Lignocellulosic Biomass-Based Carbon Dots: Synthesis Processes, Properties, and Applications
Abstract
Carbon dots (CDs), a new type of carbon-based fluorescent nanomaterial, have attracted widespread attention because of their numerous excellent properties. Lignocellulosic biomass is the most abundant renewable natural resource and possesses broad potential to manufacture different composite and smart materials. Numerous studies have explored the potential of using the components (such as cellulose, hemicellulose, and lignin) in lignocellulosic biomass to produce CDs. There are few papers systemically aiming in the review of the state-of-the-art works related to lignocellulosic biomass-derived CDs. In this review, the significant advances in synthesis processes, formation mechanisms, structural characteristics, optical properties, and applications of lignocellulosic biomass-based CDs such as cellulose-based CDs, hemicellulose-based CDs and lignin-based CDs in latest research are reviewed. In addition, future research directions on the improvement of the synthesis technology of CDs using lignocellulosic biomass as raw materials to enhance the properties of CDs are proposed. This review will serve as a road map for scientists engaged in research and exploring more applications of CDs in different science fields to achieve the highest material performance goals of CDs.
1 Introduction
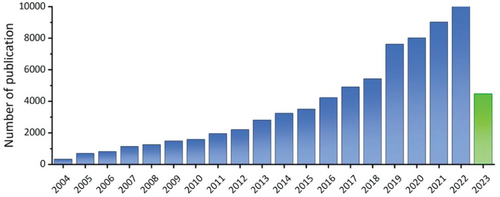
Functional environmentally friendly carbon-based nanomaterials have a wide range of applications in chemistry, materials, and other interdisciplinary fields, addressing the challenges of the impending environmental and energy crisis.[1] These nanomaterials exhibit remarkable thermal and electrical conductivity, exceptional mechanical strength, and diverse optical properties, rendering them ideal for a variety of applications. Among these carbon-based nanomaterials, carbon dots (CDs) represent a relatively new addition and are noteworthy for being among the few photoluminescent (PL) carbon nanomaterials with an appropriate emission band gap.[2, 3] In general, CDs are categorized as quasi-spherical, zero-dimensional nanocarbon materials, with a diameter that is typically less than 20 nm.[4-8] The earliest record of CDs dates back to 2004, when scientists stumbled upon a fluorescent carbon nanoparticle while purifying single-walled carbon nanotubes through an arc discharge method.[9] As research activities into CDs progressed (Figure 1), their unique properties, including fluorescence stability, small size, low biotoxicity, tunable photoluminescence, high quantum yield (QY), and excellent up-conversion luminescence, have been uncovered.[4, 10-12] When compared to traditional semiconductor quantum dots (SQDs), which are often associated with high synthesis costs, biological toxicity, and environmental pollution, CDs can be synthesized at a low cost on a large scale with less environment impact.[13] Recent research efforts have inspired a variety of innovative applications across multiple fields, such as photocatalysis, biomedicine,[11, 14] optoelectronic devices,[15-17] sensors,[18-20] information encryption,[21, 22] and composite function materials.[23-25]
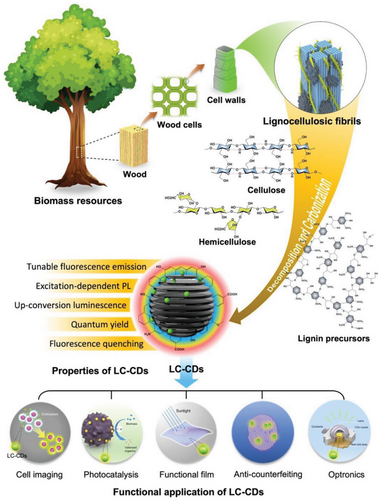
One of the most distinctive features of CDs is that their fluorescence core is primarily composed of a hybrid of carbon elements. The carbon sources employed to synthesize CDs include large-size carbons from fossil materials (such as carbon black, graphite),[26] linear organic compounds (e.g., polyvinyl alcohol),[27] small aromatic molecules (aniline structure,[28] phenol structure,[29] etc.). For the preparation of CDs, these carbon precursors are associated with a variety of imperfections, such as complex and expensive processes, unsustainable availability, and unfavorable for large-scale production. Recently, extensive interest has focused on the synthesis of CDs using biomass-based materials (such as eggs,[30] milk,[31] orange juice,[32] agricultural and forestry resources [33, 34]). Among them, lignocellulosic biomass derived from agroforests have attracted the most attention.[35-39] A large number of green, low-cost, and readily available natural compounds (such as lignin and glycan) always exhibit active and complex chemistry, guaranteeing that lignocellulosic materials can serve as ideal starting precursors of synthesizing CDs. Cellulose has a carbon content of 44%, while the carbon content in hemicellulose is slightly lower than that in cellulose. Lignin has more than 60% carbon content, with the majority existing in the form of an aromatic structure.[40, 41] Oxygen elements, which mostly exist in the form of functional groups in cellulose, hemicellulose, and lignin, can significantly enhance the molecular activity of these three compounds. Utilizing lignocellulosic biomass as precursors not only reduces the exposure of synthesis processes to chemicals but also facilitates the potential of commercializing current developed technologies of producing CDs.[42, 43] Currently, although several reviews on the synthesis process, photoluminescence mechanism, and advanced applications of CDs have been summarized,[12, 44-50] a comprehensive literature review on synthesizing CDs from lignocellulosic materials is still missing. Furthermore, most recent studies have only discussed the synthesis mechanism of CDs prepared from a single-component precursor.[36, 38, 51] It is still an open question whether synergistic interactions among different components in a lignocellulosic precursor can affect the structure and properties of CDs. The by-products formed during the process of synthesizing lignocellulosic biomass-based CDs (LC-CDs) can be diverse and complex, resulting in many unpredictable properties of CDs.[18, 39] It is paramount to comprehensively comprehend the formation mechanism of LC-CDs so that researchers may optimize the synthesis conditions and parameters to yield LC-CDs with tailored properties and minimize the generation of undesired by-products. Such a concerted effort can lead to enhanced efficiency and cost-effectiveness, and can expedite the exploration of novel applications for LC-CDs.[33]
Therefore, in this review, we focus on the methods, formation mechanisms, properties, and applications of LC-CDs. First, synthetic methods and formation mechanisms of using different components (cellulose, hemicellulose, and lignin) in lignocelluloses as precursors will be introduced in detail. Following this, the PL properties of LC-CDs from the perspective of structural and physicochemical properties are discussed. Subsequently, the applications of each type of LC-CDs in optics, energy, and biomedicine are also explored. Finally, we propose an outlook on the current challenges and opportunities of LC-CDs for future targeted design and potential applications. The overview of this review is illustrated in Scheme 1. The overarching aim of this review is to ignite greater interest in research endeavors aimed at transforming lignocellulosic biomass from various agricultural and forestry sources into high-value-added products and exploring their potential applications in energy, biomedicine, and other scientific domains.
2 Raw Materials, Synthetic Methods, and Formation Mechanism
2.1 Raw Materials of LC-CDs
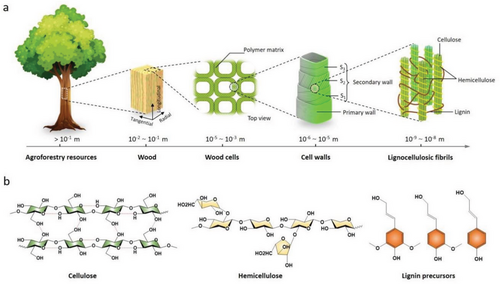
Research efforts towards reducing carbon footprints and exploring green energy sources have created a driving force promoting extensive research and development activities in the utilization of wood and forest resources.[52-54] Lignocellulose, which is predominantly derived from wood and agroforest resources, is a readily available and sustainable resource.[55-57] It constitutes a complex bio-composite material, primarily composed of cellulose (40–45%), hemicellulose (20–35%), and lignin (10–30%) that are formed during the cell wall formation process in plants such as trees (as shown in Figure 2a).[52, 58] Annually, more than 40 million tons of inedible lignocellulosic materials produced each year, including wheat stems, corn stover (stems and leaves), and wood chips, are produced worldwide.[59] Table 1 summarizes the contents of various chemical components in different lignocellulose.
| Lignocellulose | Glucan [%] | Xylan [%] | Galactan [%] | Arabinan [%] | Mannan [%] | Lignin [%] | Ash [%] |
|---|---|---|---|---|---|---|---|
| Switchgrass | 38.46 ± 0.69 | 26.34 ± 0.54 | 1.16 ± 0.18 | 3.41 ± 0.32 | 0.13 ± 0.22 | 21.40 ± 0.24 | 1.91 ± 0.10 |
| Wheat straw | 39.18 ± 2.01 | 24.62 ± 1.36 | 0 ± 0 | 1.68 ± 0.25 | 0 ± 0 | 17.17 ± 0.46 | 2.12 ± 0.87 |
| Eastern redcedar | 40.30 ± 1.50 | 8.50 ± 0.04 | 2.00 ± 0.60 | 1.40 ± 1.00 | 6.00 ± 1.20 | 35.90 ± 0.70 | 0.30 ± 0.00 |
Cellulose and hemicellulose are types of polysaccharides. Specifically, cellulose is a linear and rigid chain glycan compound (Figure 2b) mainly assembled by repeating units of d-pyran glucose through covalent bonds, and intrachain and interchain hydrogen bonds at the molecular level.[61, 62] Hemicellulose is a compound glycan containing various glycone groups (d-xylose, l-arabinose, d-galactose, d-mannose, etc.) and uronic acid groups (d-glucuronic acid, 4-O-methyl-d-glucuronic acid, d-galacturonic acid, etc.). These groups are linked together by glycosidic bonds, usually forming branched chains in the molecules.[63, 64] Lignin is a spatially intricate, three-dimensional, cross-linked phenolic polymer composed of numerous syringyl, guaiacyl, and p-hydroxyphenyl units that are interconnected via ether bonds (e.g., aryl- or phenyl ether) and carbon-carbon bonds (e.g., biphenyls, diphenyl ethane, or pinoresinol).[65, 66] Specially, lignin possesses the properties of self-association, strong ultraviolet absorption, and fluorescence emission.[67, 68] Both polysaccharides and lignin possess high carbon contents and abundant hydroxyl groups, facilitating their chemical modifications as composites. These unique properties of lignocellulosic biomass render them a viable candidate for fabricating advanced photoluminescence CDs. Hence, a thorough analysis and comprehension of the chemical structures of cellulose, hemicellulose, and lignin are critical for exploring their physicochemical properties and deducing the nucleation growth mechanism of CDs.[69] Compared with other raw materials, the utilization lignocellulosic biomass as precursors to synthesize LC-CDs presents significant potential for further optimization of their synthesis process, formation mechanism, performance regulation, and application orientation.
2.2 Synthetic Methods of LC-CDs
The synthetic methods of CDs can be grouped as two routes, “top-down” and “bottom-up” approaches based on the precursor type used in a synthesis process.[70] Figure 3a traces the synthesis process of CDs. The specific definition and classification of those synthetic methods have been fully summarized in most reviews.[71, 72] The “top-down” approach (Figure 3b) mainly uses physical or chemical methods to peel or cut large carbon into graphene quantum dots (GQDs). The “bottom-up” approach (Figure 3c) is to assemble the molecular level of carbon-based compounds,[21, 73, 74] such as organic molecules and polymers,[75, 76] into carbon quantum dots (CQDs) or carbonized polymers dots (CPDs) through assembling, polymerizing, cross-linking, and carbonizing steps under relatively mild conditions such as low temperature and less strong acid.[77, 78] In general, GQDs consist of one to three layers of graphene attached with a limited number of surface functional groups. CQDs are composed of multiple layers of graphene attached with abundant surface functional groups. Each of CPDs has an amorphous carbon core attached with abundant surface functional groups.[78]
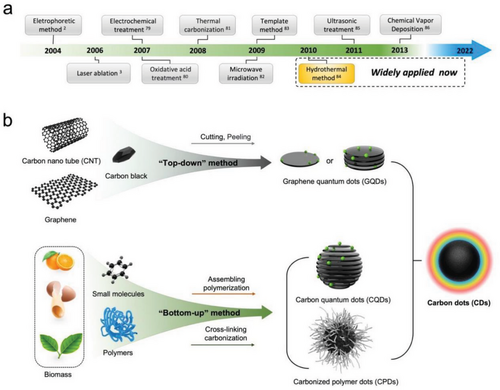
The mechanism of the “bottom-up” approach is similar to the one of synthesizing SQDs using wet-chemical reaction processes involving key steps such as reactive precursors regulation, ligands utilization, and nucleation growth and separation.[36] The structures of CDs are significantly influenced by various factors, such as the structure of precursors, the properties of doping agents, solvent used, reaction time, and temperature. Ideally, the structures and properties of synthesized CDs can be controlled through optimizing these critical reaction parameters.[35, 51, 87] Most of the current research on LC-CDs suggests that the “bottom-up” approach, which involves hydrothermal treatment, solvothermal treatment, and microwave irradiation, is the preferred method for synthesizing LC-CDs due to the unique properties of the precursor materials and the targeted requirements of LC-CDs.
