Open-Mouthed Hollow Carbons: Systematic Studies as Cobalt- and Nitrogen-Doped Carbon Electrocatalysts for Oxygen Reduction Reaction
Abstract
Although hollow carbon structures have been extensively studied in recent years, their interior surfaces are not fully utilized due to the lack of fluent porous channels in the closed shell walls. This study presents a tailored design of open-mouthed particles hollow cobalt/nitrogen-doped carbon with mesoporous shells (OMH-Co/NC), which exhibits sufficient accessibility and electroactivity on both the inner and outer surfaces. By leveraging the self-conglobation effect of metal sulfate in methanol, a raspberry-structured Zn/Co-ZIF (R-Zn/Co-ZIF) precursor is obtained, which is further carbonized to fabricate the OMH-Co/NC. In-depth electrochemical investigations demonstrate that the introduction of open pores can enhance mass transfer and improve the utilization of the inner active sites. Benefiting from its unique structure, the resulting OMH-Co/NC exhibits exceptional electrocatalytic oxygen reduction performance, achieving a half-wave potential of 0.865 V and demonstrating excellent durability.
1 Introduction
Utilizing sustainable energy storage and conversion technologies of the next generation offer a potential solution to address the challenging problems arising from global energy shortages and environmental concerns.[1, 2] In various renewable electrochemical conversion devices like fuel cells and metal–air batteries, the development of highly efficient electrocatalysts for the oxygen reduction reaction (ORR) is crucial.[3-5] However, the efficiency of these devices significantly decreases after prolonged use due to the slow kinetics of the ORR at the cathode, leading to kinetic consumption.[6] Platinum and platinum-based alloys are widely used as electrocatalysts for the ORR.[7,8] Nevertheless, the large-scale utilization of these electrocatalysts is hindered by supply limitations, price volatility, and stability concerns.[9-11] In recent years, extensive efforts have been made to explore affordable non-noble metals and carbon-based materials that exhibit ORR performance comparable to Pt-based electrocatalysts.[12-14] However, achieving feasible ORR electrocatalysts still requires appropriate pore architectures to facilitate fast mass/electron transfer and provide more accessible exposed active sites.
Hollow carbons are widely utilized as electrocatalysts in various electrochemical applications due to their large surface areas and hierarchical porous systems, which facilitate mass and electron transport and provide abundant active sites.[15-18] Specifically, hollow carbons with large open mouths on their mesoporous shells exhibit tremendous potential for electrocatalytic applications.[19] The hollow structure with open mouths enhances the accessibility and utilization of interior catalytic sites, while the mesoporous shells not only provide channels for rapid mass and charge transfer but also accommodate highly exposed external active sites.[20-23] However, the fabrication of hierarchical hollow carbons with mesoporous and/or microporous shells and large open holes remains an extremely challenging task.[24]
In recent years, metal–organic frameworks (MOFs) have gained recognition as excellent precursors for synthesizing heteroatom-doped porous carbons that contain uniformly dispersed metal single atoms or metal nanoparticles.[25-29] These carbon materials exhibit enhanced electrochemical activity in various applications related to the environment and energy.[30-32] However, the synthesis of hollow MOFs typically involves the use of sacrificial templates or additional etching with organic acid/base, both of which pose challenges due to environmental contamination and high costs.[33-35] Therefore, an efficient and environmentally friendly method is still urgently needed for producing hollow MOFs and their derived carbon-based electrocatalysts.
In this study, we successfully synthesize hollow MOFs without the use of soft- or hard-templates. These hollow MOFs are then carbonized to obtain open-mouthed hollow cobalt/nitrogen-doped carbons with mesoporous shells (OMH-Co/NC). These materials serve as highly efficient electrocatalysts for the oxygen reduction reaction (ORR). Electrochemical analysis reveals that the OMH-Co/NC electrocatalyst exhibits superior ORR performance, with a half-wave potential of 0.865 V, and demonstrats a good stability in a 0.1 m KOH electrolyte. These results are comparable to those achieved with commercial Pt/C catalysts. The excellent ORR activity of the OMH-Co/NC electrocatalyst can be attributed to two factors: i) the presence of highly exposed and accessible active sites, and ii) the hierarchically macro-, meso-, or microporous structure, which facilitates mass/charge transfer and oxygen molecule diffusion.
