizers for radiotherapy and immunotherapy.
1 Introduction
Breast carcinoma is one of the most common disease in women, and its incidence rate increases annually worldwide.[1] The main clinical treatment of breast carcinoma is radiotherapy alone or combined with other therapies.[2, 3] Radiotherapy uses ionizing radiation to kill cancer cells through structural and biological changes, and it has been widely applied in cancer therapy.[4-6] However, the damage to normal tissue adjacent to tumors and the complex immunosuppressive tumor microenvironment (TME) limit the therapeutic effect of radiotherapy, resulting in unsatisfactory treatment outcomes and metastasis.[7, 8] Therefore, radiotherapy sensitizers are urgently needed. Radiosensitization research based on nanomaterials containing high Z elements is a hot concept in recent years, such as gold, gadolinium, and bismuth[9-12] are used to enhance the intracellular radiation energy deposition due to Compton scattering effect, improve the treatment efficiency and reduce the side effects. Even so, those sensitizers achieve radiosensitization only by physical methods, the specificity of TME also limits the effectiveness of radiotherapy, such as intracellular over-expressed GSH could consume the as-generated reactive oxide species (ROS) produced through radiotherapy.[8] In addition, radiotherapy is mainly used to eliminate the primary local tumors, with limited effect on metastatic tumors far from the radiation area. Currently, anti-PD-L1(aPL-L1) immune checkpoint inhibitors as a promising treatment method for metastatic tumor therapy,[13-15] but low response rates also limit its therapeutic effect due to immunosuppressive TME. Nanomaterial mediated therapy can effectively trigger ICD which are widely synergized with immune checkpoint blockade to boost antitumor immunity for the suppression of primary and distant tumors as well as metastasis.[16, 17] Thus, designing and synthesizing nano-radiosensitization agents to reduce the effective radiation dose and overcome microenvironment resistance is a promising strategy to integrate radiotherapy and immunotherapy (immuno-radiotherapy).
Nanozyme was one of the top ten emerging technologies in chemistry in 2022 since the peroxidase (POD)-like activity of Fe3O4 nanoparticles was first reported in 2007.[18, 19] Their enzymatic activities to manipulate TME combined with the physical-chemical properties of nanomaterials have shown enormous potential for suppressing tumor growth.[20-23] Those nanozymes manipulate TME by catalyzing intracellular H2O2 to generate ·OH,[24-26] as well as reducing intracellular GSH levels[27, 28] or activating signaling pathways[29] to be beneficial for cancer treatment. Moreover, some semiconductor nanozymes with narrow bandgap energy can further enhance their enzyme-like activity through X-ray irradiation, achieving synergistic tumor-inhibition effects.[30, 31] Many nanozymes could also be combined with various external stimuli, including radiation, magnetic field, light, ultrasound, and microwave to achieve collaborative treatment in multiple ways.[32-35] Although many nanozymes can not only facilitate the eradication of local tumor by regulating the tumor microenvironment but also activate antitumor immunity to suppress metastatic tumors, figuring out the specific regulatory pathways involved is still necessary.
Tungsten elements are widely used as potential candidates for radiotherapy sensitization to enhance radiation dose deposition in cancer cells.[36] In addition, copper is a well-known essential trace element playing an important role in human metabolism, primarily as a cofactor of many metalloenzymes.[37] Nanoparticles containing multiple metal elements have been widely used in the combination therapy of cancers due to their excellent enzymatic activity and ability to respond to external stimuli.[38-41] Therefore, copper and tungsten elements were selected to synthesize Cu2WS4 nanoparticles in our work. Herein, a multifunctional hollow nanozyme of PEGylated Cu2WS4 (CWP) was designed and synthesized via a typical sacrificial template method to achieve radiotherapy-sensitization and adaptive anti-tumor immunity capacities by inducing ICD (Scheme 1). Nanoparticles containing the transition metal elements with Fenton-like properties of catalytic hydrogen peroxide generate hydroxyl radicals (·OH) that damage cancer cells and efficiently inhibit the growth has been widely researched. Multifunctional nanozymes containing one or more transition metals with excellent GSH-oxidase activity are also widely used to consume overexpressed GSH in the tumor microenvironment to enhance cancer treatment efficiency.[42, 43] These enzyme-like activities can regulate the TME and cooperate with High-Z W element to enhance the intracellular radiation energy and radiation sensitivity, effectively achieving radiotherapy sensitization through multiple pathways to inhibiting the tumor cells and triggering ICD. CWP is also a contrast imaging agent for photoacoustic tomography due to its broad absorption in the infrared region. Photoacoustic tomography (PA) imaging can guide radiotherapy mediated by CWP and collaborate with αPD-L1 to enhance immunotherapy and inhibit local or distal tumor growth. Importantly, we found that CWP does not require complex functional group modifications to enter the nucleus enhancing the damage to DNA caused by radiation therapy. Transcriptome sequencing and protein immunoblotting experiments were performed to understand the mechanism of action of CWP, finding that CWP could cause ferroptosis in 4T1 cells by regulating downstream proteins such as KEAP1/NRF2/HMOX1/GPX4 for radiosensitization. Furthermore, CWP mediated radiosensitization effectively promotes the efficacy of immunotherapy by amplifying immune response. Thus, CWP is a multifunctional sensitizer that acts synergistically with multiple treatments and is worth exploring in breast carcinoma therapy.
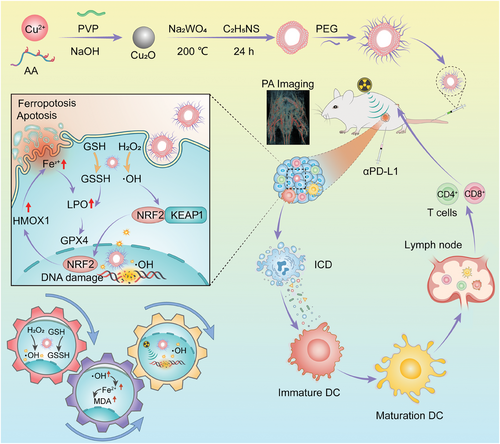
2 Results and Discussion
2.1 Structural Characterization of CWP
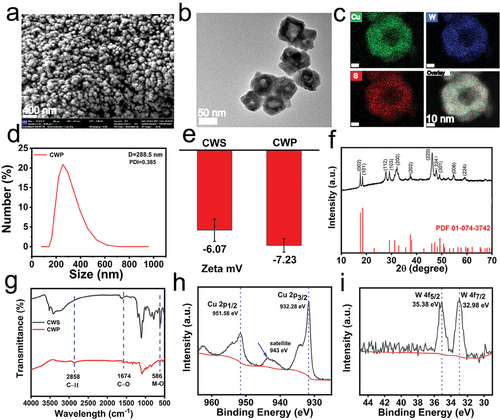
CWP was synthesized by using Cu2O nanospheres as self-sacrificial templates through a modified solvothermal method.[44] Moreover, the surface was modified with PEG to increase its biocompatibility. First, CWP had a spherical structure verified by scanning electron microscopy (SEM). Hollow nanospheres were further viewed by transmission electron microscopy (TEM), revealing a size of ≈80 nm (Figure 1a,b). The crystal lattice distance observed by High resolution (HR) TEM of CWP was ≈0.50 nm and corresponded to the (002) plane of the body-centered tetragonal CWS (Figure S1, Supporting Information). Energy dispersive spectrometer mapping further proved the coexistence of Cu, W, and S elements in the hollow CWP nanozyme (Figure 1c). Moreover, the average diameter of CWP was 288.5 nm according to dynamic light scattering (DLS) measurements (Figure 1d), and the slightly enlarged size under aqueous condition compared to the electron microscopy was due to the hydrated size of CWP (fluid dynamics diameter), usually measured to be larger than the dry nanoparticle size.[45] The zeta potentials of Cu2WS4 were −6.07 mV and changed to −7.23 mV after being modified with PEG (Figure 1e). The X-ray diffraction (XRD) analysis, shows that the diffraction data of CWP matched the crystal data of Cu2WS4 (JCPDS No. 01-074-3742).[46] The Jade software analysis further indicated that pure CWP was synthesized (Figure 1f). Fourier-transform infrared (FTIR) spectroscopy showed the successfully modified PEG on the surface of Cu2WS4 (Figure 1g). The apparent peak at 581 cm−1 in Cu2WS4 and CWP was due to the stretching vibrations of metal-S.[47] Moreover, the characteristic peaks of C─H stretching groups at 2858 cm−1 and the characteristic peaks of C═O stretching groups at 1647 cm−1 in CWP proved the existence of PEG on the surface.[48] The X-ray photoelectron spectroscopy (XPS) spectra analysis further evidenced the successful synthesis of CWP (Figure S2, Supporting Information). The characteristic absorption bands at 951.58 and 932.28 eV in the Cu2P spectrum and the satellite peaks of Cu(II) (943 eV) were also observed. These absorption bands suggest the binding energy of Cu2+, proving the existence of a Cu+/Cu2+ redox couple (Figure 1h). In addition, the characteristic absorption band of the W4f spectrum at 35.38 and 32.98 eV proved the existence of a W4+/W6+ redox couple (Figure 1i).[49] The spectrum of the S2p peak was assigned to the binding energy of 169.28 and 161.78 eV, consistent with S2P in the sulfide phases (Figure S3, Supporting Information). To evaluate the stability of as-synthesized CWP under physiological conditions, the hydrodynamic size was measured after incubation in various media (DI water, PBS, DMEM, DMEM containing 10% FBS) for 24 h (Figure S4, Supporting Information). No obvious size change was observed in all incubating media, implying an excellent stability of CWP.