2.3 Formation Mechanism of LC-CDs
2.3.1 Cellulose-Based Carbon Dots
Cellulose-based carbon dots (C-CDs) can be synthesized via various methods, celluloses through hydrothermal/solvothermal,[74, 88] microwave irradiation,[10, 24, 89] combustion carbonization,[90] and laser ablation.[91] The polymerization degree of cellulose typically ranges from 50 000 to 250 000. Various types of celluloses, such as microcrystalline cellulose (MCC),[74, 92, 93] cellulose acetate butyrate (CAB),[94, 95] pulp fibers,[96, 97] and nature celluloses refined from various agroforest materials s(e.g., corn cob,[94, 95] wood,[98] straw,[37] fresh grass[99]) have been utilized as precursor materials. Most investigations exhibit a preference for employing MCC due to its homogeneous composition and uniform polymerization degree.[88, 100, 101] However, a common issue encountered while using MCC as reactants is that the synthesized C-CDs did not meet the desired fluorescence efficiency and QY requirements.[102] Hence, the incorporation of dopants, such as amines and alkali metals,[74, 98] as reaction ligands in reactants, has emerged as a promising strategy. This approach facilitates the effective optimization of fluorescence properties of CDs by directionally inserting dopant functional groups into their structures.[24, 103] Kim et al. posited that the incorporation of nitrogen (N) into the carbon cores, dominated by carbon (C), results in the formation of graphene-like structures.[74] The carbon cores in nitrogen-doped C-CDs showcased isolated sp2 centers interspersed within an sp3 lattice, while the superior PL stability and selective sensitivity toward Fe3+ of N-doped C-CDs affirmed the efficacy of dopants. Alternatively, the heterogeneity of surface functional groups on C-CDs has also been reported.[93] Several studies have revealed that the presence of O- and N-rich groups on the surface of carbon cores creates a novel surface state, resulting in improved photon absorption efficiency and red shift of fluorescence emission.[28, 104]
However, the dopants introduced in reactants are often high-cost finishing chemicals, such as ethylenediamine.[98] This issue has prompted several studies to investigate the impact of endogenous self-doping (raw materials containing N, etc.) on the structure and PL behavior of LC-CDs. In these studies, biomass materials (such as peanut shell, cotton stalk), which contain cellulose and protein (N source), were employed to synthesize C-CDs without any dopants added through hydrothermal treatment.[38] The incorporation of endogenous doping, such as quaternary N, into the carbon cores of C-CDs has been shown to significantly enhance their fluorescent anti-interference capability.
Recently, a novel strategy for synthesizing CDs with dual-element doping has been proposed. Specifically, celluloses extracted from wood chips can be co-doped with Mg(OH)2 and ethylenediamine, resulting in the creation of green light-emitting C-CDs.[98] The dual element doping strategy endows CDs with improved fluorescence properties that can be manifested as sensitive pH responsiveness and stable fluorescence properties. The highlights the significance of selecting appropriate precursors and diverse dopants in achieving enhanced CDs properties. It is believed that heteroatoms can be doped into rigid cellulose chain macromolecules and then participate in dehydration, polymerization, rearrangement, and carbonation.[92, 105] Thus, a thorough understanding of the formation mechanism of CDs, especially for LC-CDs, is crucial as it can offer theoretical guidance for the appropriate selection of diverse precursors and dopants.
Research efforts have been carried out to explore and understand the pyrolysis mechanism and carbonization percentage of cellulose when lignocellulosic is used as a carbon source for synthesizing CDs.[106] These studies involve analyzing the proportion of each component in lignocellulose that converts to CDs based on the residues after carbonization,[37] understanding the polymerization mechanism by analyzing structural characteristics of synthesized CDs,[88] and analyzing the species of intermediate products through in-situ synthesis technology.[91] A typical approach for synthesizing C-CDs using cellulose involves initially subjecting cellulose to hydrothermal or microwave irradiation, yielding crude C-CDs, which are subsequently purified via centrifugation and dialysis techniques (as illustrated in Figure 4a).[11, 63, 96] However, the majority of these studies have only alluded to the formation mechanism of C-CDs without providing an in-depth elucidation supported by data and documentation. The process of C-CDs formation can be broadly categorized into five fundamental steps: ring opening, dehydration, polymerization, carbonization, and recrystallization. These stages are differentiated by their distinct energy conversion and synthesis processes.[37]
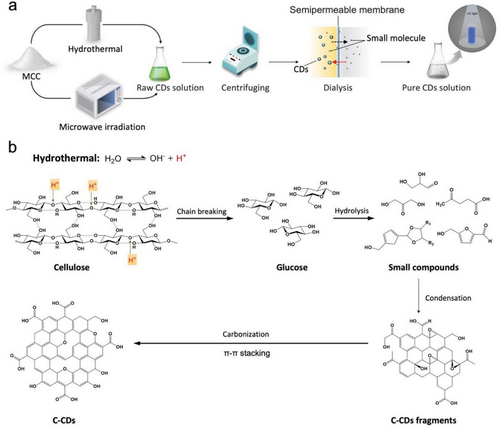
Summarizing the existing studies, the specific formation steps of C-CDs can be delineated as follows. The presence of robust intramolecular and intermolecular hydrogen bonds in cellulose chains can hinder hydrolysis reactions.[61] It is believed that water ionizing will produce H+ during hydrothermal treatment or microwave radiation at a high temperature and pressure condition (Figure 4b). Under the induction of H+, the glycosidic bonds and hydrogen bonds in cellulose chains are broken successively, forming a large number of intermediate products of oligomeric reduced sugars, such as l-glucose.[51, 107] As the thermal decomposition continues, some degraded small molecules of organic acids can be formed. These small molecules of organic acids include succinic acid, glutaric acid, and furan acid from dihydroxyacetone or glyceraldehyde via intermolecular dehydration, enol tautomerism, or intramolecular dehydration.[108] These small molecules possess minute particle size, copious polar groups, extraordinary hydrophilicity, proficient deprotonation ability, and extensive condensation, thus facilitating the rearrangement among small molecules. Ultimately, with the carbonization of rearranged small molecules, C-CDs are formed.[109, 110] Meanwhile, the ring-opening reaction of celluloses or l-glucoses and its equivalents will cause the formation of the 5-hydroxymethyl-2-furaldehyde (HMF) intermediate.[111, 112] The HMF undergoes subsequent repolymerization and dehydration, resulting in the formation of polyester molecules, which in turn undergo rearrangement to form a carbonized sheet structure.[112] During this process, dopant molecules or their decomposition fragments will also participate in the repolymerization. Aromatic dopants tend to directly participate in the formation of carbon cores, while others primarily grow along the surface sites of carbon cores. Finally, spherical carbon nanospheres will be built with the accumulation of carbon sheets.[107]
2.3.2 Hemicellulose-Based CDs
Hemicelluloses can be characterized by the presence of short lateral chains growing on backbones, consisting of different monosaccharides, either in a homopolymer or heteropolymer form. The monosaccharides in hemicelluloses mainly include glycosidic bonds linked pentose (xylose, rhamnose, arabinose) and hexose (glucose, mannose, and galactose) as well as uronic acids (e.g., 4-O-methyl glucuronic, d-glucuronic, and d-galacturonic acids).[113] Hemicelluloses typically demonstrate a reduced degree of polymerization and diminished hydrogen bonding among their molecules compared to celluloses. Therefore, the hemicellulose polymer chains can be easily hydrolyzed, and the ring opening of hemicelluloses during the synthesis of hemicellulose-based CDs (H-CDs) is more rapid than the one of celluloses.[114] The H-CDs synthesized from lignocellulosic materials rich in hemicellulose content showed a higher QY of up to 55%.[51]
Due to the presence of a variety of monosaccharide components in hemicelluloses, the small molecular compounds that form during the ring opening and hydrolysis of hemicelluloses are more complex and diverse compared to those in celluloses. In addition, the extraction and separation processes of natural hemicelluloses pose significant challenges.[115] Hence, there are few studies on the formation mechanism and structure of H-CDs. Researchers have noted that the proportion of xylan in hemicelluloses generally account for more than 50%, and therefore, it is often used as a substitute for hemicelluloses.[116] Zhou's group synthesized H-CDs using xylan as a carbon source and polyethyleneimine as a passivation agent.[73] Interestingly, it was found that N contained CDs synthesized from xylose and chitosan exhibited the same defective state-dominated PL emission behavior, as well as different photocatalytic activities when simultaneously doped with S.[117] The relatively high chemical reactive behavior of hemicellulose can make the synthesis of single-layer graphene GQDs with a larger surface area and high QY (23.8%) in an alkaline hydrothermal environment possible, and this unique feature is not available in C-CDs.[118] Beside xylan, mannose (12–15%) in hemicelluloses can also be used to fabricate H-CDs.[119, 120]
The formation mechanism of H-CDs can be inferred as follows: firstly, under thermal treatment at temperatures exceeding 225 °C, the glycans present in hemicelluloses undergo dehydration and decomposition, leading to the production of soluble compounds, such as alcohols (e.g., methanol, furfuryl alcohol), aldehydes (e.g., furfural, formaldehyde), ketones, and acids (e.g., formic acid, acetic acid, glucuronic acid, etc.). Followed by these soluble compounds being polymerized and condensed to form intermediates. Finally, carbon cores are formed through aromatization and carbonization.[51] In this proposed synthesis process of H-CDs, hydrothermal treatment is considered as the analytical model. Specifically, water ionizes produce H+ at a high temperature and pressure condition first, followed by H+ breaking glycosidic bonds and then gradually hydrolyzing broken polysaccharides into oligosaccharides (xylobiose, xylotriose, xylotetraose, etc.) and monosaccharides (glucose, xylose, etc.).[121] It is believed that in the initial reaction phase, H+ is generated from the autoionization of water, and with the participation of organic acids, the autoionization process of water is weakened, thus continuing to catalyze the hydrolysis of the remaining hemicelluloses.[122] The decomposition products in hemicelluloses resemble those in cellulose, and the subsequent dehydration, polymerization, and carbonization of small molecules follow a similar pathway as that of cellulose. However, the incomplete understanding of the formation mechanism of H-CDs remains a significant concern.
In summary, hemicelluloses are more amenable to hydrolysis into small molecules at a lower temperature (<160 °C) condition because of their unique properties such as lower molecular rigidity and multi-side chain structure. Furthermore, the degree of hemicellulose carbonization can be effectively controlled through a staged adjustment process of the reaction temperature or time, providing the opportunity for energy savings and directional synthesis of CDs. However, the hydrolyzed small-molecule fluorophores, such as water-soluble dialdehyde-based xylan, exhibit the crystalline conformation of macromolecules and robust fluorescence effects.[123] It is necessary to accurately distinguish it from H-CDs during the purification and analysis process.
