An Automated Single-Cell Droplet-Digital Microfluidic Platform for Monoclonal Antibody Discovery
Abstract
Monoclonal antibody (mAb) discovery plays a prominent role in diagnostic and therapeutic applications. Droplet microfluidics has become a standard technology for high-throughput screening of antibody-producing cells due to high droplet single-cell confinement frequency and rapid analysis and sorting of the cells of interest with their secreted mAbs. In this work, a new method is described for on-demand co-encapsulation of cells that eliminates the difficulties associated with washing in between consecutive steps inside the droplets and enables the washing and addition of fresh media. The new platform identifies hybridoma cells that are expressing antibodies of interest using antibody-characterization assays to find the best-performing or rare-cell antibody candidates.
1 Introduction
Monoclonal antibody (mAb) discovery plays a prominent role in clinical diagnostics and in the development of targeted therapies, which have been increasing at a fast pace.[1] Given the rapid market growth, demand for fast, cost-effective, and efficient discovery screening technologies has increased. In the last decades, classical hybridoma technology has been widely used for antibody discovery. This method involves the fusion of primary antigen-specific B cells with myeloma cells rendering them immortal, and generating antibody-secreting hybridoma cells, making it possible to produce and obtain pure antibodies. Conventional hybridoma dilution-based methods explore only a small subset of the entire repertoire, making the mAb discovery process labor-intensive and time-consuming (≈5–6 weeks[2, 3]). The challenge arises from the lack of automated techniques to perform such complex procedures. Therefore, various techniques have been developed to miniaturize and automate this procedure to increase throughput and reduce time and costs.
Flow cytometry-based approaches, including fluorescence-activated cell sorting[4] (FACS), enable very high throughput cell isolation of the desired cells. This technique relies on the fluorescent staining of cells, whereby either antigen baiting is used to sort surface-bound antibodies (e.g., memory B-cells) or surface markers to capture and sort antibody-secreting B cells (e.g., plasma blasts). However, the exposure to high pressure and decompression, shear forces, and high electric fields in FACS can lead to negative effects[5] in cell viability, while the sample preparation for cytometry is still very laborious. In addition, collecting the desired cells that secrete Ag-specific mAbs[6-8] is challenging with FACS and therefore is mostly used for the detection of membrane-bound mAbs on the surface of such cells.[9] Beyond the use of conventional technologies, there are miniaturized microstructure array-based platforms used for high throughput screening of antibody-producing cells.[7, 10-17] These platforms confine the Ab-producing cell in a specific location (e.g., arrays of microstructures), incubate with particular beads/target cells to capture the secreted Abs, allowing for rapid analysis and sorting of the mAbs secreting cells. These structures use gravity,[15, 18-21] capillary,[16, 22] hydrodynamic,[23-30] or light-based mechanisms[18, 31-33] to trap cells into chambers or wells. However, the first two approaches are based on Poisson statistics[22, 34] and are not efficient in terms of trapping and keeping single cells in the arrays.[18, 20] Furthermore, there are frequent Abs (secreted from the cells) losses during the addition of buffer washing or media replenishment to the wells,[18, 20] while non-specific cell adhesion has been reported for gravitational[18, 20] and hydrodynamic[23, 28] trapping preventing collection of the mAb-producing cell after screening. Although optical forces can provide deterministic single cell trapping with high efficiencies, optical devices[10, 11, 35, 36] are open systems that have associated risks with cross-contamination and loss of such antibodies. There are also high costs associated with multi-layer optical device fabrication,[35] and off-chip post-export viability is often reported to be low – ≈50% – particularly for primary cells.[36]
Droplet-based microfluidics provides high throughput screening of mAb-producing cells,[14, 17, 37-39] – i.e., B or hybridoma cells – with higher cell viability (≈80%)[17, 37, 38] due to its closed-based environment in droplets. Monoclonal Ab-producing cells are co-encapsulated with capture beads or target cells which have receptors on their membrane and can be screened at kHz frequencies.[17, 37] The system typically uses fluorescent-dye conjugated secondary antibodies and integrated optical detection with the microfluidic system to select the desired cells with target-binding antibodies which are collected for downstream sequencing and further characterization. However, existing droplet-based devices are not compatible with many mAbs characterization assays. The main shortcoming is antigen-binding assays depend on the co-compartmentalization of single B or hybridoma cells with target cells (or beads),[14, 17, 37-39] which is usually inefficient (≈16%)[40] due to Poisson-based flow-focusing encapsulation. This method also generates unwanted combinations of the two cells within each droplet, which are generally sorted to waste, resulting in the loss of specialized cells with the desired antigen-specificity.
Monoclonal antibody characterization assays are rather complex and frequently rely on a series of washing steps that require reagent removal and exchange. Most work with droplet-based microfluidics has avoided incorporating these challenging steps due to the droplets being coated with surfactant which make it difficult to break. There are several techniques that have been implemented to address the challenge of solution exchange and washing in conventional droplet microfluidics,[9, 41-44] including the use of acoustophoretic,[45-47] magnetic,[48-51] dielectrophoretic (DEP)[43] force, electro-coalescence,[51-53] pico-washing,[54] and chemical demulsifiers like perfluorooctanol.[40, 55, 56] However, chemical demulsifiers compromise cell viability[44, 52, 53] and irreversibly impact the singularity of the isolated cells, and acoustic and magnetic methods suffer from limitations in device materials and sample types, respectively. Additionally, all techniques that rely on the splitting step encounter high cell loss and low solution exchange efficiency due to the partial removal of the solution from the droplet.[43, 45-54] Although pico-washing[54] and electro-coalescence[51] techniques provide higher throughput (e.g., 100 Hz[51] or 1 kHz[54]), they face challenges such as complicated synchronization of multiple streams and droplets, particle loss, and non-uniform removal of liquid from droplets. Despite these efforts, solution exchange in a droplet-based microfluidic system still remains a challenge to implement.