2 Results and Discussion
The fabrication process of OMH-Co/NC is depicted in Figure 1. Zinc sulfate and cobalt sulfate are dissolved in methanol separately. Transmission electron microscopy (TEM) images (Figure S1, Supporting Information) confirm the formation of cobalt sulfate (Figure S1a-c, Supporting Information) and zinc sulfate spheres (Figure S1d–f, Supporting Information) in methanol, respectively. Even when moderate concentrations of zinc sulfate and cobalt sulfate are combined, the spherical structure (zinc/cobalt mixed sulfate) is preserved, as depicted in Figure S2 (Supporting Information). It is hypothesized that these spheres are formed through the self-agglomeration of metal sulfate colloids in methanol, indicating the assembly of colloidosomes, as is illustrated in Figure 1. Subsequently, 2-methylimidazole (2-MIM) is added to coordinate with the zinc and cobalt ions of zinc/cobalt mixed sulfate spheres, leading to the formation of raspberry-structured Zn/Co-ZIF (R-Zn/Co-ZIF). This structure is subsequently transformed into OMH-Co/NC.
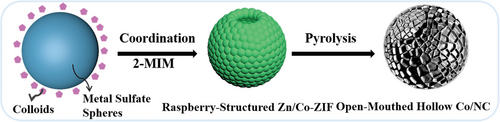
As shown in Figure 2a, the R-Zn/Co-ZIF exhibits an average diameter of ≈600 nm, with a 200 nm open hole. On the surface, Zn/Co-ZIF nanocrystals measuring less than 50 nm are assembled. For comparison, ZIF-67 is synthesized using only cobalt sulfate spheres, resulting in the formation of raspberry-structured ZIF-67 (R-ZIF-67) particles, as shown in Figure S3a (Supporting Information). Conventional Zn/Co-ZIF is also prepared using cobalt nitrate, zinc nitrate, and a methanol solution, exhibiting a typical rhombohedral structure (Figure S3b, Supporting Information). Once 2-MIM coordinates with the surface of spherical metal sulfates, the Zn/Co-ZIF nanocrystals start to accumulate, resulting in the preservation of the original spherical structure. Time-dependent TEM measurements (Figure S4, Supporting Information) show that the internal cavity develops gradually as the reaction progresses. After 4 h of processing, spheres with open-hole cavities are formed. The metal sulfate spheres undergo a hollowing process as the reaction time is extended. It is hypothesized that the metal sulfate crystallites within the spheres are smaller and less dense compared to those on the surface. Consequently, the inner crystallites are more susceptible to dissolution, followed by redeposition onto the outer surface of the spheres. Eventually, the resulting hollow spheres comprise crystallites composed of Zn/Co-ZIF.
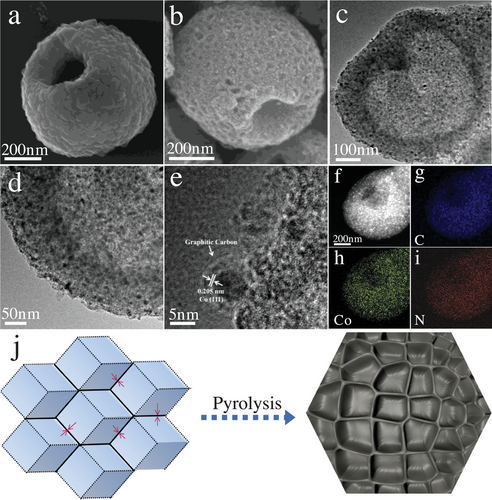
After undergoing pyrolysis, the resulting OMH-Co/NC exhibits surface pores with diameters ranging from 10 to 25 nm (Figure 2b). Figure 2j depicts the possible formation mechanism of the mesoporous structure formed on the external surface. As depicted in Figure 2a, the Zn/Co-ZIF nanocrystals are closely bonded to each other, and they gradually undergo a tight contraction at the initial stage of the carbonization process. The connecting interface between the Zn/Co-ZIF nanocrystals exhibits greater stability. Therefore, during the carbonization process, the shrinkage occurs from the central part of each individual nanocrystal, expanding outward and creating mesoporous open spaces.[36] The presence of mesopores on the external surface serves multiple purposes. First, they disperse the active sites, maximizing the exposure of these sites for efficient electrocatalysis. Second, the mesopores provide ample open spaces and shortened diffusion pathways for reactants and intermediates involved in the oxygen reduction process. This enhanced accessibility and reduced diffusion distance contribute to improved performance during the oxygen reduction reaction.[37] In addition, by preferentially stabilizing one adsorbed reaction intermediate while leaving the adsorption of the other intermediates unaffected, the nanoconfinement produced by the mesopores can be used to selectively weaken the target adsorption energies between the reactant and catalytic surface.[38, 39] As shown in Figure 2c,d and Figure S5a (Supporting Information), OMH-Co/NC maintains the hollow structure with a 100 nm shell thickness. Under the same conditions, direct pyrolysis of pristine R-ZIF-67 and typical Zn/Co-ZIF obtains hollow cobalt/nitrogen-doped carbon with mesoporous shells (H-Co/NC) (Figures S3c and S5b, Supporting Information) and Co/NC (Figures S3d and S5c, Supporting Information), respectively, which retain their original structure. Moreover, high-resolution transmission electron microscopy (HRTEM) pictures demonstrate the formation of graphitic carbons and Co nanoparticles. Lattice fringe pictures of the materials reveal the presence of graphitic carbon-covered metallic cobalt with d-spacings of 0.205 nm indicating the (111) plane of Co fcc structure (Figure 2e). The creation of graphitic carbon is due to the Co species which can graphitize amorphous carbon into graphitic carbon during a high-temperature process.[40] Furthermore, the elemental mapping images (Figure 2f–i) indicate that C, N, and Co elements are uniformly distributed in the OMH-Co/NC.