2.3.3 Lignin-Based CDs
In contrast to the clear straight-chain structure of polysaccharides (such as cellulose and hemicellulose), lignin has a complex structure of cross-linked phenolic monomers that have a high carbon content (30% higher energy density than polysaccharides) and meanwhile are rich in branched oxygen-containing functional groups. Lignin is a precious resource of aromatic compounds derived from renewable sources.[68] The global annual production of lignin is ≈60–70 million tons, mainly existing as a waste by-product from the paper-pulp industry and cellulosic ethanol bio-refinery.[124] Despite numerous reported lignin extraction strategies, none have yet succeeded in producing lignin while preserving its natural undamaged structure.[125]
Various fiber sources and pulping processes can yield diverse types of lignin, such as alkali lignin, sulfate lignin, and hydrolysis lignin, distinguished by their distinctive compositions of diverse functional groups.[126] Alkali lignin is the fragment formed by the depolymerization of natural lignin using alkali treatment, and its molecular weight is in the range from 300 to 3000 Da.[126] The phenolic hydroxyl groups in lignin benzene rings can be exposed to form active sites in its ortho positions. Sulfate lignin can be produced through the alkali lignin precipitation with acidification. The reactivity of sulfate lignin is comparatively lower than alkali lignin due to the complex structures of sulfate lignin block its many reaction sites.[125] Hydrolysis lignin is the residue of bioethanol fermentation process, and its structure is very similar to natural lignin one but without S elements. However, the lower phenolic hydroxyl content in hydrolysis lignin may reduce the activity of lignin benzene, which could ultimately affect the formation of L-CDs. Therefore, in most studies, alkali lignin is commonly used as the precursor due to its high activity.[127-129] In particular, the endogenous doping effect of sulfur (S) elements in sulfate lignin structure has been found to significantly enhance the QY of L-CDs up to 47.3%.[36]
The abundant sp2-hybridized benzene ring structure in lignin renders it an optimal precursor for assembling CDs, as compared to other constituents in lignocellulosic materials.[7, 29, 130] However, the complex structure and the strong combination of some covalent bonds, namely C─C and C─O in lignin, prevent it from being depolymerized into small molecule monomers.[131, 132] Commonly, pretreatment techniques such as acid hydrolysis and ultrasonic treatment are employed to facilitate the hydrothermal treatment of lignin.[133] The as-prepared L-CDs are usually high-quality graphene single-crystals with a thickness of 1–3 atomic layers, exhibiting a hexagonal honeycomb graphene-like network. This phenomenon can be attributed to the multifunctionality of the acids, which not only act as “top-down” scissors but also function as nitrating and oxidizing agents, thereby incorporating additional sp2 hybrid domains into the carbon cores of CDs. Consistently, Zhu et al. used the mild aromatic acid such as o-amino-benzene sulfonic acid to pretreat alkali lignin to form functionalized lignin fragments in order to obtain L-CDs with excellent crystallinity.[18] From the perspective of doping, it is instructive to treat lignin using mild aromatic acid with conjugate structures, therefore, the conjugated carbon quantity of synthesized L-CDs can be increased.[134, 135]
The mechanism of acid pretreatment is consistent with classical coking formation theories.[136] The acidic system provides necessary protons for the hydrogen transfer and dehydrogenation of hydrocarbon reactants, facilitating the condensation and rearrangement of aromatic molecules during thermal treatments.[137] However, an excessively acidic system can significantly accelerate the reactions, leading to an excessive dehydration and further accumulation of aromatic clusters and eventually forming carbon nanoparticles with larger particle sizes.[136, 138] As the aggregated carbon nanoparticle exceeds the critical quantum photoluminescence dimensions, photons can be trapped in CDs and rapidly fade away, resulting in a PL intensity decay in CDs. Other than acidity, a long higher temperature treatment of hydrothermal synthesis can also lead to the excessive carbonization of lignin and loss of PL centers.[128]
Based on the above discussion and the cleavage mechanism of lignin, the schematic of hydrothermal treatment to prepare L-CDs can be illustrated in Figure 5. Initially, water is ionized into weak acid at a temperature greater than 100 °C. Under the induction of this weak acid, the cleavage of β−aryl−ether bonds (β−O−4) together with the formation of carbocations is responsible for the depolymerization reactions of lignin to form small aromatic molecules (aromatic acids, phenols, and square aromatic ethers).[6, 139] With the depolymerization of lignin, the carbocation is produced continuously as an intermediate product.[140] During the polymerization of aromatic molecules, the generated electrophilic carbocations can react with stable and electron-rich carbon atoms in aromatic phenyl rings (aldol condensation and cycloaddition) of lignin to form aromatic fragments.[141] When the concentration of aromatic masses reaches to a critical saturation point, the rupture nucleation can take place and lead to the creation of L-CDs.[93]
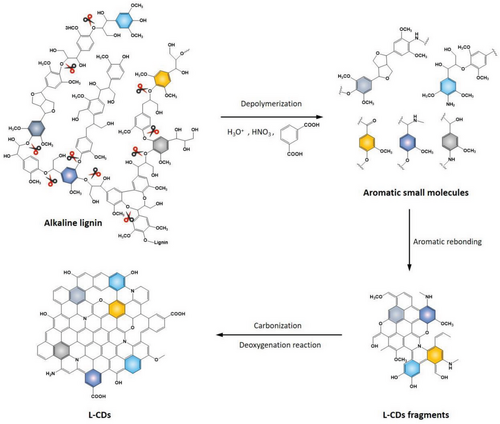
The oxidized cleavage reaction followed by the aromatic refusion of lignin molecules allows the controllable doping of heteroatoms in promoting the design and synthesis of CDs with different properties at a molecular level.[18] It is worth noting that the use of potent oxidants, namely nitric acid and sulfuric acid, is frequently employed in the synthesis of LC-CDs, which runs counter to the overarching principles of green synthesis. The exploration of eco-friendly pathways for the dissociation of lignocellulose, as well as the development of highly efficient pyrolysis techniques for its constituents, holds paramount importance in the deliberate synthesis of LC-CDs. Furthermore, the creation of LC-CDs may entail intricate processes of decomposition and polymerization, involving a multitude of natural components inherent in lignocellulosic materials. It is not rigorous to directly apply the synthesis mechanisms of C-CDs, H-CDs, and L-CDs to explain the formation path of LC-CDs without considering the synergistic or antagonism among the decomposition products.[33] In our opinion, based on different decomposition conditions of the different components in lignocellulosic materials, the synthetic process conditions such as degradation temperature and reaction time, can be adjusted in different stages. Thus, by analyzing the decomposition products acquired at different stages, it is plausible to delineate the prospective formation mechanism of LC-CDs based on the temporal dimension.
3 Structures and Properties
3.1 Structures of LC-CDs
Current research findings indicate that the structure and composition of a precursor play a decisive role in affecting the structure, chemical composition, and property of LC-CDs synthesized. [42, 51, 71, 138, 142] Cellulose and hemicellulose are both natural macromolecular polymers, sharing a common basic building block of glycans compounds. Therefore, C-CDs and H-CDs are similar in their structures and chemical compositions. Particularly, the highly stable benzene ring structure of lignin can be inherited by L-CDs, explaining the structural difference of L-CDs if compared with C-CDs and H-CDs.
Instruments such as Fourier infrared spectrometer (FTIR), X-ray Photoelectron Spectrometer (XPS) and Nuclear Magnetic Resonance (NMR) are typical means used to characterize the chemical structure of CDs,[21, 75] confirming the existence of C and O elements in LC-CDs structures. The existing forms of C elements are sp2 hybridized and sp3 carbons. The sp2 carbon is mainly realized by C═C, C═O, and other ways, while the sp3 hybridization mainly includes C─C, C─O─C, and C─OH.[143] The degree of enrichment of oxygen-containing functional groups in CDs varies with raw materials and synthesis conditions, while functional groups are mainly growing at the edge of CDs.[75] Most reports indicate that C-CDs, H-CDs, and L-CDs have abundant oxygen-containing functional groups inherited from their precursors or precursor cleavage products. Studies have shown that LC-CDs synthesized by hydrothermal treatments have a good water solubility. Chemically, the carbon core of LC-CDs typically manifests as alkyl and graphitized structures, which are hydrophobic in nature, whereas the surface of LC-CDs is characterized by the presence of hydrophilic oxygen-containing functional groups such as ─OH and ─COOH.[144] Strictly defined, the aqueous solution of CDs is a stable and dispersed hydrophilic colloid.
As is the case with the regulatory approach adopted by most carbon dots (CDs), doping strategies are commonly employed to introduce supplementary defect states and surface functional groups onto luminescent carbon dots (LC-CDs). This enables them to possess distinct and desirable properties.[145, 146] Heteroatom doping techniques, such as the utilization of urea, amine molecules, and nitric acid in conjunction with N elements,[38, 69, 145, 147, 148] sulfuric acid, or sulfates, are employed to introduce S atoms into luminescent carbon dots (LC-CDs).[67, 92, 138] This doping strategy also facilitates the integration of metal elements (such as magnesium, Mg; aluminum, Al; and copper, Cu) into the LC-CD structure through the introduction of alkaline solutions. Other studies have reported similar findings, highlighting the effectiveness of metal element doping in modifying the properties of LC-CDs.[98, 145, 149] Among these doping methods, the N-element doping strategy is particularly favored due to the diverse forms of N-element doping observed in CDs. N-element doping can occur in various configurations, including sp2-hybridized C═N─C, porphyrin C─N─C, as well as amino N and quaternary N groups, which exhibit distinct hybridization behaviors with carbon (C) atoms. This versatility in N-element doping forms contributes to the unique properties and functionalities of N-doped CDs.[28, 150] The presence of diverse surface states of nitrogen in CDs plays a crucial role in controlling their optical properties. On the other hand, the doping of other elements in CDs primarily occurs on the surface in the form of functional groups, such as C─P, C─S, ─C─SOX−, S═O, and C─C═S. These functional groups contribute to the modification of the surface chemistry and properties of CDs, thereby expanding their range of applications.[92, 117] During the doping of metal elements, metal atoms primarily interact with carbon or oxygen atoms through metal binding sites, resulting in the formation of coordinate bonds such as Mg─C and Mg─O. This process provides a new pathway for electron transfer within the structure of the CDs. The presence of metal atoms and the formation of coordinate bonds enable unique electron transfer processes and can significantly influence the optical and electronic properties of the doped CDs.[98] The polyaromatic structure and endogenous functional groups of lignin are more complex than glycan, therefore the formed L-CDs using lignin usually have an excellent graphite-like network, implying that the sp2 hybrid C═N─C and porphyrin C─N─C structures are more complete.
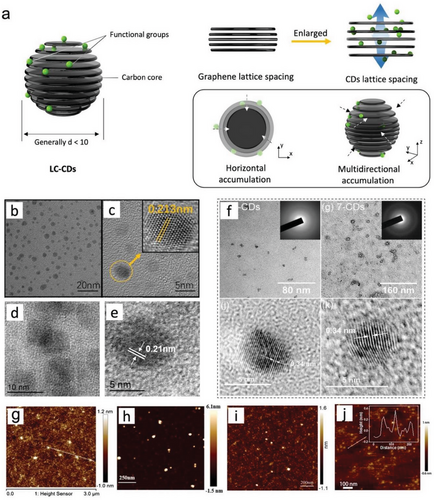
As discussed in Section 2, CDs with different morphologies such as GQDs, CQDs, and CPDs can be obtained by using different precursors and synthesis processes.[71] They are similar in chemical compositions and functional groups, and it is difficult to effectively distinguish them from their compositions and elements. Therefore, characterization instruments such as transmission electron microscopy (TEM) and atomic force microscopy (AFM) are commonly used to analyze physical morphologies and crystalline structures of LC-CDs (Figure 6).[29, 151, 152] Most reports indicated that the average particle size of LC-CDs synthesized from lignocellulosic materials is less than 10 nm.[4, 7] The crystal lattice stripes with different spacings observed under the high-resolution TEM (HR-TEM) are mainly in the ranges of 0.21 to 0.25 nm and 0.31 to 0.34 nm that are close to the (100) and (002) crystal plane spacing of graphite, respectively.[22, 88, 91, 129] The average particle sizes and lattice spacings of currently reported LC-CDs are summarized in Table 2.