We report here a new proof-of-principle method for antibody screening that allows for deterministic encapsulation of two cells and for frequent washes with minimal losses in the droplet. This new method is powered by droplet-digital microfluidics (D2), which combines droplet-in-flow techniques with digital microfluidic for the generation and the manipulation of droplets.[57-59] This integrated platform is particularly well-suited for antibody detection, as it provides control over single cell trapping, subsequently encapsulate them inside droplets, and performs on-demand merging events necessary for antibody screening. Using this device, we describe the methodology for antibody screening by deterministic trapping of hybridoma cells and then merging with target cells with high efficiency. We also show highly efficient washing of droplets and the addition of fresh media containing secondary antibodies to maintain the health of the trapped cells and remove excessive non-binding antibodies. To show the utility of our platform, we have identified hybridoma cells that are expressing antibodies of interest which were collected for downstream antibody-characterization assays.
2 Results and Discussion
2.1 Antibody Screening and Selection Using Droplet-Digital Microfluidics
Droplet-digital (D2) microfluidics has become popular for the manipulation and screening of droplets containing cells and proteins.[57-61] In such systems, electrodes are patterned below the microchannel to serve as an actuation mechanism to manipulate droplets inside the channel. In addition to being useful for manipulating droplets, the electrodes also enable individual droplet control, which allows for on-demand merging and mixing of the droplets and low-voltage droplet sorting – operations that were not possible with standard droplet-in-channel microfluidics. The on-demand functionality allows for deterministic encapsulation of cells to be retrieved and collected for downstream testing. Specifically, the use of such a device enables the confinement of multiple cells in individual droplets is a key aspect of the experimental methodology, as depicted in Figure 1.
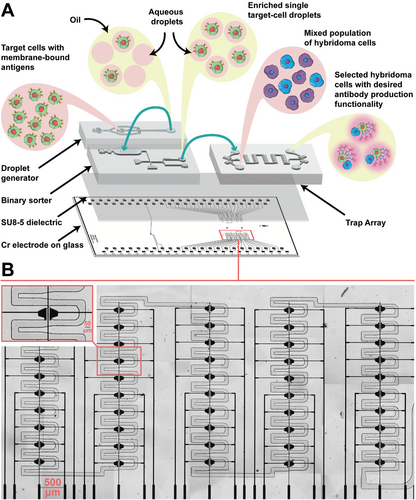
As shown in Figure 1, the device has five columns with each column consisting of 10 traps in a serpentine geometry. The new method was applied to the screening and selection of monoclonal antibodies from hybridoma cells. The integrated D2 microfluidic device has a three-layer architecture, similar to the device used by Ahmadi et al. and Samlali et al.[57-59] The device consists of chromium electrodes covered by a 7-µm SU-8 dielectric layer on which three PDMS-based microchannel devices are adhered. The PDMS-based microchannels were used i) to encapsulate the target cells in picolitre droplets (25-40 pL), ii) to sort the single target cell droplets, and iii) to execute the screening and selection assay on single hybridoma cells isolated in the traps (Figure 1A). The integrated device compartmentalizes two types of single cells (a hybridoma cell and a target cell) within a trap with a high probability (P = 0.95 ± 0.032) of having the correct combination of the two cells. This is a significant improvement over the conventional non-deterministic, flow-focusing approach, where the maximum probability (assuming λ = 1) of having a pair of two different particles encapsulated is only 13.5%.[62]
Our procedure starts with trapping single hybridoma cells in the trapping device (device 1 in Figure S1, Supporting Information). The hydrodynamic confinement of hybridoma cells follows the principle of fluidic resistance – when a hybridoma is trapped, fluid flow is blocked, enabling the next hybridoma to flow to the next open trap.[63-68] The trapped hybridomas are encapsulated in picolitre droplets through a phase change with oil and an electric potential was applied to the electrodes below the traps to generate a 40 to 150 pL droplet. A standard flow-focusing droplet generator (device 2 in Figure S1, Supporting Information) is used to encapsulate stained target cells and are sorted and collected using a binary sorter device (device 3 in Figure S1, Supporting Information). Indeed, if target cells are limited, culturing and expanding may be required to increase their numbers before antibody detection. Lastly, the collected single target cell droplets are reinjected into the trapping array where they will merge with the single hybridoma cell droplets to identify the mAb producing cells.
As shown in Figure 1B, the device contains 100 traps and, in each trap, there are two electrodes for potentiation and one ground electrode. Inspired by digital microfluidic studies in which several actuation electrodes are “bussed” together to one potential circuit,[69, 70] we electrically connected a set of five traps together to increase electrode density and reduce operational complexity. In addition, limiting the number of electrodes in each trap to only two, we could accommodate more electrodes on one chip and increase the electric fields, which is particularly important for droplet bursting and washing.
Automating droplet operations and the selection of cells on droplet-digital microfluidic devices is challenging, as it requires fluid flow and droplet control.[58] Syringe pumps are used to control the flow rates in the channel and electric potentials are needed to control droplet actuation. Here, we report an integrated automation control circuit suitable for antibody screening by droplet-digital microfluidics, depicted in Figure S2 (Supporting Information). The most important component of this fully automated system is an in-house software to control all aspects of the automation hardware, which includes the in-house automation system (comprised of a function generator, amplifier, power supply, and stacked control board of optocoupler switches), a CMOS camera connected to a microscope, a syringe pump, and a photon-counting head (Figure S2, Supporting Information). The software contains a GUI to allow the user to program flow rate to control the fluid flow in the channels, to create electrode actuation patterns for various droplet manipulations, such as merging, sorting, trapping, and releasing in the binary sorter and trapping array device, and to include spectrometer and photon counting settings for integration time, droplet travel time, and pulse time (Figure S3, Supporting Information). In addition, the software provides a real-time readout of the signals from the PMT which is used to autonomously activate the electrode for sorting and real-time view of the droplets, allowing the user to capture bright field and fluorescent images with different exposure times. Finally, the software has the capability to set multiple intensity thresholds (for different wavelengths); this is a useful advance, since we can set different fluorescence intensity thresholds for droplets containing zero, one, two, or more cells.