2.2 POD and GSH-Oxidase Enzymatic Activity of CWP
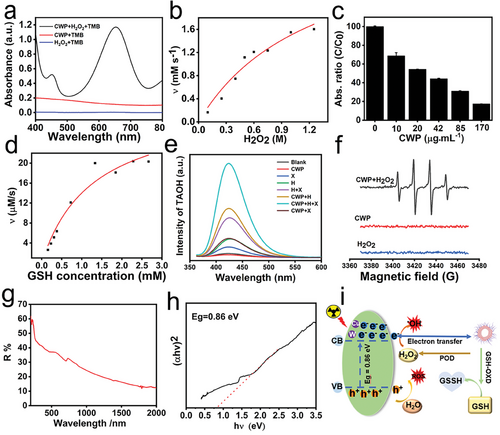
Recently, studies have reported that transition metal-doped nanozymes act as nanoreactors that specifically react with endogenous substances within the TME to adaptively enhance the effectiveness of cancer treatment. Here, the POD-like and GSH oxidase-like activities of CWP were explored, which generated ·OH and consumed glutathione. The POD-like activity of CWP was revealed by the presence of 3,3,5,5-tetraemethylbenzidine (TMB) and H2O2 (10 mm). TMB was oxidated by the ·OH generated by the hydrogen peroxide activity of CWP with absorption at 652 nm. Compared with CWP or H2O2 alone, no significant absorption was found (Figure 2a), validating the POD-like activity of CWP ascribed to the presence of multivalent elements of Cu2+ and W4+. The consumption of glutathione was enhanced with the concentration of CWP (Figure 2c), manifesting that CWP had GSH oxidase-like activity that reduced the GSH content. The catalytic efficiency of the multienzyme-like activity of CWP was evaluated according to Michaelis–Menten steady-state kinetics. The fitting curves of the POD-like activity were obtained by plotting the relationship between the initial rate of the enzymatic reaction and substrate concentration (H2O2) (Figure 2b). The Vmax values of the POD-like activity were 3.38 mm s−1, the Km was 1250 mm, and the catalytic efficiency of the POD-like activity of CWP was 122.8 µm s−1. Similarly, the values of Vmax and KM for GSH oxidase activity were 0.032 mm s−1 and 1.28 mm, respectively (Figure 2d), according to the velocity equation of Michaelis–Menten. The GSH catalytic efficiency of CWP was 1.13 × 103 µm s−1 (Table 1).
| Vmax [mm s−1] | Km [mm] | Kcat [s−1] | Efficiency [µm s−1] | |
|---|---|---|---|---|
| Peroxidase | 3.38 | 1250 | 153.6 | 122.8 |
| GSHoxidase | 0.032 | 1.28 | 1.45 | 1.13 × 103 |
Furthermore, this work successfully synthesized CWP nanozyme with the high atomic number element tungsten to enhance the X-ray energy deposition. Using terephthalic acid (TPA) as a tracing agent for generating ·OH from CWP. As shown in Figure 2, X-ray alone catalyzed H2O to produce a small amount of ·OH due to ionization and amplified this effect in the presence of CWP due to enhanced radiation energy deposition of high Z W elements. The X-rays also promoted the enzymatic catalytic activity of CWP in the CWP + H2O2 + X-rays study group better than them work alone. The ·OH generated by CWP was further tracked by electron spin resonance (ESR). A typical 1:2:2:1 peak of ·OH was observed in the CWP + H2O2 group but not in the H2O2 and CWP groups (Figure 2f). These results indicate that CWP effectively increased the concentration of ·OH and acted as an efficient radiotherapy enhancer owing to its multiple enzymatic activities and high-atomic W elements. This responsiveness of CWP to both endogenous stimuli (enzyme-like activity) and exogenous stimulus (X-ray irradiation) is mutually beneficial to realize tumor elimination more effectively. In addition to the content of high-Z metal elements, studies have also reported that X-ray can promote the catalytic activity of nanozymes due to their semiconductor structure with narrow bandgap energy, displaying outstanding electron-hole separation abilities to promote the generation of ROS.[50-52] Through the Tauc-plot method by UV–vis diffuse reflectance spectrum, the bandgap energy (Eg) value of CWP was determined to be 0.86 eV (Figure 2g,h). This value is lower than most of those reported for inorganic nanocatalysts, meaning that more electron transitions are stimulated by a lower energy.[53-55] The radiosensitizing mechanism of CWP occurred through multiple paths (Figure 2i). CWP not only contains W elements that increase the energy deposition and destroy the DNA structure of tumor cells by potentiating water radiolysis and generating ·OH, but also produces abundant ROS and GSH oxidase-like activity that decreases GSH levels, increasing the oxidative stress in the tumor and enhancing the sensibility to radiation. Moreover, CWP nanozyme was also equipped with a semiconductor structure with narrow bandgap energy, resulting in efficient electron-hole separation and enzyme-like activity.