| Raw material | Synthesis method | Average particle size [nm] | Lattice spacing [nm] | Reference |
|---|---|---|---|---|
| Cellulose acetate butyrate | Laser ablation | 2.07 | 0.26 | [91] |
| MCC | Microwave irradiation | 2.00–6.00 | 0.24 | [38] |
| Peanut shell | 2.00–6.00 | 0.31 | ||
| Cotton stalk | 2.00–6.00 | 0.22 | ||
| Cassava stem | Hydrothermal | 3.58 | 0.21 | [37] |
| Sorghum stalk | 3.37 | |||
| Sugarcane bagasse | 2.79 | |||
| Rubber wood | 3.31 | |||
| Poplar | 2.42 | |||
| Chinese fir | 2.82 | |||
| Mannose | Low-temperature carbonization | 3.57 | 0.34 | [119] |
| d-(+)-Xylose | Microwave irradiation | 6.80–16.37 | 0.34 | [22] |
| Xylan | Hydrothermal | 3.20 | 0.24 | [118] |
| MCC | Hydrothermal | 7.83 | ∖ | [153] |
| Lignocellulose | Hydrothermal | <5.00 | 0.21 and 0.24 | [98] |
| Xylose | Hydrothermal | 1.69 | ∖ | [117] |
| Cellulose | Hydrothermal | 2.54 | 0.21 | [51] |
| Xylan | 2.88 | 0.20 | ||
| Corn cobs | 2.14 | 0.21 | ||
| Lignin | 2.26 | 0.21 | ||
| Lignin | Hydrothermal | 4.68 | 0.23 | [154] |
| Waste corn stalks | Hydrothermal | 3.00–6.00 | 0.328 | [95] |
| Lignin | Hydrothermal after acid Treatment | 2.88–5.05 | 0.32 | [21] |
| Lignin | Hydrothermal after acid Treatment | 8.30 | 0.21 | [128] |
The variation in lattice spacing observed in Table 2 can be attributed to the occurrence of lattice defects in carbon nanostructures, which can be caused by chemical oxidation and exfoliation.[67] The large layer spacing, such as 0.34 nm, could be caused by oxygen-containing groups exiting on the surfaces of LC-CDs that can increase the distance between interlayers.[67] The graphite-like crystal structure is also reflected in the XRD pattern of LC-CDs showing a broad diffraction peak. The poor crystallinity observed in LC-CDs can be attributed to the significant steric hindrance of mono-benzene rings within the amorphous structure of precursors such as lignin. This hindrance weakens the interaction forces between the mono-benzene units, making their aggregation on a large scale unlikely.[88, 90, 155] Furthermore, the low crystallinity of LC-CDs can also be confirmed by analyzing their Raman spectra and calculating the high relative intensity (ID/IG) of the “disordered” D-bands and crystalline G-bands. The Raman analyses of LC-CDs revealed that they comprise both crystalline carbon and organic polymers carbon.[143] Furthermore, microscopic morphology and carbonization are also related to reaction conditions such as time, temperature, doping method, etc. The sufficient reaction time or appropriate atom doping can facilitate the conversion of polysaccharide precursors with low aromatization into LC-CDs with high graphitic crystallinity.[21] According to the formation mechanism of C-CDs, H-CDs, and L-CDs,[90] small molecules aggregating in different dimensions can form different structures. Figure 6a shows that when horizontal accumulation is more prevalent, small molecules tend to create flake GQDs, i.e., if small molecules are aggregating in all directions without any orientation, spherical CDs will be formed.
Although the lattice of CDs is not typically viewed as a critical factor affecting their properties, the integrity of the lattice is directly linked to the size of the carbon core in CDs and should therefore be regarded as an indicator for assessing the properties of CDs.[75] It has been recommended that CDs with highly graphitized carbon cores could exhibit highly stable PL properties, such as pH stability.[128] The rationale behind this observation is that an increased degree of graphitization in CDs can enhance their π electron conjugation, thereby suppressing the protonation or deprotonation processes of CDs in acidic environments. In addition, the more extensive πelectron system or the higher graphitic nitrogen content in CDs will cause a narrower energy gap in CDs, therefore shifting PL emission of CDs to the red region.[143, 158]
In the summary of the discussion on the formation mechanism of LC-CDs, the following observations can be drawn. The precursor and dopant material types will determine the elements’ composition and the types of functional groups on synthesized LC-CDs, while the degradation conditions will affect the content and structure of each functional group on LC-CDs. L-CDs often exhibit a regular graphitized lattice structure considered to be GQD or CQD because of their high degree of aromatization. The aromatization of C-CDs and H-CDs based on glycan compounds shows a “time lag”. Different degradation products of glycan compounds play different roles in the formation of LC-CDs. LC-CDs with high graphitization can also be synthesized under a sufficient aromatization time. Therefore, it can be reasonably inferred that during the process of growing LC-CDs, the highly aromatic small molecules degraded from lignin may first form carbon cores, providing suitable growth sites for small molecules degraded from glycan compounds and lignin molecular fragments. With the prolongation of reaction time, carbon cores can gradually grow into LC-CDs.[159] In another words, under different thermal treatment conditions, such as temperature and time, each of components of lignocellulosic materials can be degraded into different small molecules. Therefore, through setting different thermal treatment conditions, molecules products at different degradation stages can be analyzed.[160] This is helpful for understanding the changes in chemical components of each lignocellulosic material and the formation process of CDs’ carbon core lattice structures. Comprehending the physical architecture and chemical constitution of LC-CDs constitutes a preliminary stage in scrutinizing their properties. Owing to their singular chemical structures, LC-CDs have the potential to exhibit an assortment of physical and chemical traits.
3.2 PL Properties of LC-CDs
LC-CDs possess a distinct configuration, constituted by carbon cores and organic molecules, with the organic surface functional groups that are covalently linked to the carbon cores. This particular composition bestows upon LC-CDs an assortment of singular fluorescence and semiconductive properties.[5, 161, 162] The PL characteristics of LC-CDs such as tunable fluorescence emission properties, excitation wavelength-dependent emission, and fluorescence quenching phenomenon are summarized in the following sections. In addition, some non-universal features of LC-CDs such as up-conversion luminescence, room temperature phosphorescence are also reviewed.[128, 163]
3.2.1 Tunable Fluorescence Emission of LC-CDs
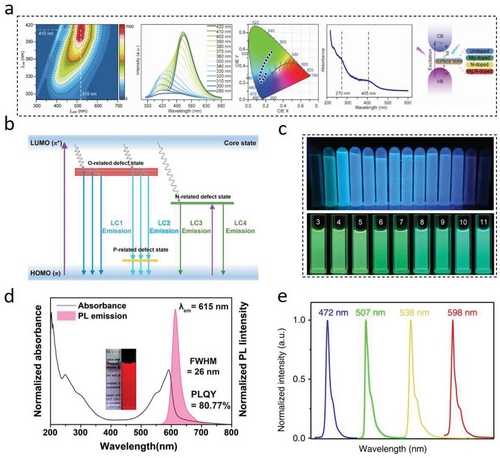
Different CDs can have suitable emission centers exhibiting tunable fluorescence emissions in different wavelength bands.[98] In daylight, CDs with different sizes or surface functional groups can have different apparent colors in aqueous solutions. Under the same excitation light irradiation, different CDs will exhibit different fluorescence emissions that cover different wavelength bands from the ultraviolet region (<395 nm) to the red emission (>650 nm; Figure 7a). The tunable fluorescence emission of LC-CDs is one of the important properties in evaluating their applications for multi-color optoelectronic devices, especially for white light-emitting diodes (LEDs). By changing the type of lignocellulose precursors, dopants, synthesis processes, etc., the fluorescence emissions of LC-CDs with different wavelengths can be achieved.[22, 119, 163] Our recent work reported that a series of L-CDs with broad spectral emissions could be synthesized by controlling their formation conditions and dopant types (Figure 7b).[128] The optimal emission wavelength of three synthesized N-doped L-CDs were located at 395 nm, 515 nm, and 602 nm that were corresponding to each of the standardized blue, green, and orange fluorescence regions, respectively.
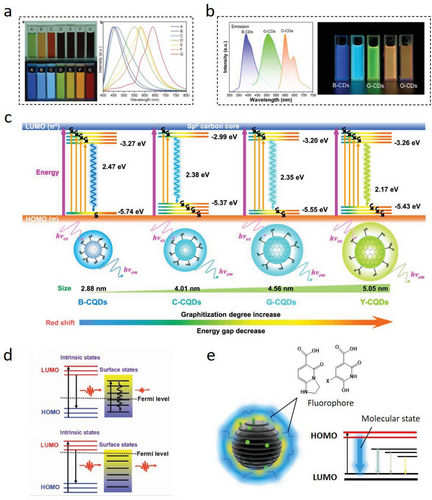
The fluorescence mechanism of CDs’ tunable emission is another topic worthy of further investigation.[164] Most research activities have been devoted to the explanation of the multi-color PL mechanism of CDs.[7, 161, 165] The PL mechanisms of the majority CDs can be classified into three aspects: quantum size effect, surface state, and molecular state.[158, 166] Among these aspects, the quantum size effect is dominated by CDs’ particle sizes. The structural analyses indicate that CDs consist primarily of carbon cores that contain densely conjugated π electron pairs.[69, 150, 161] Figure 7c illustrates that the PL spectrum can be described as a process of transitioning electrons from the lowest unoccupied molecular orbitals (LUMO) to the highest occupied molecular ones (HOMO).[167] The energy gap between two molecular orbitals depends on the particle sizes of CDs, since the energy gap decreases gradually as the size increases, resulting in different excitation and emission levels.[21, 29, 161] With the emission red-shift, the fluorescence intensity and QY of CDs will also decrease. At the carbon cores’ growth stage, the adjustment of controllable CDs’ core sizes involves the expansion of additional factors such as increased surface defects and heteroatom-doped graphite.[128, 143, 158] However, the carbon core structure is not always a graphite one, and as a result, the quantum confinement effect cannot be rigorously applied in most cases.[33] Currently, there is a lack of a controllable process for synthesizing CDs with only experimentally determinable changes in core size. Therefore, the theoretical size-dependent emission of CDs has not been unambiguously demonstrated.
The surface state of CDs is intimately related to their carbon cores, which is primarily due to the cooperative hybridization between the graphitic carbon backbone and the attached functional groups (or sub-fluorophores).[38, 143, 158, 168-170] Specifically, surface defects generated by surface oxidation act as trapping centers for excitons, giving rise to surface state-dependent fluorescence.[171] The rich electrons could occupy the energy levels of CDs’ surface states and lift CDs’ Fermi level (Figure 7b), leading to smaller self-absorption and output long emission enhancement.[158] In addition, an increase in surface oxidation or a higher type of π-electron structures caused by doping strategy can produce more surface defects and a narrower energy gap of the π–π* transitions, shifting the PL emission to the red.[87, 98, 172] Using acids as oxidizing agents proved to be an effective surface oxidation strategy.[21, 143] A higher concentration of HNO3 may lead to a higher degree of CDs’ surface oxidation, further facilitating the production of CDs with long emission wavelengths.[143] The aromatic acid can not only oxidate CDs’ surfaces, but also directly participate in the formation of carbon cores, causing a significant change in carbon core sizes. In this reaction, the energy gap reduction caused by the increase of CDs graphitization degree can lead to a more synchronous and significant redshift of synthesized CDs’ emission.[21] However, the surface oxidation effect on LC-CDs produced through acid treatment is always accompanied with the doping of heterogeneous elements (such as N and S). Therefore, it is necessary to consider this fluorescence emission introduced by the complicated synergistic effect caused by different surface states.
Different from the surface state theory, the molecular fluorescence theory can be explained by the fact that the strong fluorescence is mainly caused by the formation of organic fluorophores (Figure 7e).[173] The molecular fluorescence theory is particularly relevant for CDs that have a high degree of surface functionalization or that have undergone significant chemical modification, as these CDs are more likely to have a large number of organic fluorophores on their surface. These organic fluorophores are mostly five- or six-membered fused-ring compounds formed by the dehydration and decomposition of precursors at low temperatures. Therefore, the molecular state of synthesizing LC-CDs is related to the “bottom-up” strategy.[36, 51, 71, 162] The behavior of these fluorophores is not well-defined in the reaction system and they can exist in a free state or attach to the surface or even inside of carbon backbones, resulting in different surface states and carbon core structures.[174] With the temperature increase, the fluorophore can serve as a building block of carbon domains and directly contribute to the formation of carbon cores with a decrease in fluorescence intensity and an improvement in stability.[36, 51] Obviously, such strong organic fluorophores can interfere with the interpretation of the CDs’ fluorescence mechanism.[175] Therefore, effective filtration of organic fluorophores is critical before analyzing and characterizing CDs’ fluorescence properties. The purification methods for LC-CDs typically involve centrifugation, dialysis, column chromatography,[7, 10, 51, 176] etc. From the perspective of synthesizing LC-CDs, the precursors with relatively simple molecular structures and sufficient carbonation durations will benefit to reduce the influence of molecular states on LC-CDs’ fluorescence properties. Indeed, the complexity of lignocellulosic materials makes it impossible to disregard the molecular state during the synthesis process of LC-CDs. This is a common issue encountered in all CDs synthesis methods that utilize biomass as the starting material.