As depicted in Figure S6 (Supporting Information), the XRD patterns of the R-Zn/Co-ZIF, R-ZIF-67, and typical Zn/Co-ZIF precursors closely match the characteristic peaks of the simulated ZIF phase. After pyrolysis, the XRD patterns of the resulting OMH-Co/NC (Figure 3a) exhibit weaker characteristic peaks of metallic cobalt compared to H-Co/NC and Co/NC. The Co content for OMH-Co/NC, H-Co/NC, and Co/NC was 3.30 at.%, 3.43 at.%, and 2.60 at.%, respectively, as determined by XPS. Even though these three samples have similar Co content, the Co peak of OMH-Co/NC is noticeably weaker. This observation suggests the formation of smaller cobalt nanoparticles, which could potentially contribute to the superior oxygen reduction reaction (ORR) performance.[41] This effect can be attributed to the confinement effect of the mesopores on the outer surface, preventing cobalt particle aggregation and increasing metal dispersion within the carbon matrix.[42] Additionally, the peak at 26.4° corresponds to the C (002) plane, indicating the formation of graphitic carbon.
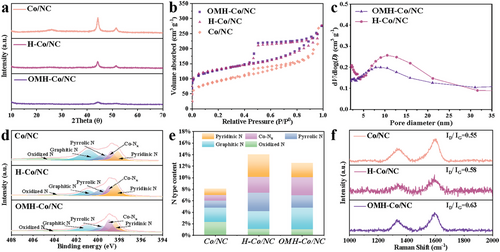
Furthermore, Figure 3b shows the isotherms with hysteresis loops for OMH-Co/NC, H-Co/NC, and Co/NC. The substantial nitrogen uptake at low relative pressure and the gradual adsorption of nitrogen in the mesopore range signify the existence of both micropores and mesopores, highlighting the hierarchical porous properties of the material. These findings are consistent with the Barrett–Joyner–Halenda (BJH) pore diameter distribution curves (Figure 3c) of OMH-Co/NC and H-Co/NC, which exhibit a peak around 10–15 nm. The OMH-Co/NC possesses a substantial Brunauer–Emmett–Teller (BET) surface area of 423.1 m2 g−1 (Table S1, Supporting Information), with a micropore area of 231.4 m2 g−1. Compared to Co/NC, the increased specific surface area of OMH-Co/NC and H-Co/NC indicates that the hollow structure contributes to the exposure of more active sites.