2.3 CWP-Mediated Radiosensitization In Vitro
Considering the effective production of cytotoxic ·OH under X-ray irradiation by CWP, subsequently investigated the anti-tumor efficacy of CWP in vitro (Figure 3a). CCK-8 was used to test the administration of CWP against human renal tubular epithelial cells (HK-2) at different concentrations. CWP did not cause significant cell death at 200 µg mL−1 or cytotoxicity (Figure S5, Supporting Information). Contrarily, the survival rate of 4T1 cells decreased as the concentration of CWP increased (Figure 3b). The higher mortality rate of 4T1 compared to HK-2 can be attributed to the synergistic effects of the peroxidase activity of CWP and the overexpression of H2O2 in tumor cells. Interestingly, the cell viability of 4T1 cells was 83.2% when treated with CWP at 25 µg mL−1 and dramatically reduced to 56.6% with the X-ray exposure. To minimize the adverse effects of nanomedicines as much as possible, 20 µg mL−1 was chosen for dosage in subsequent experiments unless otherwise stated. To demonstrate that CWP synergistic acts with the X-rays to increase the intracellular levels of ROS, DCFH-DA was used to detect them. The strongest green fluorescence was observed in the CWP + X-rays group compared with untreated cells (Figure S6, Supporting Information). Similarly, the proportion of apoptotic cells in the CWP + X-rays group increased by 30% compared with untreated cells (Figure S7, Supporting Information). As shown in Figure 3c, the survival fraction of breast cancer cells treated with X-rays or CWP + X-rays was obtained by counting the number of colonies in Figure S8 (Supporting Information). The sensitivity enhancement ratio (SER) was 1.425 calculated from the data fitting curve. The panel cloning experiment was also used to evaluate the radiotherapy sensitization effect of CWP on A549 cells, and its sensitization coefficient SERA549 was 1.312, which confirmed the radiotherapy sensitization of cancer cells by CWP (Figure S9, Supporting Information). Considering that CWP has a better sensitization effect on 4T1, which was selected as the subsequent evaluation model. And 4 Gy was the X-ray intensity for the following experiments in vitro unless stated otherwise. To fully demonstrate the performance of CWP, the double-strand breakage of DNA was also examined by the immunofluorescence staining with the phosphorylation marker γ-H2AX to demonstrate the DNA damage caused by the combined action of CWP and X-rays. The brightest fluorescence was observed in the nucleus of the 4T1 cells treated with CWP + X-rays, indicating that CWP strengthen the DNA damage with the assistance of X-rays (Figure 3d). Also evaluated the mitochondrial damage of 4T1 cells induced by CWP. The synergistic effect of CWP and X-rays further reduced the mitochondrial membrane potential of 4T1 cells, as demonstrated by the increasing intensity of the green fluorescence revealed by the JC-1 kit, indicating that the mitochondria in 4T1 cells were damaged. Moreover, the relative GSH levels detected by the DTNB reagent decreased in 4T1 cells depending on the concentration of CWP (Figure 3e). Even at concentrations as low as 20 µg mL−1, CWP reduced 25% of the intracellular content of GSH (Figure S10, Supporting Information). This ability to consume GSH within cancer cells can improve the efficiency of various ROS-dependent therapies, and considering cytotoxic ·OH produced by the POD-like activity of CWP, its enzyme-like activities work together with the X-rays to cause significant damage to cancer cells. In addition, the highest accumulation of lipid hydroperoxides (LPO) was observed in the CWP + X-rays group (Figure 3d).
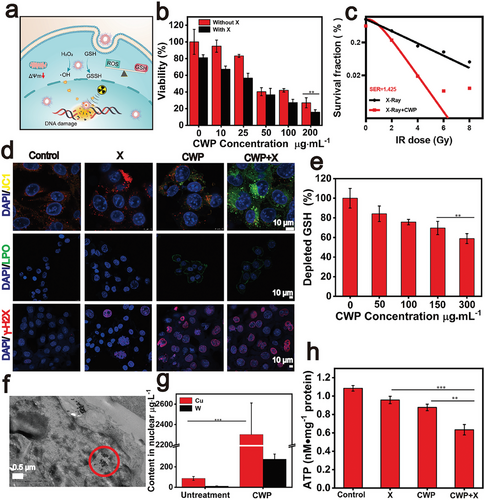
Next, TEM are used to observe the endocytosis of CWP in ultrathin sections of 4T1 cells. Surprisingly, CWP was found in the nucleus, where it may produce free radicals with the assistance of X-rays and directly damage the DNA, improving the efficiency of radiotherapy (Figure 3f). To further demonstrate that CWP entered the nucleus, nuclear extraction from different treatment groups was digested using the microwave digestion method. ICP-MS (Thermo Fisher) was then conducted to evaluate the distribution of Cu and W elements in 4T1 cell nucleus. As shown in Figure 3g, the content of W and Cu in the nucleus was far higher than that of the untreated group, which confirmed the ability of CWP to penetrate the nucleus. Such a mechanism is speculated to be related to its particle size, surface physicochemical properties. The nucleus is the subcellular compartment where genetic information is stored and transcription mechanisms are performed.[56] Therefore, drugs that can enter the nucleus enable X-rays to act precisely upon DNA molecules, killing cancer cells more directly and efficiently. For example, many drugs that tend to accumulate in the nucleus can be delivered to this subcellular compartment through different strategies, such as modification of functional groups on the surface of nanoparticles.[57, 58] However, few candidates with the capacity to enter the nucleus without using targeted antibodies have been reported.
Apoptotic bodies were also observed after cells were treated with X-rays alone, and nuclear-cytoplasmic pyknosis was observed after treatment with CWP (Figure S11, Supporting Information). Typical apoptotic marker proteins BCL-2 decreased after the treatment with CWP + X-rays (Figure S12, Supporting Information), confirming that CWP-mediated radiotherapy sensitization could also induce apoptosis in 4T1 cells.
Moreover, the fact that nanozyme-mediated therapy combined with external stimuli effectively modulates ICD has been well-recognized.[59, 60] We observed that CWP-mediated sensitization significantly increased the expression of calreticulin (CRT) on the cell membrane by immunofluorescence assay (Figure S13, Supporting Information). Notably, due to the damage caused by radiotherapy sensitization, the migration of high mobility group box 1 (HMGB1), another indicator of ICD was also tracked using immunofluorescence staining. HMGB1 was released from the nucleus after CWP-mediated radiotherapy shown in Figure S13 (Supporting Information). Intracellular ATP levels were also evaluated using an ATP kit (Beyotime). As shown in Figure 3h, the changes CWP-mediated X-ray sensitization led to the lowest intracellular ATP content. These results demonstrate that CWP-mediated radiotherapy effectively causes ICD in 4T1 cells.