In addition to the three mainstream mechanisms discussed above, other different mechanisms including different factors have also been proposed to explain LC-CDs’ tunable fluorescence characteristics such as adjusting the concentration of LC-CDs in a solution. In this situation, different internal filtering effects can change the PL emission. Other factors such as pH and solvent polarity solvent,[163, 177] surface ligand modulation,[178] cross-linking,[179] and even aggregation [180] can also have significant influences on CDs’ fluorescence properties.[93]
Nevertheless, the tunable fluorescence mechanism of LC-CDs remains a matter of debate as it is challenging to explain their fluorescence behavior using a single mechanism, given the complexity of these materials and the various factors that can affect their properties. While there are some ideal models and experimental methods that can be used to support different mechanisms, it is still not clear which mechanism is dominant and how different factors interact to contribute to the tunable fluorescence characteristics of LC-CDs.[28, 71, 75] We believe that the molecule state, carbon core state, and surface state of LC-CDs are responsible for the overwhelming majority of their PL phenomena. Especially, using cellulose, hemicellulose, and lignin or their mixtures to synthesize LC-CDs can have a more complicated effect on the fluorescence properties of synthesized LC-CDs because of the macromolecular state and complex composition of these precursors. Special attention should be given to the fluorescence phenomenon observed in L-CDs resulting from the presence of a conjugated structure within lignin. Although initial differentiation between L-CDs and lignin can be achieved based on fluorescence properties (such as emission wavelength and fluorescence stability), the complex composition of lignin's fluorescence requires a more comprehensive approach. Due to the intricate composition of lignin's fluorescence, analyzing the respective structures and morphologies of L-CDs and lignin serves as an effective method for distinguishing between the two entities.
3.2.2 Excitation-Dependent PL Emission of LC-CDs
Based on the above discussion about CDs’ tunable PL mechanisms, the defects generated in LC-CDs by oxidation or nitrification can act as exciton capture centers that will form new band gaps in LC-CDs and generate surface fluorescence.[148] Nevertheless, the surface state-dependent fluorescence does not dominate luminescence as an eigenstate, but red-shifts with increasing excitation wavelength. It is generally believed that the emission at a short wavelength is mainly caused by the π–π* transition in conjugated cores that have wider band gaps.[98] Figure 8a shows that with the increased excitation wavelength of LC-CDs, the emission wavelength gradually red-shifts, indicating a certain degree of strengthening. The emission at a long wavelength can be attributed to CDs’ surface defect states.[39, 63] The fluorescence excitation dependence property of LC-CDs is also reflected in their absorption spectrum with multiple absorptions, and CDs’ corresponding emissions are always present at different positions in their absorption spectrum.[42, 145] However, several studies have indicated that the CDs’ excitation-independent emission can be characterized by their uniform sizes.[7, 128] This excitation-free emission mechanism indicates that the CDs’ fluorescence behavior caused by their size-dependent inter-band transitions exceeds their corresponding surface state fluorescence.
Typically, CDs exhibit strong absorption peaks between 200 to 400 nm in their absorption spectrum, even accompanied by a tail extension into the visible light range.[128, 143] The absorption peaks at lower wavelengths (usually 200 to 250 nm) are assigned to the π–π* (C═C─C═O/C═C) transition.[22, 181] The energy required for the π–π* transition caused by the high-integrity π-conjugated carbon will fluctuate within a certain range because of the change in energy levels caused by heteroatom doping.[18, 163] The absorption peaks at intermediate wavelengths (300–400 nm) are generally attributed to the n–π* transition of C═O/C═N.[87] The broad absorption peaks at longer wavelengths (>580 nm) are often observed in CDs with red or NIR emissions that could be caused by a π-electron conjugation between the O/N groups and sp2 domains.[29, 181, 182]
The rich surface functional groups and diversified atomic doping contribute to the unique excitation dependence of CDs (Figure 8b).[33, 38, 162] We found out that the optimal excitation wavelengths of most LC-CDs (including using cellulose, hemicellulose, and lignin as a single precursor, respectively) were below 600 nm with a wider full width at half maximum (FWHM), and fluorescence emissions of most LC-CDs were blue-green (Figure 8c).[18, 35, 91, 154] The short-wavelength emission states of LC-CDs imply their weak fluorescence penetration and single-color production. Meanwhile, the wider FWHM of LC-CDs indicates a low level of color purity that indicates it is insufficient for using LC-CDs in such applications as optoelectronic devices and living biological imaging. Some researchers (Figure 8d,e) have been devoted their research efforts into the narrow bandwidth and long emissions of CDs.[7, 130] Up to date, only L-CDs synthesized using lignin with a highly aromatic structure can achieve such long and narrow emission, while the wider wavelength emission of glycan CDs still needs further breakthroughs. We believe that suitable aromatization precursors, precise atomic doping, and efficient purification play critical roles in synthesizing narrow emissive LC-CDs.
3.2.3 Fluorescence Quenching Phenomenon of LC-CDs
The fluorescence quenching phenomenon of CDs can be described as the CDs’ fluorescence intensity significantly disappears or decreases under a self-stacking or external interference condition in which the aggregation-caused quenching (ACQ), electron transfer quenching, and Förster energy resonance quenching are mainly involved.[18, 118, 138, 183] The ACQ of LC-CDs always can be observed when LC-CDs are in a solid aggregation state. As the presence of a large number of π electrons in LC-CDs, LC-CDs usually suffer from self-quenching in a solid aggregation state mainly because of their severe π–π interactions or excessive energy transferring. Specifically, when the distance (D) between two adjacent CDs is small enough, i.e., D < R0, where R0 is the Förster distance, the spontaneous Förster resonance energy transferred between two CDs will be induced.[142] Overcoming CDs’ ACQ is a prerequisite for a possibility of using these CDs in optical devices and functional materials. An effective solution of introducing some steric hindrances such as aqueous solutions, and nano-carriers,[89, 129, 184] polymer-carriers and surface coating,[29, 98] etc. among CDs’ particles can encourage a comfortable interval among CDs. The surface functionalization of CDs can reduce their self-aggregation behavior caused by electrostatic adsorption, and the nano-carrier can also be used to keep CDs maintaining an appropriate distance through the physical or chemical adsorption (Figure 9a,b).[185] Jin et al. utilized eco-friendly cellulose derivatives with different charges to surround CDs, forming a quasi-core shell structure and effectively inhibiting the CDs’ ACQ.[183] Similar processing strategies, such as a delignified wood with hierarchical pore structure and abundant free hydroxyl groups being used as a carrier to load L-CDs in order to realize the fluorescent wood, were also reported in our recent work.[129]
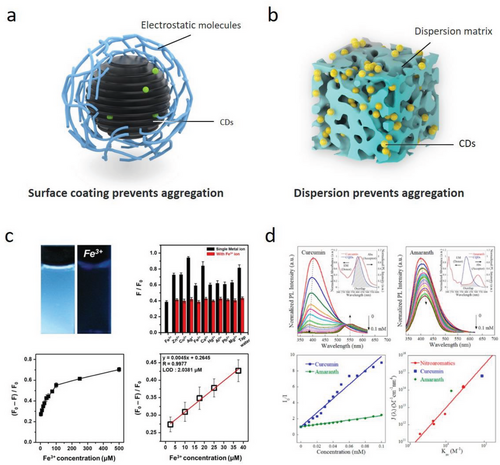
Electron transfer quenching involves the transfer of an electron from the excited state of the CDs to a quencher molecule, which then deactivates the CDs. The electron transfer quenching mechanism is commonly applied to explain the CDs’ fluorescence quenching phenomenon caused by metal ions. Metal ions are believed to form efficient chelation or coordination among CDs, thereby increasing nonradiative transitions and fluorescence quenching.[23, 37, 74, 187] Theoretically, the main driving force for CDs’ electron transferring is the energy gap between the LUMO of donors and acceptors, and the CDs’ fluorescence quenching is more likely to occur with the stronger electron absorption ability of acceptors.[147, 156] In Fe3+-induced fluorescence quenching (Figure 9c), the part of electrons or charges in excited LC-CDs is induced to pass on the unsaturated Fe3+ 3d electron orbitals, leading to an ineffective nonradiative transition that causes the fluorescence quenching. After that, the chelation between LC-CDs and Fe3+ can be reduced through introducing a reducing agent because of the reduced Fe3+ valence, and the fluorescence intensity of LC-CDs will gradually recover.[188-190] However, it is insufficient to explain some LC-CDs’ quenching phenomena using the electron transfer mechanism alone. The quenching mechanism of Förster resonance energy transfer (FRET) is a reasonable explanation for CDs’ quenching caused by such organic molecules (Figure 9d).[187, 191] FRET is a non-radiative energy transfer process between two chromophores, where the excited state energy of the donor chromophore is transferred to the acceptor chromophore through dipole-dipole interactions. This mechanism has been approved to be responsible in the QDs’ fluorescence quenching by nitroaromatics.[186] The trigger of FRET quenching is in fact that the energy emission spectrum of donor fluorophores (LC-CDs) must overlap with the energy absorption spectrum of acceptors (quenching agent), and the efficiency of energy transferring depends on the overlap degree between LC-CDs spectrums and quenching agents.[138] In summary, different fluorescence quenching mechanisms can explain that LC-CDs can be used as a fluorescence switch to achieve the targeted detection of particular ions, organic molecules, and drugs precisely.
3.2.4 Up-Conversion Luminescence of LC-CDs
The up-conversion luminescence (UCL) observed in CDs is commonly attributed to anti-Stokes transitions. The ground-state multiplicity of the carbene in CDs provides energy levels in both π and σ orbitals. When CDs' π orbitals are excited by low-energy photons, they can transition to higher energy states such as the LUMO. Subsequently, they transition back to lower energy states (σ orbitals), resulting in the emission of UCL by the CDs.[130, 192] Put simply, the UCL process converts the long-wavelength emission of CDs into a shorter-wavelength one. Ding et al. reported that L-CDs possessed not only their common PL emission but also exhibited their distinct up-conversion emission (located at ≈410 nm) and excitation-dependent up-conversion PL emission behavior at the excitation wavelength range from 500 to 800 nm.[42] Although the UCL process is unusual in LC-CDs, it offers new opportunities for LC-CDs used in the solar energy harvesting, cell imaging with two-photons luminescence microscopy, and efficient catalysts design.