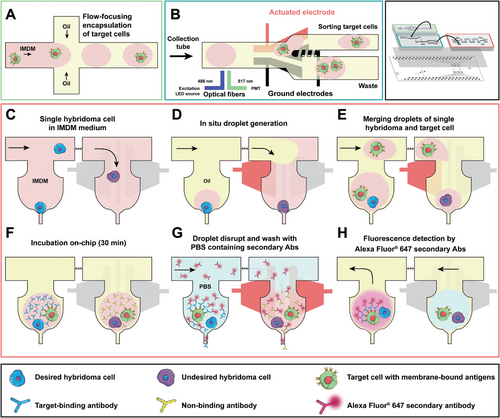
Figure 2 (and see Video S1, Supporting Information) summarizes the automated operations for two-cell screening. Briefly, in step A, single target cells are encapsulated in ≈pL droplets using a standard flow focusing droplet generator. In step B, we used a co-planar binary electrode sorter[59] to select the single target cells using fluorescence-based labels. In steps C and D, the trapping array was primed to decrease cell adhesion against the channel walls, and a continuous flow of cell media containing hybridoma cells were injected into the device. The design of the trapping array for hydrodynamic confinement was inspired from our previous works,[63-68] the cells are trapped via fluidic resistance.[64, 67, 68] The resistance of an open trap (i.e., without cell) is lower than the bypass channel (Figure S4, Supporting Information) and the resistance increases when a cell is inside the trap, blocking the outlet of the trap and enabling subsequent cells to the next empty trap.[64, 67, 68] Using this technique, 50 cells can be trapped using our device (Figure S5, Supporting Information). In step E, the hybridoma cells in the traps will be encapsulated in droplets in situ by an application of an electric potential to the electrodes to hold the aqueous phase containing the cell inside the trap, while flowing an oil phase to shear off the aqueous phase in the trap to generate a ≈pL droplet. In step F, the collected single target cell droplets (from step two) are re-injected into the trapping array and single target cell droplets are merged with a hybridoma droplet inside the trap by actuating the electrodes below the trap (Figure S6, Supporting Information). In steps G and H, the merged droplets are incubated, then disrupted via electrical potential to the droplets, washed, and screened for the desired mAbs. All non-desired mAbs are washed out from the traps, while the mAbs secreted that are specific to the target cell antigens are inside the trap during the wash. Droplets containing the desired mAbs that are bound to the specific antigen on target cell membrane become fluorescent in the presence of a conjugated secondary antibody and can be retrieved from the trap with a reverse flow of the oil.
2.2 Characterization of Droplet Operations for Antibody Screening
Antibody screening requires confining cells of interest, identification of cells that produce the desired antibodies, and reagent addition or removal of constituents – which are operations that are particularly challenging with droplet-in-channel devices.[43] The serial nature of droplet-in-channel devices and the lack of individual controllability of the droplets makes the process of encapsulating exactly two cells and adding the reagents or removing the constituents while keeping the cells at the same location difficult. We leveraged our electrode-based droplet system to enable individual and control of each droplet containing a cell inside the trap to allow for droplet bursting and media exchange. Thus, a primary goal reported here was to characterize the efficiency of co-encapsulation of two cells, washing using our system at different flow rates and voltages, and validated any cell loss at the different flow rates.
2.2.1 On-Demand Deterministic Co-Encapsulation
An automated three-step droplet-digital microfluidic method was developed to co-encapsulate two cells in the trap. This portion of the experiment is depicted by microscopy images shown in Figure 3A–C. Using hydrodynamic methods,[58, 63-68] trapping single hybridoma cell is demonstrated by flowing media in the device (Figure 3A). Using our geometry, the cells will enter the nearest open trap, and when the cell occupies a trap, the flow will be mainly directed through the serpentine channel (Figure S4, Supporting Information). Single cell trapping efficiency is highly dependent on the media flow rate. As shown in Figure 3D, the single cell occupancy with probability over 50% is ensured for flowrates between 0.5 and 30 nL s−1 with the maximum efficiency (98 ± 3.92%) achieved at 1 nL s−1. At a constant flow rate, cells experience a higher pressure in traps closer to the inlet (Figure S4B, Supporting Information). The increased pressure causes cells to experience compression, leading to a reduction in their diameter,[71] and consequently, the cells in the first column of the traps (nearest to the inlet) could be released easily through the constriction. The pressure drop through the device leads to empty traps in the column closer to the outlet at flow rates <0.1 nL s−1, because the hydrodynamic pressure of the continuous flow does not provide sufficient pressure to move cells through the serpentine. At flow rates >50 nL s−1, more single cells are trapped in the last columns, but the cells in the first column experience a higher pressure and squeeze through the constriction, leaving the traps empty. A balance is struck at the optimal flow rate such that the pressure is enough to trap the cells near the outlet, but not high enough to push the cells out of the trap near the inlet.
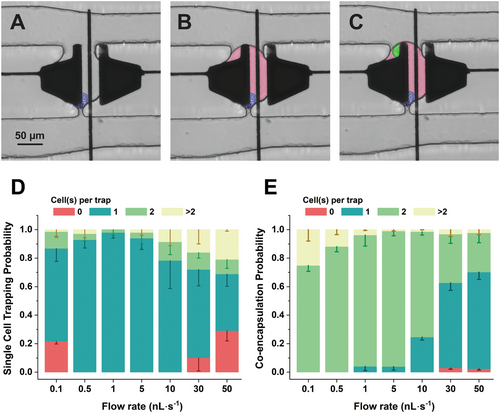
Following trapping of a single cell, we evaluated the on-demand generation of a droplet containing a single cell within the traps (Figure 3B). At flow rates below 1 nL s−1, the applied pressure was not sufficient to overcome the resistance of the serpentine channel. As a result, the oil flow was erratic near the last column of the traps, leading to an absence of droplet formation. A solution is to increase the flow rate >1 nL s−1, which causes the hydraulic pressure at the traps to overcome the droplet pinning force (i.e., the force generated by the applied potential), resulting in pushing the droplets out of the trap through the constriction side. Therefore, the flow rate should be high enough to provide the phase exchange along the serpentine channel, but not high enough to cause droplet losses via the constriction. According to our findings, a flow rate of 1 nL s−1 yielded over 95% of droplets successfully generated within the traps.