To further investigate the elemental states of the electrocatalysts, X-ray photoelectron spectroscopy (XPS) analysis is conducted (Figure S7, Supporting Information). The N 1s region (Figure 3d) reveals peaks corresponding to pyridinic N, Co-Nx, pyrrolic N, graphitic N, and oxidized N, with respective energies of 398.3, 399.1, 399.8, 401.0, and 404.0 eV.[43] The N species content of the electrocatalysts, as determined by XPS (Figure 3e and Table S2, Supporting Information), shows that OMH-Co/NC has the largest content of Co-Nx. Notably, Co-Nx coordination is considered the major active site in ORR. The presence of metallic cobalt and cobalt oxides is indicated by the high-resolution Co 2p spectrum (Figure S8, Supporting Information), with distinctive peaks associated with Co0 (779.5 eV), Co3+ (781.2 eV), and Co2+ (783.2 eV).[44, 45]
Raman spectra of OMH-Co/NC, H-Co/NC, and Co/NC (Figure 3f) are obtained to analyze the defective structure. All the electrocatalysts exhibit D and G bands at around 1340 cm−1 and 1580 cm−1, respectively, corresponding to disordered carbon defects and ordered graphitic carbon.[46] The ID/ IG values show an increasing trend of Co/NC (0.55) < H-Co/NC (0.58) < OMH-Co/NC (0.63), indicating that OMH-Co/NC has the highest number of structural defects due to the introduction of open holes and hollow structures. The presence of numerous defect sites can influence the surface state of the electronic structure, including charge and spin densities, leading to structural distortions in the carbons. This optimization of the adsorption energies plays a crucial role in enhancing the performance of electrochemical catalytic processes, particularly for the oxygen reduction reaction (ORR).[47] Additionally, these defect sites provide accommodation for active sites, facilitating the transport properties of species relevant to ORR. This accommodation effect promotes the capacity for efficient transport and contributes to the overall performance improvement in ORR.[48]
To evaluate the catalytic activity, cyclic voltammetry (CV) measurements under O2-saturated conditions are initially conducted in a 0.1 m KOH solution (Figure S9, Supporting Information). As expected for ORR, the investigated catalysts exhibit cathodic peak currents under O2-saturated conditions. From the linear sweep voltammetry (LSV) curves shown in Figure 4a, it is evident that OMH-Co/NC displays superior ORR activity with a half-wave potential (E1/2) of 0.865 V, significantly more positive than H-Co/NC and Co/NC. Notably, the electrocatalytic activity of OMH-Co/NC surpasses that of commercial Pt/C (E1/2 of 0.824 V) and many Co-based electrocatalysts listed in Table S3 (Supporting Information), highlighting the significant advantages of the mesoporous hollow structure with open holes in enhancing the ORR process. Moreover, OMH-Co/NC exhibits a limiting current density of 5.7 mA cm−2 at 1600 rotations per minute (rpm), which is comparable to that of commercial Pt/C (Figure 4b). To determine the actual specific activity of the electrocatalysts, the observed LSV curves were normalized by the BET specific surface area. As illustrated in Figure 4c, the specific activity (estimated based on the specific surface area) of OMH-Co/NC at 0.850 V is 0.042 A m−2, which is significantly higher than the specific activities of H-Co/NC (0.033 A m−2) and Co/NC (0.028 A m−2). This data clearly demonstrates that the open-mouthed structure of OMH-Co/NC provides a larger number of exposed active sites as well as the elemental state of OMH-Co/NC provides a superior catalytic activity. As shown in Figure S10 (Supporting Information), the OMH-Co/NC shows the lowest Tafel slope compared to other samples, indicating the fast catalytic kinetics of OMH-Co/NC.
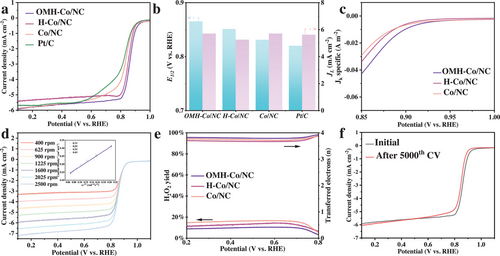
To investigate the optimum pyrolysis temperature, OMH-Co/NC obtained under 800, 900, and 1000 °C are further tested for the ORR performance (Figure S11, Supporting Information), which shows OMH-Co/NC-800 is the best electrocatalyst compared to the other electrocatalysts. Furthermore, to further confirm the role of Co nanoparticles as the main active phase, an acid etching experiment is conducted on OMH-Co/NC using 0.5 m H2SO4. The half-wave potential of OMH-Co/NC after acid etching exhibits a significant decrease compared to that of pristine OMH-Co/NC (Figure S12, Supporting Information). This observation provides further evidence that the Co nanoparticles serve as the main active sites in the electrocatalytic process.