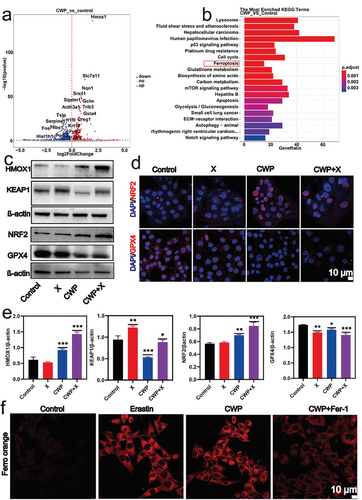
2.4 CWP Inducing Ferroptosis via KEAP1/NRF2/HMOX1/GPX4 Pathway
Given the evidence that CWP is a potential radiotherapy sensitizer, we conducted a transcriptome sequencing study to probe the underlying mechanism. The volcano plot in Figure 4a shows the significant differences in gene expression between the groups with and without CWP (Figure 4a). The expression levels of several genes changed after 4T1 cells were treated with 20 µg mL−1 CWP for 24 h. The most obvious change expression of HMOX1, an enzyme that catalyzes the heme catabolism and regulates intracellular iron metabolism and oxidative stress responses.[61, 62] Based on the analysis of KEGG pathway enrichment results, ferroptosis pathway may be an important signaling pathway induced by CWP (Figure 4b), and HMOX1 is involved in the regulation of ferroptosis pathway. Research has shown that KEAP1/NRF2/HMOX1/GPX4 is an important pathway for regulating ferroptosis pathway.[63, 64] To further validate this result, Real-time quantitative PCR and western blotting (WB) were conducted. A quantitative polymerase chain reaction (q-PCR) validated that the relative expression of HMOX1 mRNA was higher than that of the untreated group (Figure S14, Supporting Information). Western blot results also demonstrated that CWP treatment led to the upregulation of HMOX1 (Figure 4c). The expression of NRF2 was upregulated after CWP treatment from western blot and immunofluorescence results as shown in Figures 4c,d. Confocal microscope images indicated that the red fluorescence of NRF2 in the nucleus was enhanced by CWP treatment compared to that of the untreated group. NRF2 is primarily regulated by KEAP1, a substrate adaptor for a Cul3-containing E3 ubiquitin ligase.[65] KEAP1 can be covalently captured by electrophiles oxidized to sulfenic acid from disulfides, releasing NRF2 from the low-affinity binding site.[66] Therefore, the ·OH generated by CWP-mediated radiotherapy leads to the oxidation of KEAP1, resulting in the enhanced fluorescence of NRF2 observed in the nucleus, confirmed by the downregulation of KEAP1 (Figure 4c). The downregulation of GPX4 showed by immunofluorescence (Figure 4d) and western blot (Figure 4c) also proved that CWP effectively caused ferroptosis in 4T1 cells. These results demonstrate that X-rays strengthen the ability of CWP to produce ROS and consume GSH, inducing ferroptosis by disturbing the KEAP1/NRF2/HMOX1/GPX4 signaling pathway. The strong red fluorescence in 4T1 cells treated with CWP was the same as cells treated with iron death agonist erastin, and the fluorescence of probes decreased after adding the iron death inhibitors fer-1, further confirming that CWP could induces ferroptosis (Figure 4e). Furthermore, we evaluated the cell survival rate after treatment with ferroptosis inhibitors using the CCK-8 assay kit (Figure S15, Supporting Information), compared with the control group, after 24 h of combined treatment with CWP and X-ray on 4T1 cells, the cell survival rate was 57%. The survival rate increased to 72% after being treated with ferroptosis inhibitor, which elucidates the important role of ferroptosis in CWP mediated radiotherapy.
2.5 Pharmacokinetics and Biodistribution of CWP
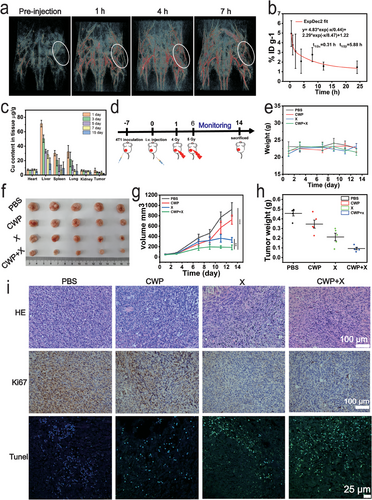
Accurate tumor delivery is an important strategy to increase treatment efficiency. The near-infrared absorption of CWP endows it with the potential to be a contrast agent for photoacoustic imaging (PA). CWP exerted clear PA signals at 808 nm laser stimulation compared with aqueous solutions (Figure S16, Supporting Information). Thus, the PA signal in 4T1 tumor-bearing nude mice was detected after intravenous injection of CWP at different times. As shown in Figure 5a, a strong PA signal was visualized in the blood vessels in the tumor area at 808 nm laser excitation after CWP injection 7 h. The PA signal was also monitored in the tumor area at 1640 nm laser excitation owing to the broad infrared absorption of CWP (Figure S17, Supporting Information). The circulation of CWP in the body after its intravenous injection was determined by the W levels in the blood at different times using ICP-MS. The systemic circulating half-life for the distribution and elimination phases of CWP nanozyme was 0.31 and 5.88 h, respectively (Figure 5b).[73] In addition, the distribution of CWP was also estimated during a long period postinjection. The contents of Cu and W in the primary organs (the heart, liver, spleen, lungs, and kidneys) and tumor tissue were quantitatively analyzed by ICP-MS on different days (1, 3, 5, 7, and 15 days) (Figure 5c; Figure S18, Supporting Information). Both Cu and W elements were mainly accumulated in the endothelial reticular system (e.g., the liver and spleen). Attributed to the fact that nanomedicine was mainly engulfed by macrophages in the liver and spleen of the endothelial network system after intravenous injection.[67] The accumulation of Cu (6.57 µg g−1 of tissue) and W (1.35 µg g−1 of tissue) at the tumor site peaked 1 day after CWP injection, owing to its enhanced permeability and retention (EPR) effect. And the content of Cu and W in the tumor decreased with time, attributed to the fact that the tumor without any treatment, resulting in a rapid increase in tumor volume and mass. Although CWP was mainly concentrated in the liver and spleen after intravenous injection, both Cu and W in the main organs decreased over time. The Cu (71.3 µg g−1 of tissue) in the liver, (30.2 µg g−1 of tissue) spleen, and (32.33 µg g−1 of tissue) lungs was found 1day postinjection, and in the liver (15.6 µg g−1 of tissue), spleen (9.3 µg g−1 of tissue), and lungs (5.3 µg g−1) at day 15 postinjection, the W (3.2 µg g−1 of tissue) in the liver, (2.4 µg g−1 of tissue) spleen, and (1.7 µg g−1 of tissue) lungs was found 1day postinjection, and in the liver (0.8 µg g-1 of tissue), spleen 0.2 µg g−1 of tissue), and lungs (0.3 µg g−1) at day 15 postinjection. which was attributed to the detoxification of the organism itself. These results indicate that CWP could circulate to the tumor by the EPR effect and be slowly expelled from the body. EPR-dependent drug delivery is complicated by high tumor interstitial fluid pressure (IFP), irregular vascular distribution, and poor blood flow inside tumors.[68] To improve its delivery efficiency enhancing treatment effectiveness is the issue we need to consider next.
2.6 CWP Mediated Radiosensitization In Vivo
Considering the remarkable radiosensitization effect of CWP in vitro and its accumulation in the tumor, we next evaluated the radiosensitization effect of CWP on a subcutaneous tumor model of breast cancer in mice. The mice with a unilateral tumor were randomly assigned to the PBS, X-rays, CWP, and CWP + X-rays groups. The injection time of CWP was considered day 0, and the tumor region was exposed to X-ray irradiation two times based on a schedule (Figure 5d). To minimize the radiation dose and inhibit tumor growth effectively as much as possible, we determined a total irradiation dose of 9 Gy by two times (4 and 5 Gy).[50, 73] The weight of mice in different treatment groups did not fluctuate significantly during the treatment period (Figure 5e), and the histopathological analysis of vital organs (Figure S19, Supporting Information) suggested negligible systemic toxicity in the treatment groups.
Considering the growth curve of tumor volume, the group treated with CWP alone had a slight anti-tumor effect. The X-ray irradiation group showed a 58.2% tumor inhibition, whereas the CWP + X-rays group showed the greatest tumor growth inhibition (TGI) index at the end of the treatment period (79.7%) (Figure 5f,g). Moreover, the tumor weight analysis confirmed the tumor eradication capacity of CWP (Figure 5h). The therapeutic effects of different treatment groups were further analyzed by histopathology. The CWP + X-rays group showed a significant increase in nuclear pyknosis and tissue necrosis in the HE staining film compared to the untreated group (Figure 5i). The most significant signal reduction of Ki67-positive cells occurred after the CWP + X-rays treatment; this tumor proliferation suppression tendency indicates the positive effect of CWP-guided radiotherapy sensitization. Cell damage markers, such as terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) immunofluorescent staining were used to evidence the severe damage caused by the treatment with CWP + X-rays. Furthermore, ICD indicators, such as the increased expression of CRT on the membrane of 4T1 cells and the transfer of HMGB1 from the nucleus, were also detected. Figure S20 (Supporting Information) shows that the expression of HMGB1 was downregulated in mice treated with CWP + X-rays. The CRT translocation in tumorous tissue was notable in the immunohistochemistry samples from the CWP + X-rays groups, validating that this treatment amplifies the ICD effect in mice. The maximum downregulation of Bcl-2 was also observed through immunohistochemistry (Figure S21, Supporting Information), indicating that CWP-mediated radiosensitization causes tumor cell apoptosis in vivo. Moreover, ferroptosis associated with GPX4 expression was significantly downregulated in the tumor tissue sections from the CWP + X-rays group (Figure S22, Supporting Information). These results indicate that the multifunctional nanozyme CWP combined with radiotherapy effectively inhibits tumor growth. Furthermore, ferroptosis and apoptosis play an essential role in CWP-mediated radiosensitization. Moreover, the damage-related molecular patterns released by the ICD associated with this treatment initiate a series of cellular responses, ultimately activating both innate and adaptive immune responses.