To co-encapsulate two cells, the collected single-target cell droplets are re-injected to the trapping array device. In initial experiments, the most common failure point is the merging of two sequential target cell droplets. To mitigate this issue, we employed two separate inlets — one containing the re-injected droplets and the other housing the oil phase and surfactant (flow rate: 1 nL s−1) – to create spacing between the droplets as they move toward the trapping array. When a target cell drop comes to the immediate vicinity of a trap (visualized by microscopy), a potential (32 Vrms at 15 kHz) is applied to either one of the two potential electrodes (see Figure S6, Supporting Information for the electrode actuation pattern) merging the target cell droplet with the hybridoma cell droplet inside the trap (Figure 3C). Controlling the droplet reinjection flow rate is critical at this step. As shown in Figure 3E, we measured the co-encapsulation probability for different flow rates. Flow rates >10 nL s−1 increases the hydrodynamic pressure on the trapped hybridoma droplet and forces the trapped cell to squeeze through the constriction. This leads to a common failure point – coalescence of the target cell droplet with an empty droplet in the trap. At the lower flow rates, the target-cell droplets in the serpentine microchannel tend to coalesce (even with the oil spacing) considering that the flow rate does not generate sufficient pressure to overcome the resistance within the channel. The optimal point (overcoming resistance and maintaining the hybridomas in the trap), with the highest efficiency of 95 ± 3.2%, for co-encapsulation of the two cells is at a flow rate of 5 nL s−1. To our knowledge, this is the highest co-encapsulation efficiency reported that neither requires preliminary sorting of both cells to achieve single cell encapsulated droplets[14, 62, 72-74] nor depends on complicated microvalve system to capture cells[75] (see Table S1, Supporting Information). The method for co-encapsulation for 100 doublet cells requires 60 min to complete with our automated system.
2.2.2 On-Demand Droplet Disruption and Media Exchange in Traps
Two crucial steps in mAb identification and characterization assays are reagent addition for labeling cells and washing away the non-specific antibodies.[9] While array-based microfluidic[18, 30, 34] allows for facile reagent addition and removal in the microwells, there is a risk of non-specific cell adhesion during the wash step. Washing in droplet-based microfluidics is limited to relying on continuous droplet splitting to dilute the unwanted reagents.[43, 45, 47, 48] Here, we demonstrate an alternative that completely removes the liquid droplet inside that traps after co-encapsulation while keeping the hybridoma cell with the target cells in the trap using electrostatic forces. In contrast to previous reports,[76-78] which use an emulsion destabilizer or a diluted surfactant (0.001% wt[77]) in the oil phase, we individually disrupt droplets by electrostatic forces avoiding unwanted droplet coalescence and prevent any change in the medium/oil constituents that might adversely affect the viability or functionality of the cells.[78]
In initial experiments for droplet rupturing, we experienced challenges with the surface coating of the channels during phase exchange with PBS since from the initial observations the surface tension increases between the PBS and the walls in the second phase exchange. Therefore, a proper surface coating (see Note S1, Supporting Information) of the trapping array device becomes very important at this step, as it allows smooth exchange of the two phase in the main channel. In addition, maintaining the PBS flow rate <1 nL s−1 prevents droplets from leaving the trap or droplets being randomly formed inside the channel. When an aqueous solution reaches the trap area (Figure 4A), the electrodes below the trap (see Figure S7A, Supporting Information) are actuated and an electrostatic force is generated perpendicular to the interface between the aqueous stream and the trapped droplet (see Figure S7B, Supporting Information). When the electric field intensity is between 2 and 4 × 105 V m−1, the field causes the droplets to burst inside the trap. Aside from the actuation voltage, there is an optimal flow rate range to achieve successful disruption of the droplet interface. As shown in Figure 4B, the lowest flow rate 1 nL s−1 with an applied voltage as low as 32 Vrms is sufficient to break droplets with almost near perfect efficiency. Interestingly, as flow rates increase, a lower potential can be applied to obtain the same droplet disruption probability. The higher flow rates create a larger hydrodynamic pressure and shear force on the droplet interface, making it more susceptible to break in the presence of electric field. In addition, we measured the washing efficiency after droplet disruption. Figure 4C is the normalized fluorescence intensity of the droplet after washing. Traps were filled with 10 mM fluorescein 150 pL droplets and were disrupted and washed in non-fluorescent PBS solution for five minutes (see Note S2, Supporting Information for methodology). As shown, after only one wash step, we observe a 96.8% reduction in the normalized fluorescence intensity. For the data in Figure 4C, we used a fluorescein solution that was prepared in 1M NaOH and suspect that 100% washing efficiency was not achieved due to the removal of the surface coating of the device that was caused by the corrosive NaOH solvent during the droplet generation and incubation inside the trap. Consequently, there was residual fluorescein remaining on the damaged surface, rendering its removal challenging. In the future, other rinse solutions or solvents may prove to be useful for washing. Regardless, the data in Figure 4C demonstrate that our platform can be used for washing, which may be useful for other non-antibody related applications.