Experiments using rotating disk electrodes are conducted at various rotation rates, ranging from 400 to 2500 rpm, to gain a deeper understanding of the electron transfer process during the ORR reaction (Figure 4d). The Koutecky–Levich (K-L) plot of OMH-Co/NC shows a linear relationship, as depicted in the inset of Figure 4d. Furthermore, rotating ring disk electrode tests (RRDE tests) were performed to determine the electron transfer number of the ORR reactions. The results indicate a range from 3.8 to 4.0 and a low H2O2 yield of 0–10% (Figure 4e), suggesting a dominant four-electron pathway in the oxygen reduction process. Durability is another essential characteristic of a functional electrocatalyst. To evaluate the stability of the OMH-Co/NC electrocatalyst, the cyclic voltammetry (CV) method is employed. Figure 4f reveals that the half-wave potential of OMH-Co/NC has changed minimally with a decrease of 15 mV after 5000 CV cycles in O2-saturated 0.1 M KOH electrolyte, indicating an excellent ORR durability. In addition, after a long-term durability test (Figure S13, Supporting Information), the OMH-Co/NC shows great stability compared to the Pt/C. The graphitic carbon coating likely contributes to the higher corrosion resistance.[49]
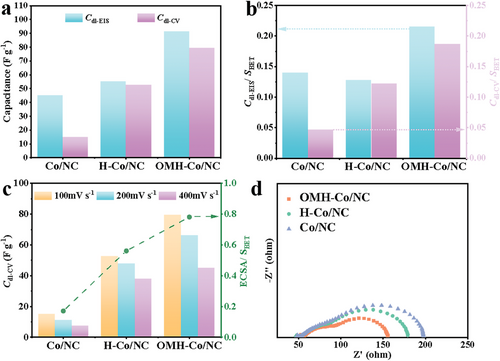
To investigate the influence of the hollow structure and open holes on the electrochemical performance, an electrochemical impedance spectroscopy (EIS) study is conducted. To avoid limitations in charge and mass transfer resulting from a high catalyst loading amount, electrodes are prepared with minimal catalyst loading (0.2 mg cm−2). This approach ensures the utilization of the entire electrochemically wettable and accessible surface area (ECWA and ECSA, respectively) of the porous carbon materials.[50] By comparing the values of Cdl-CV (Figure S14, Supporting Information) and Cdl-EIS, the impact of structural changes on the corresponding area changes can be determined. As shown in Figure 5a, both Cdl-EIS and Cdl-CV exhibit an increasing trend of Co/NC < H-Co/NC < OMH-Co/NC. This suggests that both the mesoporous hollow structure and open holes contribute to the exposure of more active sites and the enhanced utilization of Co-Nx species under dynamic condition. The introduction of open holes enhances the accessibility and utilization of interior catalytic sites. Consequently, the unique spatial structure of OMH-Co/NC helps reduce its electrochemically redundant/inactive surface area. This is further supported by the values of SBET normalized Cdl-EIS and Cdl-CV (Cdl-EIS/SBET and Cdl-CV/SBET, respectively) shown in Figure 5b. The OMH-Co/NC demonstrates much higher Cdl-EIS/SBET and Cdl-CV/SBET values, showing that mesopores and open pores are beneficial to the maximization of ECWAs and ECSAs. Furthermore, the Cdl-CV values of Co/NC, H-Co/NC, and OMH-Co/NC are obtained at high scan rates (100, 200 and 400 mV s−1) to gain insights into the rate capability when ion and electron transport are hindered by the high scan rates. In contrast to Co/NC and H-Co/NC, OMH-Co/NC shows considerably superior Cdl-CV values (Figure 5c) at higher scan rates, indicating the potential benefits of mesopores and macropores in the rate capability.[51] Moreover, the ECSAs obtained by ranging the scan rate from 5 to 200 mV s−1 are further normalized by SBET. The results reveal that OMH-Co/NC can keep the rate capability at a higher level than those of H-Co/NC and Co/NC (Figure 5c). Hence, surface mesopores, hollow structure, and open-holes play an important role in maintaining electrochemical active areas at high charge-discharge rate, which is due to the unique structure resulting in rapid ion transport. Figure 5d shows the EIS data in the mixed-diffusion region (0.80 V versus RHE). The EIS consists of two semicircles, which represent charge-transfer and mass-transport processes, respectively.[52] It can be seen from Figure S15 (Supporting Information) that the OMH-Co/NC presents the smallest charge transfer resistance of 45.4 Ω and lowest mass transport resistance of 58.1 Ω compared to H-Co/NC and Co/NC, which indicates that the introduction of open-hole can improve the ability of mass transport.