2.7 CWP-Mediated Radiotherapy Sensitization Synergistic PD-L1 Blockade
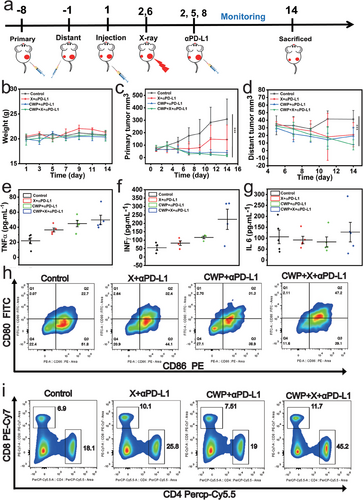
Our results demonstrate that CWP-mediated radiosensitization not only inhibits tumor growth, but also mediated ICD activation. Next, we evaluated the inhibitory effect of CWP-mediated radiation sensitization combined with PD-LI blockade against distal tumors using a BALB/c mice bilateral tumor model. The primary tumor of 4T1 cells was inoculated into the right dorsal on day −8 and the distant tumor was inoculated into the left dorsal on day-1 to establish a bilateral subcutaneous tumor model. Figure 6a illustrates the treatment process in detail. Randomly grouped bilateral tumor model mice received an intravenous injection of CWP when needed. Subsequently, the original tumors that required irradiation treatment were subjected to 4 and 5 Gy on days 2 and 6, respectively. If necessary, anti-PD-L1 (75 µg kg−1) was administered through an intraperitoneal injection at corresponding time point. No evident changes in mouse body weight at the end of therapy were observed (Figure 6b), indicating that the treatments mediated by CWP have no significant toxicity. Compared with the control group, the aPD-L1 + X-rays and CWP + aPD-L1 groups treatments slowed down tumor growth with a TGI of 53.2 and 87%, respectively, whereas the CWP + X-rays + aPD-L1 treatment showed the best tumor growth inhibition effect in the primary tumor with a TGI of 95.1% (Figure 6c; Figure S23, Supporting Information). The PD-L1 antibodies further enhanced the immune system to attack the tumors. The best inhibition effect on the distant tumor also occurred in the CWP + X-rays + aPD-L1 group (Figure 6d; Figure S24, Supporting Information). Cytokines are proteins that regulate immune function, and their concentration changes are used to evaluate the immune status of the body. The levels of immune-associated cytokines in serum were determined after different treatments by enzyme-linked immunosorbent assay, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interferon-γ (IFN-γ). These cellular inflammatory factors increased after the CWP + X-rays + aPD-L1 treatment (Figure 6e–g). Moreover, these cytokines increased in the early stage of the treatment, indicating that this synergistic therapy can inhibit tumor cell growth by activating the immune system. Next, the percentage of mature dendritic cells (DCs) (CD80+ and CD86+) in the lymph nodes near the primary tumor was determined (Figure 6h). The frequency of mature dendritic cells increased by ≈10% in the CWP + aPD-L1 and X-rays + aPD-L1 groups, whereas the mature DCs (CD80+ and CD86+) increased by 24.5% after the CWP + X-ray + aPD-L1 treatment, indicating that DCs are effectively stimulated to mature by the antigens released by ICD (Figure 6h). The population of activated cytotoxic T lymphocytes (CTLs) in the spleen of mice was also explored further to illustrate the anti-tumor immune mechanism of this synergistic therapy. Flow cytometry showed that CWP + aPD-L1 and X-rays + aPD-L1 treatments recruited 3.2% and 0.61% more tumor-infiltrating CD8+ T lymphocytes (CD3+ and CD8+), respectively, than those of the untreated group, whereas the CWP + X-rays + aPD-L1 treatment recruited 6.8% more CD8+ T lymphocytes that those of all the other groups. T helper cells (Th) cells (CD3+, CD4+, and CD8−) infiltration also increased by 7.7%, 0.9%, and 27.1% in the CWP + aPD-L1, X-rays + aPD-L1, and CWP + X-rays + aPD-L1 groups, respectively, compared with those of the untreated group (Figure 6i). These flow cytometry analysis results indicate that the proportion of cytotoxic T and Th cells was the highest after the CWP-mediated radiotherapy sensitization synergistic immunotherapy, demonstrating its effectiveness and feasibility.
To date, nanoparticles containing high atomic number (Bi, W, and Au) have been reported for cancer radiotherapy and with elicitation of robust immune response.[9, 10] But the specific mechanism of radiotherapy sensitization is unknown. Transcriptome and WB demonstrate that X-rays strengthen the ability of CWP to produce ROS and consume GSH, inducing ferroptosis by disturbing the KEAP1/NRF2/HMOX1/GPX4 signaling pathway. This effective lower radiation dose and ferroptosis-inducing radiosensitizer is a promising candidate to integrate radiotherapy and immunotherapy (immuno-radiotherapy) And the therapeutic strategy of enhancing ferroptosis or necroptosis and immunotherapy stimulated by a multifunctional Nano platform is a promising way reported recently.[69-71] SO, CWP is a multifunctional nanosensitizers for radiation and immunotherapy, providing more evidence for potentiating cancer therapeutic strategy.
2.8 Biosafety Assessment of CWP
Then, we systematically administered CWP at different dosages (0, 10, 20, 25, and 50 mg kg−1) into female Blab/c mice and recorded their weight change during the observation period. The main organs were evaluated by pathological observation of their tissue sections. The HE staining results did not show noticeable lesions after 14 days of treatment (Figure S25, Supporting Information). There were no evident pathological abnormalities in all treatment regimens, verifying CWP biosafety. No significant pathological weight changes in mice were observed during the observation period even at doses of 50 mg kg−1 (Figure S26, Supporting Information). After injecting different doses of CWP for 14 days, whole blood was collected for blood routine analysis, and there was no significant difference in all indicators compared to mice without CWP treatment (Figure S27, Supporting Information). The hemolysis experiment also proved that no hemolysis was observed even when the co-incubation concentration reached 250 µg mL−1 (Figure S28, Supporting Information). These results imply that CWP possesses ideal biocompatibility characteristics and is a candidate for clinical translational applications in cancer treatment. In general, CWP alleviates the radiotherapy resistance generated by the TME and reduces side effects, key objectives of an improved treatment. Thus, we designed and successfully fabricated a biocompatible, multifunctional, nanozyme sensitizer (CWP) with radiotherapy and immunotherapy synergistic effects for cancer treatment. First, the enzyme-like activity of CWP that enables the overexpression of hydrogen peroxide and GSH in the TME improves the radiotherapy effects. Second, high-atomic W elements respond to X-ray irradiation by promoting energy deposition, and their access to the nucleus increases DNA damage. Moreover, the ·OH produced by CWP initiates ferroptosis by regulating the KEAP1/NRF2/HMOX1/GPX4 signaling pathway. CWP has a narrow bandgap energy of 0.86 eV that effectively promotes electron-hole separation under the external energy stimulation of X-rays, increasing its enzyme-like catalytic activities. Owing to these therapeutic advantages, CWP collaborates with X-ray treatment to trigger an effective anti-tumor immune response through ICD, further enhancing the anti-tumor immunotherapy with aPD-L1. Thus, CWP has considerable clinical potential as a PA imaging-guided radiosensitization therapeutic agent.