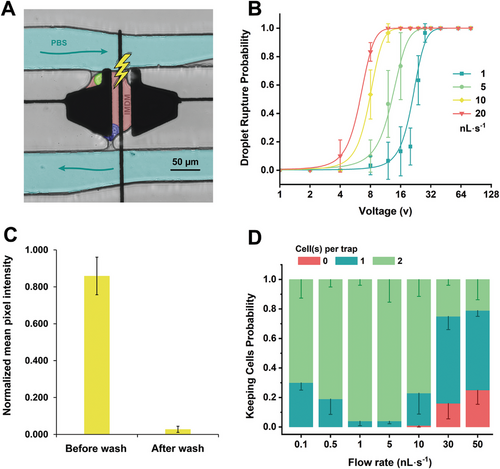
A final goal of this work was to demonstrate that after droplet bursting and washing, the cells can be kept inside the trap. MAbs that bind to the membrane-bound antigens on the target cells, which maintain both the target cell and the corresponding mAb producing hybridoma cell in the trap after droplet breakage is essential for downstream analysis (e.g., sequencing). Figure 4D depicts that there is an optimum range for the aqueous flow rate (between 1 and 5 nL s−1) to keep both cells in the trap with average success probability of 96 ± 9.6%. The hydrodynamic pressure of the aqueous stream must be high to prevent the cells from escaping through the trap entrance. Simultaneously, the pressure should not exceed a threshold pressure such that one or both cells are squeezed out of the constriction opening. For example, at higher flow rates (over 10 nL s−1), although less voltage (<12 Vrms) is required for droplet breakage, either one or two cells are squeezed through the trap's constriction. We also tested the viability before and after the washing and bursting operations and our results in Figure S8 (Supporting Information) suggest that there is minimal effect on the cells, which we believe our integrated platform can be applied to non-immortalized B cells and toward applications that require two (or more cells) to be co-cultured and simultaneously selected and analyzed.
2.3 Screening of Mixed Population of Hybridoma Cells
To develop a D2 microfluidic system for selecting antibody-secreting cells, we prepared a range of hybridoma cell line mixtures consisting of two cell lines: one producing monoclonal antibodies (mAbs) that bind to specific membrane-bound antigens on target cells and the other producing non-specific mAbs in a 1:1 ratio. In this specific experiment, we mixed the Alexa fluor 647 secondary antibodies with the IMDM solution containing target cells to be able to detect mAbs production through fluorescence imaging. In addition, we evaluated the effect of washing on detection and isolation of desired hybridoma by measuring the signal-to-noise ratio of the fluorescence distribution before and after the washing step.
We co-encapsulated the two cells inside traps using our method and incubated the cells for 30 min. The hybridoma and target cell lines were stained with calcein-AM red-orange (shown as red) and Celltrace CFSE (shown as green) respectively, prior to loading on the chip (Figure 5A; left). During the incubation time, desired hybridoma cells produced antigen-specific monoclonal antibodies that were bound to the membrane receptors of the target cells. The Alexa Fluor 647 secondary antibodies bound to all secreted mAbs including those captured on the membrane of the target cells, generating a red fluorescent signal (λex = 627 nm and λem = 665 nm). Before washing, all droplets containing one hybridoma cell emitted a uniform red signal (Figure 5B; right), indicating the production of mAbs. In the case of producing the desired mAbs, there is an intensity peak around the target cell due to the accumulation of mAbs on their membrane.
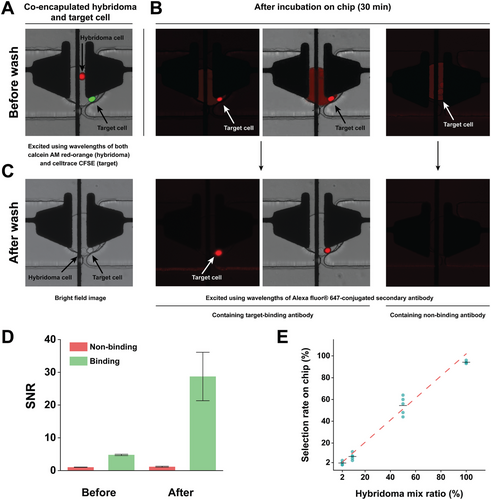
After washing the droplets, which removed the non-binding antibodies from the trap, the overall background signal was reduced (Figure 5C). As shown in Figure S9 (Supporting Information), we quantified the fluorescence measurement across the image, and there is background fluorescence being generated by the non-binding antibody. The high fluorescence given by the background could lead to false positives in the detection. By defining the signal as the mean fluorescence value of the cells and the noise as the mean fluorescence value of the droplets, we plotted the signal-to-noise ratio before and after the washing process, as depicted in Figure 5D. Remarkably, the signal-to-noise ratio (SNR) value exhibited a substantial increase after a post-washing step. However, in the absence of washing, both specific and non-specific antibodies displayed a remarkable similarity in their signal-to-noise ratios (SNR) (P > 0.05), raising the prospect of detecting secreted and expressed IgGs. It is only following the washing step that we observe a noteworthy enhancement in the SNR, effectively discerning the desired monoclonal antibodies (mAbs) with elevated SNR values, while concurrently maintaining low SNR values for the undesired mAbs. The significant improvement in SNR resolution post-washing contributes to the accuracy and reliability of our detection process.
We further evaluated the effects of washing by conducting a screen of mixed hybridoma populations producing the specific and non-specific mAbs as shown in Figure S10 (Supporting Information). Figure 5E depicts the results showing the success of detecting of these specific hybridoma cells when mixed at different ratios with non-specific hybridoma cells (94.00%, 54.40%, 7.60%, 1.60% measured selection rate for 100:0, 50:50, 1:10, 1:50 ratios, respectively). As shown, we were able to select cells at the higher ratios (100:0) with 94.00 ± 1.41% selection rate. Furthermore, our platform also showed remarkable sensitivity to populations comprising merely 2% (1:50) of the total hybridoma cell population screened on the chip, which is the typically relevant concentration of antigen-specific B cells in the blood samples from immunized donors.[14, 79, 80] For the lower ratio (i.e., 2%) of the rare cells starting from an initial concentration of 1 × 106 cells mL−1, we expect to find ≈10 rare cells. To guarantee the capture of an adequate number of rare cells, we implemented multiple rounds of injections. This approach aimed to provide a more precise representation of these rare cells, taking into consideration their expected scarcity. Additionally, we employed this repeated injection strategy to overcome the limitations inherent in droplet-based systems, including issues like droplet pinning within the channels and dead volumes in the tubing, which make a one-time injection of cells unfeasible for our purposes. Desired cells at much lower frequencies (e.g., 1:100 and 1:1000) can potentially be detected by screening a larger population of cells over multiple iterations or simultaneously operating multiple devices in parallel. Nevertheless, we contend that improving the surface coating of the device is imperative for identifying desired cells at lower ratios.