3.2.5 Quantum Yield of LC-CDs
The CDs’ QY is defined as the ratio of the number of molecules generated or consumed by the reaction system under the excitation of monochromatic light to the number of absorbed photons, reflecting the utilization efficiency of photons by CDs.[193] The QY is one of the key indicators used in evaluating LC-CDs’ optical properties. There are two methods for the measurement of CDs’ QY: absolute QY (a-QY) and relative QY (r-QY).[128] The absolute QY is measured and calculated based on the fluorescence spectrometer attached to an integrating sphere, while the relative QY can be determined using substances with their QY known such as quinine sulfate.[194] Because the QY of the same LC-CDs determined by two different test methods can be quite different, and also currently reported test methods in various studies are also different, it is not a good approach to compare the QY values of LC-CDs reported in different studies. Presently, employing the strategy of heteroatom doping (N, S) to increase the pathway of LC-CDs’ surface state excitons back to the ground state is considered as an effective method to enhance the LC-CDs’ QY property.[145] The QY of some reported LC-CDs are listed in Table 3. In addition, we believe that two methods, which can limit LC-CDs’ particle sizes for obtaining a higher specific surface area and more excitons for LC-CDs as well as explore a method that is capable of directional separation and purification of LC-CDs, can have significant effects on the improvement of LC-CDs’ QY properties.
| Raw material | Dopant | Ex/Em [nm] | a-QY [%] | r-QY [%] | Reference |
|---|---|---|---|---|---|
| Carboxymethylcellulose | Linear-structured polyethyleneimine | 350/465.5 | / | 44 | [104] |
| Mannose | Ammonium citrate | 365/450 | / | 9.8 | [119] |
| MCC | NaOH | 360/436 | / | 21.4 | [88] |
| d-(+)-Xylose | m-phenylenediamine | 440/518 | / | 73.6 | [22] |
| Xylan | NaOH and urea | 350/434 | / | 23.8 | [118] |
| Lignocellulose | Ethylenediamine and magnesium hydroxide | 410/518 | 2.78 | / | [98] |
| Lignin | p-phenylenediamine | 500/605 | 4.62 | / | [163] |
| Hemicellulose | Nitrogen-rich deep eutectic solvent | 360/436 | / | 23.45 | [63] |
| MCC | Ethylenediamine solution | 360/436 | / | 51 | [199] |
| Mannose | Ammonium citrate | 365/450 | / | 8.8 | [200] |
| Xylan | Branched polyethyleneimine | 340/463 | / | 8.0 | [73] |
| MCC | Ethylenediamine | 365/461 | / | 10.1 | [93] |
| Xylan | NH4OH | 320/403 | 16.18 | [146] | |
| Cellulose | / | 360/435 | / | 1.05 | [51] |
| Xylan | / | 360/435 | / | 0.96 | |
| Lignin | / | 360/470 | / | 1.25 | |
| Lignin | Urea | 350/395 | 41.41 | / | [128] |
| Lignin | 2,4-diaminobenzene sulfonic acid | 360/450 | / | 30.5 | [21] |
| Lignosulfonate lignin | NaBH4 | 440/475 | 47.3 | [36] | |
| Xylan | NH4OH | 360/443 | / | 3.3 | [172] |
| Polyethyleneimine | 380/466 | 7.9 | |||
| Polyethyleneimine and acetic acid | 320/466 | 13.7 | |||
| NH4OH and polyethyleneimine | 370/466 | 5.4 | |||
| Alkali lignin | Citric acid | 377/454 | / | 43 | [127] |
| Ethanol-water pulping lignin | Ethylenediamine | 320/424 | / | 16.9 | [145] |
| Mg(OH)2 and ethylenediamine | 405/510 | 46.38 |
In addition, other PL properties of CDs such as room-temperature phosphorescence (RTP) [182, 195, 196] and two-or multi-photon excitation,[130, 197] electrochemical luminescence (ECL) and ultralong phosphorescence,[7, 198] have also been explored and revealed in some works. These properties are not common for LC-CDs and need to be studied in more depth.
4 Functional Applications
The continuous deepening of our understanding of various properties of LC-CDs, such as their strong photoluminescence, good photostability, excellent water solubility, and low toxicity, has made them promising candidates for potential applications in many fields, including sensors, information encryption, bioimaging, optical devices, and photocatalysis (Figure 10).[201, 202] The potential applications of various LC-CDs are reviewed and summarized in the following sections.
4.1 LC-CDs in Optical Applications
4.1.1 LC-CDs in Sensors
The continuous development of modern industries has led to the accumulation of metal ions in the living environment, posing a potential threat to public health.[92, 97, 203] Therefore, it is necessary to monitor metal ions in biological systems. The unique LC-CDs’ fluorescence quenching properties in responding to metal ions and organic molecules enable LC-CDs to be used as fluorescence sensors in detecting various pollutants at a low cost. LC-CDs have been extensively studied for their fluorescence detection properties due to their fast response times, high sensitivity, and simplicity of operation. These properties make them attractive candidates for a wide range of applications, including environmental monitoring, biomedical research, and chemical analysis.[19] Importantly, most recent research reports have indicated that the LC-CDs’ fluorescence intensity quenching in aqueous solution states has a strong linear relationship with different concentrations of metal ions, indicating the great potential of using LC-CDs in quantitatively detecting harmful metal ions in the environment. In addition, LC-CDs derived from different precursors and synthesis processes are capable of being equipped with selective identification and directional detection of different metal ions, such as Cu2+,[203, 204] Cr3+, Cr(VI), [20, 118] Hg2+,[23, 24] Ag+,[63] and Fe3+.[96, 188] Table 4 summarizes the reported LC-CDs’ fluorescence response properties to various metal ions in the detection of these metal ions. It can be found that LC-CDs have a strong recognition and fluorescence responsiveness to Fe3+ (Figure 11a).[92, 147]
| Raw material type | Types of CDs | Synthesis method | Target ion | Limit of detection | Ex/Em [nm] | Reference |
|---|---|---|---|---|---|---|
| Lignocellulosic Biomass | LC-CDs | Hydrothermal | Fe3+ | 0.91 µM | 360/440 | [37] |
| Alkali lignin | L-CDs | Hydrothermal | Fe3+ | 0.66 µM | 340/408 | [203] |
| Xylan | H-CDs | Hydrothermal | Cr6+ | 0.10 µM | 360/434 | [118] |
| Linter cellulose | C-CDs | Hydrothermal | Hg2+ | 0.2 µM | 370/450 | [23] |
| Cotton fibers | C-CDs | Hydrothermal | Hg2+ | 3 nM | 360/470 | [24] |
| Cellulose-based biowaste | C-CDs | Combustion | Fe3+ | 0.03 µM | 310/370 | [107] |
| Hemicelluloses | H-CDs | Solvothermal | Ag+ | 21 nM | 360/436 | [63] |
| Durian shell waste | C-CDs | Hydrothermal | Mn (VII) | 46.8 nM | 345/415 | [205] |
| MCC | C-CDs | Hydrothermal | Cr6+ | 17 nM | 397/455 | [190] |
| Pine | LC-CDs | Hydrothermal | Fe3+ | 0.35 µM | 330/447 | [19] |
| Alkali lignin | L-CDs | Hydrothermal | Fe3+ | 0.44 µM | 300/400 | [147] |
| Glucosamine | H-CDs | Hydrothermal | Fe3+ | 1.42 µM | 350/442 | [206] |
| Flax straw | LC-CDs | Hydrothermal | Co2+ | 0.38 µM | 360/449 | [207] |
| Cr6+ | 0.19 µM | |||||
| Cellulose | C-CDs | Hydrothermal | Hg+ | 7.53 nM | 230/420 | [39] |
| Xylose | H-CDs | 8.33 nM | ||||
| Lignin | L-CDs | 6.82 nM | ||||
| Alkali lignin | L-CDs | Hydrothermal | Fe3+ | 1.49 µM | 370/453 | [189] |
| Alkali lignin | L-CDs | Hydrothermal | Cr6+ | 0.054 µM | 300/346 | [140] |
| 0.049 µM | 330/428 | |||||
| 0.077 µM | 490/514 | |||||
| Linter Cellulose | C-CDs | Hydrothermal | Hg+ | 0.2 µM | 370/450 | [23] |
| Microcrystalline cellulose | C-CDs | Hydrothermal | Fe3+ | 0.21 nM | 360/436 | [199] |
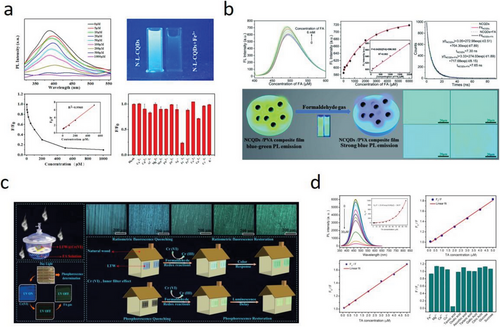
LC-CDs can also be used as a sensor to detect organic molecules such as Sudan I,[138] tannic acid, tetracycline,[208] paracetamol,[209] l-cysteine,[63] etc. Yang et al. used xylan and branched polyethyleneimine as precursors to synthesize aminated H-CDs that can be employed as a novel fluorescent probe for the sensitive and selective detection of tannic acid (TA). It was concluded that the fluorescence quenching of H-CDs induced by TA was caused by the ineffective transfer of electrons between the two (Figure 11d).[73] Some innovative sensor applications such as detecting formaldehyde (FA) (Figure 11b and c) were also proposed.[105, 154] Wang et al. synthesized N-doped L-CDs with a good fluorescence response to FA gas.[105] The study utilized the fast reaction between the surface functional groups of L-CDs and FA to produce Schiff bases, which altered the surface defects of CDs and led to a blue shift in the fluorescence emission. In addition, this work realized the real-time detection of gaseous substances using CDs in a wood porous material, providing a new application idea for CDs used as solid-state sensors.
It is anticipated that in the future study, different fluorescence sensors can be designed based on different LC-CDs’ fluorescence behaviors. Furthermore, the irreversible fluorescence quenching behavior of most LC-CDs limits their usages only in disposable products as single-use detection, and not suitable for low-cost application. Therefore, exploring the quenched fluorescence recovery method and developing a portable composite fluorescence sensor can be the next challenging research topics related to LC-CDs. For example, LC-CDs can be combined with stimuli-responsive polymers, which can change their conformation or properties in response to external stimuli such as temperature, pH, or specific molecules. This can lead to a reversible quenching or recovery of the LC-CDs' fluorescence, enabling their repeated use in sensing applications. Additionally, the use of protective coatings or encapsulation can also help to protect the LC-CDs from irreversible quenching, improving their stability and reusability.
4.1.2 LC-CDs in Information Encryption
Information encryption can effectively protect valuable data from being forged. The fluorescent patterning is one of the common forms of photoluminescent materials used in anti-counterfeiting applications.[201] Compared to traditional anti-counterfeiting materials such as fluorescent printing, metal–organic complexes, or organic fluorescent materials, LC-CDs with different emission properties are also ideal for information encryption and fluorescent anti-counterfeiting applications (Figure 12a) due to their unique characteristics such as environmental friendliness, non-toxicity, ease of design, and low cost.
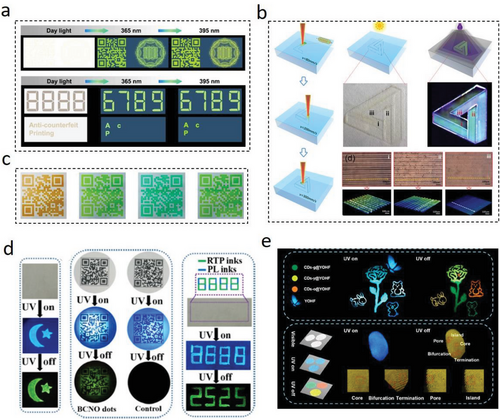
Yang et al. successfully mixed xylose and m-phenylenediamine in a phosphate solution, followed by microwave irradiating the mixture to synthesize H-CDs with the green light-emitting property.[22] The fluorescence patterns printed out in H-CDs inks displayed the blue-to-green fluorescence in different pH environments. However, such environmental-responsive fluorescent patterns could not guarantee to provide clear information indication in complex application environments. As a result, there is a growing demand for CDs-based fluorescent patterns that are durable and resistant to environmental factors such as water, photobleaching, and pH changes. Achieving water resistance, photobleaching resistance, and pH stability in CDs-based fluorescent patterns requires careful selection of CDs with appropriate surface functionalization, as well as optimization of the synthesis and processing conditions to ensure maximum durability and stability of the patterns. Zhu et al. investigated the stable yellow-green fluorescence of different L-CDs in different pH conditions, and found that these L-CDs did not fade out when exposed to sunlight bleaching, indicating a great potential in fast and intelligent fluorescent anti-counterfeiting printing applications (Figure 12c).[21] Guo et al. developed an in-situ fluorescence patterning technique based on C-CDs luminescence, i.e., using lasers to etch a three-dimensional fluorescence pattern directly on solid cellulose films (Figure 12b).[91] This technique was reported as an efficient and low-cost technology for the large-scale production of fluorescent patterns. However, this in-situ solid-state PL achieved through the efficient anchoring of CDs in additional precursor matrices prescribed a limit to CDs’ precursors. The complex components and intertwined distribution in LC-CDs precursors made it difficult to avoid the interactions among the components. However, recent investigations on in-situ synthesis of fluorescence patterns based on oxidized lignin luminescence on wood surfaces have indicated that specific fluorescence mechanisms and controllable synthesis processes still require further study.[195]
In addition, synthesizing CDs with RTP performances is also proposed.[134, 211] Embedding CDs in suitable matrices can enhance their efficient intersystem crossing (ISC) from the lowest excited singlet state (S1) to a triplet state (Tn) while suppressing their nonradiative transition behavior can obtain CDs with RTP.[182, 191, 196] The LC-CDs’ RTP behavior can provide long-lasting times of afterglow perceived by human naked eyes. Different matrices and LC-CDs concentrations can result in different RTP behaviors in final CDs’ composites with higher levels of multiple information encryption because of different LC-CDs’ afterglow features (Figure 12d,e).[176, 210] However, the difficulty is that some host matrices in matrix and LC-CDs composites are limited to certain CDs species for achieving a required RTP property, indicating host matrices are lacking their universality in all CDs species. In addition, unstable reversible interactions between CDs and matrices can potentially impact the RTP properties of CDs and matrix composites, which can hinder their practical applications.