3 Conclusion
In summary, a nucleus-entering, multifunctional CWP nanozyme was constructed via the sacrificial template method to achieve radiotherapy sensitization and amplify the anti-tumor immune response. Taking advantage of the narrow bandgap energy and multivalent elements (high-atomic W and Cu), CWP shows efficient POD-like and glutathione oxidase-like activities to generate ·OH and consume GSH, mitigating the oxidative stress of the TME and being beneficial for radiotherapy. The high atomic number elements and nucleus-entering properties of CWP further improve the efficiency and accuracy of radiotherapy. Moreover, CWP initiates ferroptosis in 4T1 cells by its multienzyme-like activity and further upregulates HMOX1 expression. CWP-mediated radiosensitization effectively causes immunogenic death in cancer cells, synergistically acting with PD-L1 therapy to promote the suppression of both primary and distant tumors. This novel CWP sensitizer has potential applications in tumor synergistic therapy.
4 Experimental Section
Materials
Copper chloride (CuCl2), polyvinylpyrrolidone (PVP), ascorbic acid (AA), (+)-glucose, ethylene glycol (EG), sodium tungstate (Na2WO4·2H2O), glutathione (GSH), 5, 5’-dithiobis (2-nitrobenzoic acid) (DTNB),terephthalic acid (TPA), and thioacetamide (C2H5NS) were purchased from Shanghai Aladdin Bio-Chem Technology Co. Ltd. (China). Sodium hydroxide (NaOH), trichloroacetic acid (TCA), and hydrogen peroxide (H2O2, 30%) were bought from Shanghai KeShi Technology Co. Ltd. (China). Dulbecco's modified eagle's medium (DMEM), penicillin (10000 U mL−1)/streptomycin (10 000 µg mL−1) mixture, fetal bovine serum (FBS) was obtained from CST Company (USA). 2’, 7’-dichlorofluorescein diacetate (DCFH-DA), TUNEL apoptosis assay kit, dimethyl sulfoxide (DMSO), 3,3′,5,5′-tetramethylbenzidine (TMB), ATP assay kit, lipid peroxidation MDA assay kit, and mitochondrial membrane potential assay kit (JC-1) was purchased from Beyotime Biotechnology (China). TrypLE Express and Hoechst 33342 were acquired from Thermo Fisher Scientific, Inc. (USA). The nuclear extraction kit was purchased from Solarbio Life Sciences Nanjing Institute (China). All cell lines were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Female BALB/c mice (8 weeks, ≈20 g) were supplied by Chongqing Teng Xin Bill Experimental Animal Sales Co., Ltd (China). FITC anti-mouse CD80 antibody, PE anti-mouse CD86 antibody, APC anti-mouse CD11c, PE/Cyanine7 anti-mouse CD8a antibody, PerCP/Cyanine5.5 anti-mouse CD4, FITC anti-mouse CD3 antibody, and purified anti-NRF2 antibody were purchased from BioLegend. Mouse IFN-γ, TNFαand IL-10 were ELISAKIT obtained from Solarbio Bioengineering Institute (China). InVivoMab anti-mouse PD-L1 was acquired from BioXcell (Malaysia). Anti-HMGB1, anti-CRT, anti-Bcl-2, anti-GPX4, anti-Ki67, and anti-KEAP1, anti-HO-1 were acquired from Proteintech (China).
Characterization of CWP Nanozyme
Scanning electron microscopic (SEM) images were carried out using a ZEISS GeminiSEM 300 (German). Transmission electron microscopic (TEM) images and high-angle annular dark-field scanning TEM (HAADF-STEM) images were captured by a FEI TALOS G2 high-resolution transmission electron microscope operating at 200 kV. The zeta-potential and size of the nanoparticles were measured in a Malvern Zetasizer, NANO ZS90. The FT-IR characterization was recorded on a Thermo Scientific Nicolet iS20 FT-IR spectrometer. The elemental analysis was performed by an iCAP TQ system (Thermo Scientific, Waltham, MA, USA). The X-ray photoelectron Spectroscopy (XPS) spectra were analyzed by Thermo Fisher Scientific ESCALAB 250Xi Spectrometer Electron Spectroscopy (America). The crystalline form of the nanostructure was characterized by X-ray diffraction (XRD) using an X-ray diffractometer (XRD-7000, Shimadzu) with CuKα radiation (λ = 1.5406 Å). The UV–vis absorption measurement was recorded using a UV-3600 UV–vis spectrophotometer (Shimadu, Japan). Fluorescence spectra were detected by a near-infrared (NIR) fluorescence spectrometer (Thermo Fisher Scientific, USA) fluorescence spectrometer with a Peltier temperature control accessory. The confocal laser scanning microscopy (CLSM) characterization was acquired by using a Leica TCS-SP5 confocal system (Leica, Germany). The flow cytometry data was obtained by a Sony ID 7000 analyzer. The ESR was acquired by (EMX Nano, Bruker). X-rays (160 kVp, 25 mA, 0.3 mm copper filter). Photoacoustic (PA) imaging was conducted using a LOIS-3D PA imaging system (TomoWave Laboratories, USA).
Synthesis of Hollow Cu2WS4 Nanozyme
CuCl2 (0.171 g) and PVP (3.333 g) were dispersed in 100 mL of deionized water under magnetic stirring. Then, NaOH (2 m, 10 mL) and ascorbic acid (0.6 m, 10 mL) were added to the solution and stirred for 1 h. The yellow precipitate Cu2O nanospheres were collected after centrifugation (12 000 rpm, 10 min), and the Cu2O nanospheres were washed with ethanol and DI water three times, respectively, to remove the remaining foreign objects. Then, the samples were dried in a vacuum oven at 60 °C overnight. The hollow Cu2WS4 was synthesized by using the above procedure prepared Cu2O template. Briefly, 40 mg of Cu2O powder and 0.12 g glucose were mixed in 20 mL of ethylene glycol and dispersed by sonication. Then, 70 mg Na2WO4·2H2O and 0.12 g C2H5NS were added into the solution and sonicated for another 30 min. The mixed solution was transferred into a 20 mL Teflon-lined stainless-steel autoclave, and the autoclave was placed in an oven maintained at 200 °C for 24 h. The products were collected by centrifugation at 12 000 rpm for 10 min and were washed thrice with absolute ethyl alcohol and DI water. For PEGylated Cu2WS4, Cu2WS4 was mixed with NH3-PEG5000-COOH, and the reaction proceeded for 24 h under stirring. Finally, Cu2WS4-PEG (CWP) was collected by centrifugation, washed with deionized water, and dried in a vacuum oven at 60 °C overnight when necessary.
Peroxidase Mimicking Activity
The POD-like catalytic activity of hollow Cu2WS4 nanozyme was assessed according to the protocol published by Yan et al.[72] Specifically, CWP were dispersed in 2 mL of Hac-NaAc buffer (0.2 m, pH 4.5). Subsequently, 10 µL of freshly prepared TMB solution (10 mg mL−1) and 10 µL of H2O2 (10 mm) were added to the reaction. Then, the absorbance of the above-mentioned solution was recorded via a UV–vis spectra spectrophotometer. The specific steps of fitting the Michaelis–Menten equation for the multienzyme activity of CWP are provided in the supporting information.