There are several avenues for enhancing the utility of the droplet-digital microfluidic system. Improving cell imaging procedures, eliminating multi-layer fabrication and alignment complexities, and augmenting the number of traps and electrodes represent key areas for advancement. As depicted in Figure 1B, the device consisted of chromium, non-transparent electrodes and was capable of trapping 100 cells, an improvement over our previous work[58] that consisted of only 12 traps. Droplet and cell imaging was performed with non-transparent, chromium electrodes, which restricts the field of view within the traps. Exploring alternatives such as incorporating an indium tin oxide (ITO) substrate for transparent electrodes or designing smaller electrode shapes can greatly improve cell imaging procedures. Another area of improvement would be to address the surface fouling in the channels, which becomes more prominent after repeated injections, and is crucial for maintaining device performance and ensuring reliable cell recovery. Using lubricated porous films[81] or longer longevity surfactants[82] are promising strategies to achieve efficient cell retrieval. Finally, our current system's throughput (100 cells h−1; ≈1000 cells per day) is below current droplet microfluidic standards (>10k cells per day), however, to further enhance our system's throughput, we recommend integrating our technique with other droplet manipulation methods[83, 84] capable of bolstering the multiplexing capabilities of the droplet-digital system. For example, a strategy could be to design (based on thin-film transistors) 1000s electrodes per device and integrate ten sets of 50 traps on such a device. This would equate to ≈500 trapping events on a single device. To increase the throughput further, multiple devices could simultaneously be operated by vertically stacking them via “slot-connection” automation system, which could allow for 1000s of total trapping events per hour to potentially occur in parallel and surpassing current droplet microfluidic methods.
3 Conclusion
We used a droplet-digital microfluidic platform to achieve a precise co-encapsulation of two different cell lines and to perform on-demand droplet disruption and media exchange within a trapping array device. We demonstrated the reliable encapsulation of single cells and cell doublets, achieving an impressive efficiency rate of over 90%. This efficiency significantly surpasses that of nondeterministic co-encapsulation devices adhering to Poisson statistics, where the encapsulation efficiency stands at a mere 16%.[14, 62, 72-74] Furthermore, we successfully disrupted the droplets' interfaces and exchanged the media while keeping the cells confined within the traps, a crucial step for performing various biochemical assays on microfluidic platforms. These droplet manipulations and detection were automated using our in-house software, which led to efficient detection and capture of rare antibody populations.
With further advancements to our system, we envisage the versatile platform finding application not only in isolating hybridoma cells but also in facilitating the production of therapeutic and diagnostic antibodies from antibody-secreting B-cells. The potential applications could encompass biomarker identification for disease diagnostics, metabolite screening for drug discovery, and various cell-based screening applications. Furthermore, this platform holds promise for significantly advancing fundamental biological research by offering an automated platform for cell signaling studies and immunoassays. Therefore, we propose that the droplet-digital microfluidic platform will pave the way for the broader adoption of automated microfluidic systems in biological assays that demand precise encapsulation and media exchange.
4 Experimental Section
Reagents, Materials, and Equipment
Microfabrication Materials: Materials for hybrid microfluidic devices included a high resolution 25400 dpi transparent photomask (Artnet Pro Inc., Bandon, OR), AZ-1500 positive photoresist coated glass slides (Telic, Valencia, CA, USA), CR-4 chromium etchant (OM Group, Cleveland, OH, USA), AZ-300T photoresist stripper (Integrated Micro Materials, Argyle, TX, USA), MF-321 developer, SU-8 5, SU-8 2035, and SU-8 2075 resists, and SU-8 developer (Kayaku Advanced Materials, Westborough, MA, USA), <100> Si wafers (Silicon Valley Microelectronics Inc., Santa Clara, CA, USA), polydimethylsiloxane (PDMS) (DOW Silicones Corporation, Midland, MI, USA), chlorotrimethylsilane (Sigma-Aldrich, Oakville, ON, CA) and polylactic acid (PLA) (Shop3D, Mississauga, ON, CA). Additional solvents and chemicals used for microfluidic chip fabrication included acetone (cleanroom lab grade) and isopropanol (IPA, cleanroom lab grade) and were purchased from Sigma-Aldrich (Oakville, ON, CA). Polylactic acid (PLA) material for 3D printing was purchased from Shop3D (Mississauga, ON, CA). DI Water used for microfabrication had a resistivity of 15 MΩ cm−1.
Reagents used for surface treatment of PDMS, and device operation included 3 m Novec HFE7500 engineering fluid, the surfactant 3 m Novec 1720 (M.G. Chemicals, Burlington, ON, CA), PEG fluoro-surfactant dissolved in HFE7500 (20 g of 5% wt) (Ran Biotechnologies, Beverly, MA, USA), Fluorinert FC-40 (Sigma-Aldrich), Pluronics F-127 (EMD Millipore Corp, Billerica, MA, USA), and Triton X-100 (Sigma-Aldrich).
Microfabrication Equipment: Harrick Plasma PDC-001 (Ithaca, NY, USA), Quintel Q-4000 mask aligner (Neutronix Quintel, Morgan Hill, CA), Laurell spin coater (model WS-650Mz-8NPPB, Laurell Technologies Corporation, North Wales, PA, USA), and mask aligner UV-KUB 3 (Kloe, Montpellier, Fr).