4.1.3 LC-CDs in Functional Films
The topic of employing CDs’ fluorescence properties to construct functional films has been attracting much attention at present.[129, 212] Junka et al. modified cellulose nano fibrillar films using covalent EDC/NHC coupling in CDs for anti-counterfeiting and biosensing applications.[151] Polyvinyl alcohol (PVA), a macromolecule commonly used to prepare various functional films, has abundant hydroxyl groups that can be combined with CDs’ surface functional groups to form a large number of hydrogen bonds. This hydrogen bonds’ formation can realize the “solid solution” for the LC-CDs. Tao et al. produced transparent fluorescent films with excellent fluorescence and flexibility through dispersing L-CDs into PVA,[98] and investigated the practicability of these films as sensors for the real-time detection of pH variations of human sweat during exercising (Figure 13b). The attention of using CDs as sensors to build smart wearable devices in the study is innovative. However, in the real situation, the signal acquisition and translation process can directly affect the detection accuracy of L-CDs’ sensors because of the complex dynamic fluctuations of human bodies. Han et al. synthesized C-CDs that exhibited a significant Stokes shift at a short wavelength excitation, as well as having the maximum concentration-dependent fluorescent excitation and emission. These C-CDs were dispersed into a PVA matrix to form a composite film with an excellent transparency property that can effectively block blue light (Figure 13c).[93] However, the photobleaching properties of C-CDs and the aging resistance of the resulting composite films have not been thoroughly discussed.
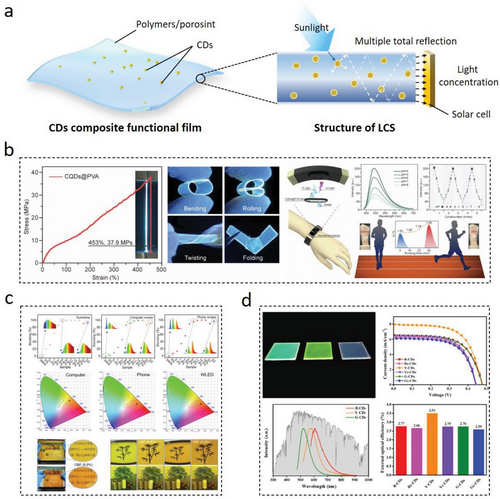
In addition, CDs-based transparent fluorescent films can also be further developed into the applications in the area of luminescent solar concentrators (LSC).[212, 213] LSC is a device that consists of light-emitting materials coated or embedded in transparent polymer films and can be used to broaden the light absorption range and increase the light intensity per unit area of solar cells. LSC can convert the absorbed short-wavelength light into long-wavelength light under the sunlight illumination. On account of the total internal reflection of light, the most of re-emitted light will be trapped within the transparent polymer film and then waveguided to the films’ edge where the re-emitted light will be absorbed and converted into electricity (Figure 13a right).[214] CDs have their unique properties for constructing LSC films such as high photostability, adjustable absorption and emission spectra, and non-toxicity. Wang et al. researched LSC films made through embedding L-CDs with different fluorescence emission wavelengths into epoxy resins and found out that LSC films with yellow-fluorescence CDs exhibited a high power conversion efficiency (ηPCE) and external optical efficiency (ηopt), and had a significant dependence on the long wavelength and high PL QY of L-CDs (Figure 13d).[213]
In addition, LC-CDs also can be mixed with polymers or hydrogels to prepare functional films.[20] However, the study only focused on the PL function of LC-CDs-based films, and the utilization of other properties of LC-CDs is limited. Especially, the utilization of LC-CDs’ efficient photoelectron transfer ability and rich surface functional group active bonding ability is lacking. Using matrix materials with different confinement properties can create environments for CDs used in different functional applications.
4.2 LC-CDs in Energy Conversion
With the shortage of fossil fuels and the implementation of carbon peaking and carbon neutrality strategy, the exploration of renewable, environmentally friendly, stable, and efficient energy conversion and storage technologies is of vital importance to the development of global economy.[88, 117, 215] Credited to their advantages such as low cost, large specific surface area, excellent electron acceptor/donor characteristics, superior electronic conductivity, and coordinated optical characteristics, LC-CDs have attracted much attention in energy fields such as photocatalysis, light emitting devices, photothermal energy storage materials, and supercapacitors.[44, 216, 217]
4.2.1 LC-CDs in Catalysts
It is a flexible strategy to use solar energy as a photodynamic catalyst for various photochemical reactions. LC-CDs have been proposed as photocatalysts to improve catalytic performances.[152, 184, 218] LC-CDs show excellent catalytic abilities in photocatalytic CO2 reduction, water decomposition, and pollutant degradation because of their broad spectral absorption ranges, excellent photo-stabilities, and electron separation and transfer capabilities.[184, 218]
In the hybrid LC-CDs, heteroatoms located along the edges of aromatic cores can achieve thoracic interfacial electron transferring and act both as light absorbers and electron acceptors in the heterojunction to enhance the photocatalysis. The large electron storage capacity of LC-CDs can facilitate the shuttle of photoexcited electrons from other semiconductors to their conductive network, thus hindering the recombination of photogenerated carriers at the junction interface.[117] Therefore, the combination of LC-CDs with catalysts such as graphene,[152, 219] metal materials,[220] or metal oxide-based semiconductors can increase catalytic activities,[184] providing an excellent strategy for designing high-performance photocatalysts. Sharma et al. evaluated CDs decorated with TiO2 nanohybrid TiO2/CDs as a catalyst and found out that there was an enhanced photocatalytic activity in the degradation of alkali lignin (up to 99%) under the sunlight.[184] The superlative photocatalytic activity was attributed to the presence of CDs in the nanocomposite, preventing the recombination and rapid transferring of photogenerated charge carriers and effectively utilizing the entire solar spectrum. Furthermore, in addition to acting as electron acceptors and donors, facilitating photogenerated charge transferring, LC-CDs can also serve as a role in enhancing visible light harvesting. When LC-CDs are excited by visible light, the electrons on the π orbital will be transitioned to the high-energy π* orbital, and the electronic instability of the excited state will return to the low-energy σ orbital through radiative relaxation, resulting in the emission of short-wavelength light.[94, 192] Xie et al. fabricated a magnetic and environmentally friendly photocatalyst Fe3O4/BiOBr/C-CDs decorated with C-CDs, and found that its removal efficiency for carbamazepine (CBZ) was 99.52%.[94] On one hand, this high catalytic efficiency is because of the addition of C-CDs with unique up-conversion photoluminescence properties that can enhance the optical absorption capacity of optical tests. On the other hand, the benign design of the Z-type structure of photocatalyst further improved the separation and transferring of photogenerated charges (Figure 14a,b).
LC-CDs with large specific surface areas and abundant oxygen-containing groups can be used as a carrier to load metal material catalysts (such as platinum, Pt) onto their surfaces to catalyze the electrooxidation of methanol.[220] A large number of oxygen-containing functional groups on LC-CDs can facilitate the uniform and stable deposition of Pt catalysts, and therefore increasing the nucleation density of Pt, preventing the aggregation of Pt catalyst during reduction, exposing more Pt catalyst to the reaction system, and finally enhancing the catalytic efficiency.
In the process of photocatalytic degradation of methylene blue (MB), it was found that the minor steric hindrance of C-CDs with small particle sizes can promote efficient photon and OH transferring (Figure 14c). The large specific surface area and more acidic sites brought by the smaller particle size of C-CDs can increase the adsorption capacity of MB and improve the degradation rate.[88] The better PL emission characteristics and QY of ultra-small C-CDs were also considered as CDs’ important properties that can enable CDs composites to generate more exciting electrons, and thus the photocatalytic reaction rate can be improved (Figure 14d). However, CDs do not always exhibit improved catalytic performance. Therefore, the precise working mechanism of CDs-based catalysts is still being investigated, and great effort is needed to make the breakthrough.
4.2.2 LC-CDs in Optronics
The CDs’ abundant and tunable PL emission characteristics make them great prospects in light emitters and LEDs applications of replacing the expensive rare-earth-based phosphors and toxic metal-based semiconductor QDs in traditional LEDs.[15, 17, 29, 221] Typically, such materials are encapsulated in high light-transmitting polymers as active fluorescent conversion layers for LEDs (Figure 15a).[16]
The solid-state PL characteristics of CDs are a prerequisite for their utilization in light-emitting devices. Several reported strategies for achieving the CDs’ solid-state PL involve dispersing CDs in a matrix like transparent polymers (such as PVA,[5, 27] epoxy resin, [16, 212] etc.), starch, silica, and even porous materials with the purpose of preventing the excess ACQ effects from accumulation (Figure 15b and c).[29, 165] The effectiveness of this approach in fabricating multi-color or even white LEDs using CDs has been widely demonstrated. In our reported work,[128] multi-color (including white light) fluorescent transparent woods had been prospected as a fluorescence conversion layer for constructing LEDs. While the use of a fluorescent conversion layer for constructing LEDs has shown promise, there are some challenges to overcome. Firstly, the process of fabricating the luminescent layer involves multiple steps. Additionally, thicker fluorescent conversion layers can lead to a reduction in luminescence intensity of the exciting chips.[222] CDs with fluorescent properties can be directly coated on the inner wall of LEDs lamp caps or serve as an active luminescent layer in a multi-layer structure to avoid the fluorescence damage and reduce the cost of fabricating CDs-based LED.[183, 223] To date, despite the progress made in the development of CDs-based LEDs, there remain significant challenges in achieving high color development index, multiple fluorescence emission, and luminescence efficiency. Most of these challenges are closely related to the development of CDs that have a wider color range, narrow FWHMs, and high QY.
Recent advances have been made in electroluminescent LEDs based on CDs (Figure 15d). Yuan et al. synthesized polychromatic high-performance triangular CDs (NBE-T-CQDs) with the narrow bandwidth emission (FWHM of 30 nm) and QY up to 54 to 72%. Moreover, these multicolored CDs-based LEDs demonstrated excellent stability, high color purity, and high performance (Figure 15e). [7] Although this work has pushed CDs-based LEDs a step toward more practical applications, the research on CDs-based LEDs, especially LC-CDs-based, is still at their early stage if compared to more mature semiconductor quantum dot LEDs. CDs-based LEDs have more room for the improvement in brightness, external quantum efficiency, and color purity.