GSH-OXD Activity
The GSH-oxidase activity of CWP was evaluated by the reaction of the DTNB reagent with thiol groups measured at 412 nm. CWP (0, 10, 20, 42, 85, 170 µg mL−1) was dispersed in a HAc-NaAc buffer (0.2 m, pH 4.5). The concentration of GSH was 0.4 mm, the reaction tube was placed in a shaker, and the temperature was set at 37 °C to simulate the internal environment of the organism. Subsequently, the CWP was separated by centrifuge (12 000 rpm, 10 min). Next, 400 µL of DTNB (4 mg mL−1) were added to the supernatant and the reaction continued in the dark for 30 min. Finally, the absorption of reaction products was evaluated at 412 nm using a microplate reader.
ROS Generation Capacity
Hydroxyl radicals can oxidize terephthalic acid (TPA) to produce fluorescent products; thus, TPA is often used to detect the presence of hydroxyl radicals. The TPA was also used to evaluate the ·OH radicals produced by CWP catalyzing H2O2. Specifically, 0.5 mm TPA reagent was mixed with the solution of CWP (20 µg mL−1) in the presence or absence of 10 mm H2O2, providing X-ray stimulation when needed (6 Gy). After continuing the reaction for 30 min, the fluorescence spectrum of each product was recorded using a fluorescence spectrophotometer. In the ESR experiment of ·OH detection, CWP (20 µg mL−1) was mixed with or without H2O2 (10 mm) in PBS buffer. Then, 5 µL DMPO reagent was added as a spin trapping to detect ·OH signals quickly.
Biocompatibility of CWP
The biocompatibility of CWP was first evaluated using Human Kidney-2 (HK-2) cells. HK-2 cells were cultured in 96-well plates, and different concentrations of CWP were added to the HK-2 cells for 48 h, then their cell viability was tested using a CCK-8 assay. The whole blood of two healthy mice was placed in anticoagulant tubes, centrifuged at 2000 rpm for 5 min three times washed with PBS, and to obtain a red blood cell solution (4% in PBS). Subsequently, different concentrations of CWP were mixed with the red blood cell solutions and allowed to rest at room temperature for 6 h. The lysis of red blood cells in the supernatant was observed and measured using a microplate reader. To observe the toxicity of CWP in mice, different doses of CWP (0, 10, 25, and 50 mg kg−1) were injected intravenously into female Balb/c mice, observed their status, and recorded their body weight daily. After 14 days of observation, the whole blood of the mice was collected and tested for various indicators using an automatic hematology analyzer (URIT-5160 Vet, URIT, China). The main organs of these mice were embedded in paraffin and sliced, and HE staining was used to observe whether there were significant pathological changes under a fluorescence microscope (Leica, Germany).
Cell Endocytosis of CWP
4T1 cells in a T25 culture bottle were treated with CWP (20 µg mL−1) for 6 h. Then, the cells were thoroughly washed with PBS to remove the free CWP, and the different groups were treated with 4 Gy X-rays when needed. Subsequently, the different treatment groups were cultured for 12 h in an incubator at 37 °C and 5% CO2. The cells were washed with PBS, digested with trypsin, and fixed with 1.5 mL 2% glutaraldehyde solution. Ultrathin sections obtained with an ultramicrotome were stained and imaged using TEM (HT7800, Hitachi, Japan). To further verify that the CWP nanozyme can be endocytosed into the nucleus, 4T1 cells were cultured in a 6-well plate overnight according to standard cell culture procedure. Then, cells were cultured with CWP (20 µg mL−1) for 24 h,after the nucleus was extracted following the manufacturer's instructions (Solarbio). The extracted nucleus was digested by microwave, and the obtained solution was diluted with DI water and subjected to ICAP-TQ.
Cytotoxicity Study Under Different Treatments
Human lung adenocarcinoma cancer (A549) and mouse breast tumor (4T1) cells were cultured in an incubator at 37 °C and 5% CO2. For colony formation assessment, cells were cultured in a 6-well plate at a density of 500 cells per well. Then, after co-incubation with 20 µg mL−1 CWP for 6 h, the wells were washed with PBS to remove the free CWP, and fresh culture medium was added. Then, cells were exposed to X-ray irradiation (0, 2, 4, 6, and 8 Gy) continue cultured for 10 days, and stained with crystal violet dye solution after being fixed with glutaraldehyde, the cells were air-dried at room temperature. The stained cell colonies were photographed using a digital camera and counted. The surviving fraction curves were calculated by counting the colonies, and the sensitizer enhancement ratio (SER) of CWP was obtained. The CCK-8 assay was employed to assess the cytotoxicity of CWP mediated radiosensitization. 4T1 cells were cultured in 96 well plates according to standard procedures and incubated with different concentrations of CWP for 6 h. After washing the cells with PBS, a fresh culture medium was added, and the cells were subjected to radiation stimulation (4 Gy) when necessary. Finally, the cell survival rate was calculated. Flow cytometry was used to analyze the cell apoptosis after treatment with CWP alone or combined with X-rays (4 Gy) using the Annexin V-FITC apoptosis detection Kit (Beyotime). Briefly, 4T1 cells were cultured in 6-well plates and incubated overnight. After being treated with or without CWP (20 µg Ml−1) for 6 h, the groups allocated to X-rays (4 Gy) were irradiated. After 24 h, cells were collected by trypsinization and centrifugation, washed twice with free-calcium PBS, resuspended, and stained following the instructions of the apoptosis kit.
Regarding intracellular ROS detection, cells were inoculation in a 6-well plate. The blank, X-rays, CWP, and CWP + X-rays groups were established. After 6 h of incubation with CWP (20 µg mL−1), cells were treated with DCFH-DA according to the instructions of the reagent kit. Then, the green fluorescence of ROS was observed using a fluorescence microscope (Olympus). In addition, the intracellular ATP content in different groups was spectrophotometrically measured according to the manufacturer's protocol (Beyotime).
To evaluate the mitochondrial damage, 4T1 cells were inoculated in confocal dishes and allocated to the blank, X-rays, CWP, or CWP + X-rays group. Then, following the instructions of the reagent kit of the JC1 probe, a laser confocal microscope (LSCM) was used to observe and take photos of the cells. For DNA double-strand break analysis, cells were cultured in 6-well plates overnight. After the corresponding treatment, cells were incubated for another 24 h. Then, cells were fixed using paraformaldehyde for 10 min, washed with PBS, and Trition-X100 was used to enhance the permeabilization of the cells and treated with a blocking buffer. Cells were incubated with γ-H2AX antibody and marked with an adaptive fluorescent secondary antibody. Finally, a confocal microscope was used to obtain the images of the cells. Intracellular lipid peroxides were also examined using the lipid peroxide probe Liperfluo (Dojindo, China) and viewed by LSCM.
Detection of Intracellular GSH Content
4T1 cells were cultured in 96-well plates and cultured overnight. Different concentrations of CWP (0, 50, 100, 150, and 300 µg mL−1) were used to treat the cells for 24 h. Then, 50 µL of 6.5% TCA solution was added to cause the cell lysis. Next, 400 µL of DTNB (2 mg mL−1) were added and allowed to react for 30 min in the dark. Then, a microplate reader was used to record the absorbance of the reaction products at 412 nm. The GSH content in 4T1 cells after being treated with CWP was also detected by an ELISA Kit (Shanghai Enzyme-linked Biotechnology Co., Ltd). Cells were cultured in 6-well plates, cultured overnight, and treated with CWP for 24 h. Finally, the GSH content was detected according to the manufacturer's protocol.