Cell Culture and Antibody Screening Reagents: The cell culture medium was purchased from Wisent Inc (Saint-Jean-Baptiste, QC, CA). The cell culture reagents include Iscove's Modified Dulbecco's Media (IMDM) for the hybridoma cells, Dulbecco's Modified Eagle's Medium (DMEM) for the target cells, fetal bovine serum (FBS), recombinant mouse IL-6 (carrier-free), Geneticin (G418), penicillin/streptomycin and phosphate buffer saline (PBS) (Ca2+/Mg2+ free). The CellTrace CFSE and Calcein Red-Orange dyes were purchased from Thermo Fisher (Mississauga, ON, Canada), and the Alexa fluor 647-conjugated goat anti-mouse IgG (H+L) from Jackson Immuno Research Labs (West Grove, PA, US), respectively. The cell dissociation solution non-enzymatic 1X was purchased from Sigma-Aldrich. The cell viability reagents include Fluorescein diacetate (FDA) (5 µg mL−1) and Propidium Iodine (PI) stock solutions (2 µg mL−1).
Fluidic System: Gastight syringes purchased from Hamilton (Reno, NV, USA) include 500 µL and 2.5 mL glass syringes with fittings and tubing (see Table S3, Supporting Information for a complete list of items) purchased from IDEX Corporation (Lake Forest, Illinois, USA). A low-pressure neMESYS pump system (Cetoni, Korbussen, DE) was used for flow control. The 1.87 mm magnetic stirring disk and the syringe stirrer were purchased from V&P scientific (San Diego, CA, USA).
Optical Setup, Data Acquisition, and Control System: The optical setup (Figure S11, Supporting Information) consisted of an inverted microscope (Olympus IX73; Tokyo, Japan) mounted on a vibration-dampening bench (Thorlabs; Newton, New Jersey, USA), a photon counting head, filter block, SMA connector (Hamamatsu Photonics H11890; Township, NJ, USA), multichannel LED light source (Ocean Optics; Orlando, FL, USA), bandpass filters, attenuator filter, fiber optics (Thorlabs; Newton, New Jersey, USA), and a Hamamatsu ORCA-Flash 4.0 (Township, NJ, USA). See Table S4 (Supporting Information) for a complete list of items with their specifications. Data acquisition and PMT control was performed through a USB interface by the Python based graphical user interface. The software is open-source and available to download at https://bitbucket.org/shihmicrolab/f_ahmadi_2023_uflowcontrol.
Preparation of Hybridoma and Target Cells
Two hybridoma cell lines (names are confidential) were provided by National Research Council Canada (NRC) and cultured in IMDM supplemented with 10% heat inactivated FBS (56 °C, 30 min), mouse interleukin-6 (1 ng mL−1), and 1x penicillin-streptomycin in a cell incubator at 37 °C and 5% CO2. Cells were passaged every three days at a final concentration of 8 × 106 cells mL−1 in 10 mL of medium in T-25 treated flasks. Cells were used for experiments when the cells were in the mid-logarithmic phase which was typically three days after the last passage. For screening and selection of the hybridoma cells, the cells were resuspended in the IMDM cell culture medium containing only 10% FBS and mouse interleukin-6 (1 ng mL−1) without antibiotics at a final concentration of 1 × 106 cells mL−1.
Target cells were provided by NRC and cultured in DMEM supplemented with 10% heat inactivated FBS (56 °C, 30 min), and G418 (0.4 mg mL−1) in a cell incubator at 5% CO2 and 37 °C. Cells were passaged every three days at a final concentration of 3 × 106 cells mL−1 in 14 mL of medium in T-75 treated flasks. Passaging and preparation followed the same protocol as the above for hybridoma cells except pre-warmed (37 °C) cell-dissociation solution was added and incubated for 2 to 10 min or until cells started to detach and restore expression of a surface receptors. For single encapsulation of target cells, the cells were gently resuspended in PBS solution mixed with CellTrace CFSE to a final concentration of 5 µm at a final concentration of 1 × 106 cells mL−1. The cells were stained after 20 min incubation at 37 °C followed by washing with PBS to remove excess dye. For the screening assay, the target cells were resuspended in the IMDM medium containing only 10% FBS and mouse interleukin-6 (1 ng mL−1), – i.e., no antibiotic – at a final concentration of 1 × 106 cells mL−1.
Droplet-Digital (D2)-Microfluidic Chip Operation for Antibody Production
Three microfluidic chips were assembled together on a single patterned chromium-coated glass substrate for antibody identification assay. Device 1 (Figure S1A, Supporting Information) consisted of a trapping array with a total of 50 traps on each side of the substrate to deterministically trap single hybridoma cells. For trapping cells, a protocol was followed similar to the previous work in Samlali et al.[58] and Video S1 (Supporting Information) is available to show the workflow. Briefly, the device was first primed with PBS for 5 min from inlet 3 (Figure S12, Supporting Information) with a flow rate of 50 nL s−1, while outlet 1 was closed using a 1″ PEEK 1/32″ OD tubing hot glued on one end. Next, the PBS flow rate was reduced to 1 nL s−1 and outlet 1 was opened by removing the blocker tubing. The hybridoma cells (stained with CellTrace calcein red-orange(AM)), at a density of ≈1 × 106 cells mL−1, were introduced to the device (1-5 nL s−1) from inlet 1 using a tubing with 0.03˝ OD/0.015˝ ID. Once all traps were filled with cells via microscopy inspection, the tubing connected to the cell suspension syringe was removed from inlet 1 and the tubing connected to 2% Ran-HFE oil was inserted into inlet 2. The oil flowed through the microchannel, removing the IMDM medium, and generating droplets of IMDM containing single hybridoma cells which are situated inside the traps. Droplets generated in situ vary in size depending on the oil flow rate (≈40–150 pL).