3 Conclusion
In summary, we have successfully synthesized an open-mouthed hollow cobalt/nitrogen-doped carbon with mesoporous shells (OMH-Co/NC) structure through the self-conglobation effect of metal sulfate in methanol, followed by pyrolysis. The incorporation of open-mouthed and hollow structures in the OMH-Co/NC electrocatalyst results in a favorable surface area, abundant mesopores, and high accessibility to inner active sites. These features contribute to its remarkable electrochemical activity, facilitating mass and charge transfer during the oxygen reduction reaction (ORR) in alkaline electrolytes. The OMH-Co/NC electrocatalyst demonstrates excellent ORR performance, achieving a half-wave potential of 0.865 V, and exhibits remarkable stability. This work presents a novel method for synthesizing hierarchical hollow carbons with mesoporous or microporous shells and large open pores, opening new possibilities for the development of efficient electrocatalysts for oxygen reduction reaction.
4 Experimental Section
Chemicals
Zinc nitrate hexahydrate, cobalt nitrate hexahydrate, cobalt sulfate heptahydrate, zinc sulfate heptahydrate, and potassium hydroxide pellets were purchased from Sinopharm Chemical Reagent Co., Ltd. Pt/C (20 wt.%) was obtained from Shanghai Macklin Biochemical Co., Ltd. 2-Methylimidazole was obtained from Aladdin Industrial Corporation. Nafion D-520 dispersion (5% w/w in water and 1-propanol) was purchased from Alfa Aesar Chemical Reagent. All the chemicals used in this work were used without further purification.
Preparation of Typical Zn/Co-ZIF
Zn(NO3)2⋅6H2O (0.4 mmol), Co(NO3)2⋅6H2O (1.6 mmol), and 2-methylimidazole (16 mmol) were dissolved in methanol (50 mL), respectively. Then, 50 mL of the 2-methylimidazole methanol solution was added to the metallic nitrate methanol solution. After 10 min of stirring, the obtained solution was left undisturbed for 24 h. The obtained precipitate was filtered, washed with methanol, and dried at 70 °C overnight.
Preparation of R-ZIF-67
CoSO4⋅7H2O (20 mmol) and 2-methylimidazole (160 mmol) were dissolved in methanol (400 mL), respectively. Then, 400 mL of the 2-methylimidazole methanol solution was added to the cobalt sulfate methanol solution, which was agitated for a further 4 h. The purple product was filtered, washed multiple times with methanol, and then dried overnight at 60 °C.
Preparation of R-Zn/Co-ZIF
ZnSO4⋅7H2O (4 mmol), CoSO4⋅7H2O (16 mmol), and 2-methylimidazole (160 mmol) were dissolved in methanol (400 mL), respectively. Then, 400 mL of the 2-methylimidazole methanol solution was added to the metallic sulfate methanol solution, which was agitated for a further 4 h. The purple product was filtered, washed multiple times with methanol, and then dried overnight at 60 °C.
Preparation of OMH-Co/NC
R-Zn/Co-ZIF was placed in a crucible and carbonized in a tube furnace at 800o C for 2 hours with a heating rate of 5o C min−1. The final product was designated OMH-Co/NC. Under the same conditions, H-Co/NC and Co/NC were obtained by carbonizing R-ZIF-67 and Zn/Co-ZIF, respectively.
Characterization
The sample morphology was evaluated by SEM using the Hitachi S4800 equipment at 10 kV acceleration voltage. At an accelerated voltage of 200 kV, FEI Tecnai G2 F30 was utilized to test TEM and EDX. The HAADF-STEM pictures were acquired using the JEM-ARM300. The specific surface area and pore size of BET were determined using a Micromeritics ASAP 2020V4.01 H instrument and a N2 adsorption/desorption isotherm at 77.5 K. The specific surface area was calculated using the multi-point BET technique at relative pressures ranging from 0.03 to 0.60 based on the adsorption data. The inVia Reflex from Renishaw was used to study the structure of carbon at room temperature using Raman spectroscopy. XPS (ESCALAB250Xi) was evaluated with Al K radiation.
Electrochemical Measurements
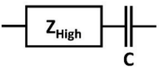
The impedance in the high-frequency range, which includes electric and ionic resistances and capacitance in series, is represented by the equivalent circuit shown above. In the low frequency domain, however, ZHigh’s impedance is constant, allowing Cdl_EIS to be easily estimated from the raw data.
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (22005099), JST SPRING (JPMJSP2128), the International UQ-Yonsei Research Project, and the JST-ERATO Yamauchi Materials Space-Tectonics Project (JPMJER2003). This work was supported by the Researchers Supporting Project RSP2023R405, King Saud University, Saudi Arabia. This work used the Queensland node of the NCRIS-enabled Australian National Fabrication Facility (ANFF).
Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