Western Blot
4T1 cells were cultured overnight in T25 culture bottles and exposed to CWP for 6 h. Cells were treated with X-ray irradiation (4 Gy) after being washed with PBS. The supernatant from different treatment groups was collected by centrifugation after ice bath lysis, and the protein concentration was determined using a BCA kit. The proteins were separated using a commercially available separation gel. After transferring the proteins to a PVDF membrane, it was treated with a quick-blocking solution. Then, the membrane was incubated with the corresponding anti-NRF2, anti-GPX4, anti-KEAP1, anti-HMOX-1, anti-BCL-2, and anti-β-actin antibodies at 4 °C overnight. The PVDF membrane was then exposed to the corresponding secondary antibodies and developed.
RNA Extraction and RT-qPCR Analysis
4T1 cells cultured in 6-well plates were exposed to CWP for 6 h. Then, cells were treated with or without X-ray irradiation (4 Gy). All cells were harvested after being cultured for an additional 24 h. Total RNA was extracted from the cells using the TRIzol reagent (TaKaRa Biotechnology). The isolated total RNA was reverse transcribed to complementary DNA (cDNA) using the ABScript III RT Mix (ABclonal Technology). Quantitative PCR was performed in a Bio-Rad CFX96 Real-Time system using SYBR Green Fast qPCR Mix (ABclonal Technology). β-actin was used as an internal control. Relative RNA abundances were calculated using the comparative Ct method (2-ΔΔCt).
Immunofluorescence Staining
To investigate the effect of CWP nanozymes mediated radiotherapy assistance, the expression of related proteins was evaluated by immunofluorescence. 4T1 cells cultured in confocal dishes overnight were exposed to 20 µg mL−1 CWP for 6 h. Then, cells were treated with X-ray irradiation (4 Gy) after being washed with PBS. Cells were cultured for 24 h, fixed, and permeabilized with Triton X-100 (0.2%) for 10 min when required. Then, the cells were blocked using a quick-blocking buffer and incubated with anti-NRF2, anti-HMGB1, anti-CRT, and anti-GPX4 antibodies. Afterward, cells were incubated with the corresponding fluorescent secondary antibody. The fluorescence was evaluated using LSCM.
Biodistribution of CWP Nanozyme In Vivo
A 4T1 subcutaneous tumor was inoculated in the right hind leg of Blab/c mice, and 20 mg kg−1 CWP was intravenously injected. Three mice were randomly selected at different time points for euthanasia, and the main organs and tumor tissue were weighed and dried. The tissues were fully dissolved in aqua regia, and the concentrations of Cu and W were measured using ICP-MS. Then, the content of Cu and W in different tissues was calculated. To evaluate the circulation time of CWP in the body, 10 µL of the tail blood of mice was taken at different times after the intravenous injection of CWP. After a thorough dissolution with 1 mL of nitric acid and digestion using the microblog digestion device, the concentration of W elements was measured after dilution with deionized water using ICP-MS.
PA Imaging Properties of CWP
For PA imaging in vitro, CWP was dissolved in an aqueous solution at various concentrations (0, 25, 50, and 100 µg mL−1) and transferred into individual tube phantoms. The PA-contrasting capacity of CWP was evaluated using a NIR PA imaging system excited by an 808 nm NIR laser. For PA imaging in vivo, 4T1 tumor-bearing nude mice were intravenously injected with CWP (20 mg kg−1) and imaged at various time points using a NIR PA imaging system excited by an 808 or 1064 nm NIR laser. The PA amplitude in the tumor regions was quantified using TomoView (TomoWave).
Anti-Tumor Effect of CWP-Mediated Radiotherapy Sensitization
All the female Balb/c mice bearing a 4T1 tumor in the right flank were randomly allocated to the saline, X-rays, CWP, or CWP + X-rays group. The intravenous injection of therapeutic agents into different groups of mice was performed according to different treatment plans (day 0), whereas those mice of the X-rays and CWP + X-rays groups received X-ray irradiation (4 and 5 Gy) on days 1 and 6, respectively. Subsequently, the tumor size was measured every other day and their body weight was recorded. After 14 days of treatment, the main organs and tumor tissues of the mice were collected, paraffin-embedded, and sliced for pathological analysis, including immunofluorescence and immunohistochemistry staining. All animal procedures were approved by the institutional animal care and use committee of the College of Preventive Medicine, Army Military Medical University.
CWP-Mediated Radiation Sensitization Synergy of PD-L1 Blockade
To verify the therapeutic effect of CWP-mediated radiotherapy combined with PD-L1 antibody on distal tumors, a bilateral tumor model using female Blab/c mice was established, with the first dose of 4T1 cells (1 × 107 mL−1, 100 µL) administered to the right hind leg and the second dose (3 × 106 mL−1, 100 µL) administered to the left hind leg 7 days later.[69, 73] Then, the mice were randomly allocated to the 1) saline, 2) X-rays + anti-PD-L1, 3) CWP (20 mg kg−1) + anti-PD-L1, or 4) CWP (20 mg kg−1) + X-rays + anti-PD-L1 group. Then, X-ray irradiation (4 and 5 Gy) treatment or intraperitoneal injection of PD-L1 antibody (75 µg kg−1, on days 2, 5, and 8) were administered according to each treatment scheme. Subsequently, the tumor size was measured and weighed every other day. At the end of therapy, the tumor tissue was collected to be weighed and photographed.
Dendritic Cell Maturation and T Cell Infiltration
First, the same bilateral tumor model was established according to the previous method. Then, CWP (20 mg kg−1) was intravenously injected into the corresponding mice when needed (day 1). At 24 h post-injection, the tumor site was exposed to X-ray irradiation with the help of lead plates on day 2. Then, PD-L1 antibody (75 µg kg−1) was injected into the corresponding treatment group through intraperitoneal injection on day 3. On post-injection day 5, mice were sacrificed. The blood, lymphaden, and spleens of mice were harvested. Blood was allowed to stand at 4 °C for 6 h and then centrifuged at 12 000 rpm for 10 min to obtain the serum. The content of IFN-γ, TNFα, and IL-6 in serum was measured using an ELISA kit. Lymphaden and spleens were homogenized in PBS free of calcium and magnesium.
The tissue was ground and washed with PBS to remove erythrocytes and obtain a single cell suspension. Single cells were obtained by a cell sieve (70 µm) and dispersed in a flow cytometry staining buffer. To investigate T cell infiltration within the spleens, the cell suspensions were stained with FITC-conjugated anti-mouse CD3, PerCP/Cyanine5.5-conjugated anti-mouse CD4, and PE/Cyanine7 conjugated with anti-mouse CD8a on an ice bath for 30 min, and then analyzed using a Sony ID7000 flow cytometry. To further assess the maturation of dendritic cells, the cell suspensions of the lymphaden were stained with PE-conjugated anti-mouse CD86, APC-conjugated anti-mouse CD11c, and FITC-conjugated anti-mouse CD80. Then, cells were washed with a flow cytometry staining buffer twice and analyzed using a Sony ID7000 flow cytometry system.
Statistical Analysis
All data were reported as the mean ± standard deviation (SD). All the results have been performed at least three times by independent experiments. The level of statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001, n = 5) was defined by one-way analysis of variance using OriginPro (Ver. 9.0).
Acknowledgements
The authors thank Knorigene Technologies (Chongqing, China) for providing the gene analysis. All animal experiments were approved by the Ethics Committee of the Third Military Medical University (Accreditation number, AMUWEC20230069, Chongqing, China). [Correction added on May 29, 2024, after first online publication: Affiliations has been updated in this version.]
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