Devices 2 and 3 were used to prepare droplets containing single target cell. Device 2 (Figure S1B, Supporting Information) consisted of a flow focusing droplet generator used for single cell encapsulation of target cells following Poisson distribution. The throughput of this device was ≈3 kHz with monodisperse droplet size of ≈30 pL. The droplets from device 2 were transferred to device 3 using a tubing with 0.014˝ OD/0.006˝ ID bridged between the two devices. Device 3 (Figure S1C, Supporting Information) was a binary sorter with co-planar electrodes and was used to isolate droplets containing a single target cell (see Note S3 and Figure S13, Supporting Information) generated from device 2. For sorting, the target cells were stained with CellTrace CFSE before starting the encapsulation. All droplets prior to sorting were excited with a 470 nm wavelength light and fluorescence emission (520 nm) counts were recorded using the photon counting head. If the fluorescence counts were above the threshold line of droplets containing one cell and below threshold line of droplets with two or more cells, the actuation system triggered the electrodes to sort the single target cells droplets. The selected droplets from device 3 were collected in a PCR tube and transferred to device 1 through inlet 2 (Figure S12, Supporting Information). The hybridoma cells held in traps were co-compartmentalized with target cells expressing a membrane-bound antigen through merging with the transferred droplets by actuating the electrodes below the traps (see Table S5, Supporting Information for detailed information on parameters used for the droplet-digital microfluidic operations). The electrodes were actuated (32 Vrms and 15 kHz frequency) when the single-target cell droplets were in the proximity to the traps. The tubing used for oil flow in all three devices had 0.03˝ OD/0.005˝ ID.
After merging, two cells were incubated together on the chip for ≈30 min. PBS solution containing Alexa Fluor 647-conjugated secondary antibodies was loaded to the trapping array from inlet 2 with a flow rate of (1-5 nL s−1), while oil flow from inlet 1 was stopped (Figure S12, Supporting Information). The PBS/Alexa Fluor solution exchanged the oil flow inside the channels, and voltage (64 Vrms and 15 kHz frequency) was applied on the electrodes below the traps to disrupt the droplet interface. The two cells in each trap were washed with this solution for a total duration of ≈5 min to wash all 50 traps. The flow rate of the PBS/Alexa Fluor 647 solution was decreased to 0.1 nL s−1 and the 2% Ran-HFE oil flow rate was increased back to 5 nL s−1. The phase-change generated droplets in situ in traps with the two cells.
Hybridoma cells producing antigen-binding monoclonal antibodies were identified by fluorescent microscopy imaging of the trapping array (λex = 627 nm and λem = 665 nm, 300 ms exposure). Droplets containing the desired hybridoma cell and monoclonal antibodies were released from the traps by starting a reverse flow (flow rate = 4 nL s−1) of the 2% Ran-HFE oil from outlet 1 was initiated while inlet 3 was opened for a few seconds to remove previous droplets accumulated in that channel (Figure S12, Supporting Information). Droplets containing desired hybridomas were collected from inlet 1 with flow coming from outlet 1 (and inlet 3 closed).
“Spiked” Ratios of Desired versus Non-Desired Antibody Producing Cells
To replicate a rare population of hybridoma cells, different concentrations were created for two distinct hybridoma cell lines. One of these cell lines produced the desired monoclonal antibodies (mAbs) that specifically bound to membrane-bound antigens on target cells, while the other cell line produced non-binding mAbs. The cell lines were mixed at various ratios: 100% containing the desired mAbs (and 0% of the non-desired mAbs), as well as 50%, 10%, and 2% to represent the rare antibody population (Figure S10A, Supporting Information). Prior to device operation, the total cell concentration of the mixed populations was adjusted to 1 × 106 cells mL−1 and the cells were stained with CellTrace calcein red–orange (AM) following the manufacturer's protocol. Repeated injection and removal of the cells were repeated five times to ensure trapping cells from the “rare” populations (see schematic in Figure S10B, Supporting Information). For each injection, cells were encapsulated in droplets and washed following the protocol above. After washing, the desired cells that produced Ag-specific mAbs, their droplets were the only detectable fluorescence signal. All other droplets that had no detectable fluorescence were removed by visual observation via microscopy (non-fluorescent cells) and releasing the droplets from the trap via actuation and flow using the potentials and flow rates, respectively, as above.
Cell Retrieval
To collect selected droplets, a previously documented protocol [58] was adhered to. Briefly, a glass capillary filled with HFE 7500 was placed over the outlet to draw in the released droplet into the capillary. Next, a 10–20 µL droplet of complete media (IMDM) was situated on top of a PTFE membrane (Thomas Scientific, New Jersey, USA), secured by a custom-design 3D printed holder. Subsequently, the capillary contents containing the collected droplet with cell and HFE oil were expelled using ≈1 mL of FC-40. The introduction of FC-40 disrupted the surfactant-stabilized emulsion, allowing for absorption of the oils by the membrane. The droplet containing the cells and complete media were merged, and the resulting droplet combination was transferred to a culturing platform (e.g., well-plate) using a pipette for further expansion or downstream processing.
Acknowledgements
The authors would like to thank the Human Health Therapeutics Research team at National Research Council Canada for providing with cell lines. The authors thank the Natural Sciences and Engineering Research Council (NSERC), the Fonds de Recherche Nature et technologies (FRQNT), and the Canadian Foundation of Innovation (CFI) for funding. F.A. thanks Concordia University Department of Electrical and Computer Engineering for FRS and FRQNT PBEEE for funding. SCCS thanks Concordia for a University Research Chair.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
F.A. and S.C.C.S. designed the experiments. F.A. designed and fabricated the hybrid microfluidic devices (droplet generator, binary sorter, and trapping array), carried out experiments, and analyzed the data. F.A. performed all proof-of-concept experiments and characterization. H.T. wrote the interface software for the automation system, optical detector (PMT), syringe pump, and microscope camera to operate the experiments on device. N.L. assisted in cell culture and staining. S.R.L. helped Fatemeh in fabrication of the DMF substrates. F.A. carried out all antibody discovery assay on chip and analyzed the data with S.C.C.S. F.A. and S.C.C.S. wrote the paper, and all authors reviewed the final version of the manuscript before submission.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