4.2.3 LC-CDs in Photothermal Conversion
Similar to carbon-based materials such as graphene and carbon nanotubes that have found widespread use in photothermal conversion systems,[224] CDs can be harnessed as a valuable tool for fabricating efficient photothermal conversion systems through taking the advantage of their unique properties of high solar light absorption, stability, and scalability.[225, 226]
The consensus is that CDs have a graphite-like crystal structure, and their lattice structures generally correspond to the crystal form of graphene (1 0 0) that has graphite-like sp2 carbon clusters. Therefore, CDs have been proven to be able to enable effective lattice vibration by solar irradiation and generate thermal energy.[26, 227] In addition, when CDs absorb photons with a particular energy, CDs’ electrons will undergo energy-level transitions. During this process, electrons in CDs often undergo nonradiative transitions, such as intersystem crossings and vibrational relaxations, which result in the release of some energy as heat, and ultimately transition back to the ground state, exhibiting fluorescence behavior.[181]
Chao et al. designed a novel and green photothermal evaporation system with ecological and economic advantages through using L-CDs and wood. The system achieved an evaporation performance of 1.18 kg m−2 (1.09 kg m−2 h−1) and a photothermal conversion efficiency as high as 79.5% under single solar irradiation (1 kW m−2).[228] In addition, CDs also can be used for the design of energy storage composites, thus achieving effective heat energy collection. Yang et al. employed a simple solvothermal method of first synthesizing L-CDs with optical properties such as red (650 nm) and near-infrared (710 nm) emissions under visible light excitation (580 nm), followed by combining these L-CDs with phase change materials to construct photothermal energy storage materials.[229] CDs with significant Stokes shifts, especially the NIR emission where the non-radiative transition plays a dominant role in the energy transformation, can have a prominent photothermal effect. This significant effect can lead to a new prospect for LC-CDs being used in photothermal energy storage, photothermal therapy, etc.[181]
4.2.4 LC-CDs in Supercapacitors
Hybrid CDs can be used to better improve the electrochemical performance of supercapacitors compared to other carbon materials, polymers, or metal oxides due to their efficient electron-transferring ability.[230-232] The formation of constant conductive 3D carbon frameworks with large specific surfaces and good wettability is because of CDs’ continuous interconnected core-shell structures and abundant functional groups. A possible way of producing 3D carbon frameworks could facilitate the fabrication of stable large-capacity energy storage devices. In a study by Yu et al., the surface of lignin-based activated carbon was uniformly coated with CDs to create 3D composites that exhibited high-performance capacitor behavior. The introduction of CDs led to a significant improvement in the capacitance of the lignin-based activated carbon, increasing from 125.8 to 301.7 F·g−1.[233] The presence of CDs dramatically increased the surface polarity of the composite, leading to improved affinity of the electrode surfaces for aqueous electrolytes. The resulting increase in capacitance was attributed to a greater availability of electroactive sites due to an increase in available surface area and a reduction in the ion diffusion path.
4.3 LC-CDs in Biomedical Science
The most extensive development of CDs is their applications in biomedical fields such as in vivo imaging, cellular imaging, and drug delivery.[14, 103, 234] First, in vitro cytotoxicity studies indicated that CDs had low or no toxicity even at their higher concentrations. In vivo experiments also have shown that no obvious inflammatory symptoms were found in mice fed with CDs aqueous solution.[144] Second, CDs’ special characteristics such as small sizes, down-conversion PL, stable fluorescence performance, high brightness, and multi-photon PL make them attractive candidates for replacing traditional fluorescent probes and shine in the biology field.[14, 235]
4.3.1 LC-CDs in Bioimaging
In vitro imaging analysis process, CDs can be applied to human tissue cells,[136] cancer cells,[127, 133] and bacteria.[200] Research on the in vitro imaging of CDs has focused primarily on animal cell imaging and cytocompatibility assessment, which have been extensively discussed in literature.[28, 90] Usually, 3-(4,5)-dimethylthiahiazo (-z-y1)−3,5-di-phenytetrazoliumromide (MTT) assay is commonly used to assess the cell viability.[14] Shi et al. performed cell imaging experiments on mouse macrophage cell lines (RAW 264.7) using aminated L-CDs to evaluate the feasibility of L-CDs bioimaging. It was found that CDs had a low cytotoxicity and the CDs’ fluorescent state was not altered by the cellular environment.[157] The in vitro imaging study of animal cells is an exploration of its potential application in human cells, and human cell imaging is the target application of LC-CDs in biological imaging. Xue et al. used L-CDs as fluorescent probes to image the distribution of human breast cancer cell lines (HeLa cells).[127] Large amounts of L-CDs’ nanoparticles were found to be internalized into the cell body by the endocytic machinery (Figure 16a). It was observed that the bright fluorescence of CDs in cells was mainly concentrated in the cell cytoplasm, and a weak emission signal was distributed in the nucleus that was determined by the stricter selectivity of the nuclear membrane if compared to the cell membrane (Figure 16b). Therefore, the rapid labeling of cell membranes and cytoplasm by LC-CDs enables the isolation of nuclei, which is of great importance in imaging and observing cancer cells.
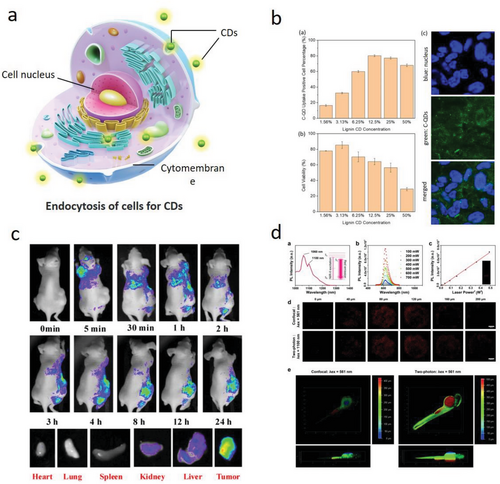
However, for the observation of cancer cells, in vitro imaging technology can only be considered as the basis for in vivo imaging. In vivo imaging is the highlight of biological imaging that is of great significance for tracking cancer cells in vivo and realizing the accurate localization of cancer cells, rapid detection, and targeted therapy.[5, 181] Currently, the research on in vivo imaging using LC-CDs is not yet extensive. Our reported work investigated the H-CDs performance in vivo optical imaging by injecting 200 µL (0.2 µg mL−1) of H-CDs into tumor-bearing mice via tail vein injection.[103] As time progressed, fluorescent signals were detected in all parts of mice bodies and gradually aggregated to the location of the tumor, indicating that H-CDs can internally circulate and accumulate in mice bodies (Figure 16c). Upon reflection, there are still some issues that needed to be addressed in this research. First, in addition to the bright fluorescence at tumor sites, fluorescence was also observed in some organs of mice, suggesting that although the fluorescence imaging technology using LC-CDs had a high contrast, the selective imaging was not satisfactory. Secondy, the relationship between the directional aggregation behavior of CDs in organisms and their intrinsic properties had not been clearly established. The observed fluorescence in non-tumor organs in our LC-CDs study highlights the potential uncertainty and risk associated with biological applications of LC-CDs. It is therefore imperative to develop strategies to achieve targeted aggregation of LC-CDs, enabling selective imaging of specific cells and tissues. This targeted aggregation approach would enhance the specificity and accuracy of LC-CDs in biological imaging applications and represent a significant advancement in the field.
In addition to the “targeted imaging” limited by the minimum autofluorescence and light scattering of tissues, the development of LC-CDs with a long-wavelength emission (red/ NIR emission) or multi-photon emission for in vivo imaging is also of positive practical significance. The red emission or NIR emission has a strong penetration ability and high photothermal conversion efficiency (Figure 16d), that will yield a significant application value in deep tissue imaging and photothermal therapy.[130, 181, 236] Such advancements would represent important practical breakthroughs in the field of biological imaging, facilitating a wide range of applications with improved sensitivity and specificity. Furthermore, the precise mechanism underlying the interaction between CDs and organisms, including their distribution, metabolism, and excretion mechanisms, remains poorly understood.
CDs also have exploitable potentials applications in drug or gene delivery.[237] LC-CDs with small size, biocompatibility, and tunable surface chemistry can be employed to load targeted drugs that can gradually incorporated into diseased tissues and subsequently release to target cells, thereby improving the overall efficiency of drugs delivery.[36, 238] Compared with traditional drug delivery systems, CDs have the advantages of low toxicity, good targeting, and excellent drug efficacy.[239] Rai et al. used L-CDs as nanocarriers for the anticancer compound curcumin imaging and therapy of cancerous cells (A549 and SW480). The L-CDs’ drug loading efficiency was calculated to be 67.4%, and the maximum drug release efficiency was 82.7% at physiological pH (7.4).[36] Importantly, this study also revealed that the main attachment mechanism between L-CDs and curcumin is the hydrophobic (primary) and hydrogen bonding interactions. Therefore, the L-CDs’ drug loading capacity can be attributed to the functional groups (─OH, ─COOH, ─SO3, ═CO) on L-CDs’ surfaces, providing the guidance for designing CDs with multiple layers and high-drug-loading.
Presently, the application of using LC-CDs for drug delivery is still in the exploratory stage.[71] While the potential benefits of using CDs for drug delivery are promising, the loading mechanism and release conditions for targeted drugs are still not fully understood. One of the primary challenges is the difficulty of acquiring LC-CDs with the desired targeted structure. Additionally, there is a need to expand the identification range of LC-CDs for more targeted cells to improve the overall efficacy of drug delivery. Further research is required to fully explore the potential of CDs-based drug delivery systems, and to overcome the challenges associated with their development and implementation.
In addition to the aforementioned applications of CDs, recent research has also explored their potential in the field of agriculture.[240] In the work Wang et al., it was demonstrated that the application of CDs solution on soybean leaf surfaces, coupled with the light conversion properties of nitrogen-doped CDs, can enhance photosynthesis and nitrogen metabolism in soybeans cultivated in arid environments. This enhancement in physiological processes ultimately results in improved crop yield and quality.[241] Similar studies have also provided preliminary findings on this topic.[242] Undoubtedly, these advancements serve as valuable contributions to the progression of nanoscale agricultural technologies, offering potential solutions to mitigate the impact of climate change on the food industry. However, the application of LC-CDs in agriculture remains an area with limited available research. By embracing the concept of “taking from agriculture, using for agriculture,” LC-CDs hold the potential to facilitate crop growth and establish a sustainable closed-loop system of LC-CDs and agricultural practices. Nonetheless, it is important to note that previous studies have reported variations in the effects of carbon dots on different crop species, highlighting the need for further investigation and understanding.[243] Therefore, achieving comprehensive coverage of emission wavelengths remains a challenge for LC-CDs. Furthermore, research is needed to gain a comprehensive understanding of the structure-activity relationship and optimize the application of LC-CDs in diverse agricultural contexts.
5 Challenges and Prospects
LC-CDs are distinguished by their utilization of green carbon sources such as wood or agricultural waste, promoting sustainability and reducing reliance on fossil fuels. These CDs possess abundant oxygen-containing functional groups, including hydroxyl, carboxyl, and carbonyl groups, which enhance their water solubility, surface reactivity, and potential for functionalization. Notably, they exhibit adjustable emission properties within the visible and near-infrared spectrum, enabling customization for specific applications. LC-CDs derived from lignocellulosic sources demonstrate biocompatibility, rendering them suitable for biomedical applications, while their environmentally friendly nature contributes to reduced carbon emissions and waste accumulation.
Undoubtedly, lignocellulosic-based CDs exhibit promising characteristics. However, their development is accompanied by several challenges that require attention. One significant challenge lies in the current synthesis methods of LC-CDs, where the thermal decomposition of macromolecular raw materials and repolymerization of small molecular components are intricately intertwined and difficult to separate. The degree of decomposition and polymerization is influenced by various factors such as raw materials, process temperature, and time. Particularly in lignocellulosic materials, the three main chemical components of cellulose, hemicellulose, and lignin are tightly bonded. In current research, the synergistic effects of degradation products with different components are not usually considered when LC-CDs come to nucleation growth, as CDs often are “random synthesis” and difficult to “synthesize on demand”. However, the targeted design of LC-CDs is an essential requirement for enhancing their performance in various potential applications. Future studies should focus on elucidating how the contributions and interactions of the three components, i.e., precursor molecules, surfactants, and solvents, impact the nucleation and growth of LC-CDs.
Up to now, two approaches have been employed to regulate LC-CDs’ fluorescence emission properties. The first common method is to dope LC-CDs with heteroatoms or functional groups to increase the number of fluorophore groups in LC-CDs, especially electron-donating groups that affect brighter fluorescence emission. However, whether the introduction of multiple groups will or not have a synergistic or antagonistic effect on the fluorescence performance of LC-CDs remains to be seen. Another challenge is to identify the specific functional group responsible for emission at each wavelength, as this is crucial for accurately regulating the PL behavior of LC-CDs. The second method is in-precise separation and purification techniques. The accurate separation of LC-CDs of various sizes (especially the filtration of strong fluorescent organic clusters) can significantly improve the QY and color purity of CDs. It is still necessary to improve conventional processes (such as dialysis and chromatographic columns) because of their high cost, low efficiency, and low yield.
Besides the above-mentioned synthetic methods and PL mechanisms, future research also should focus more on the application-oriented design of LC-CDs. Generally, LC-CDs can only be served as a solution for quantitative detection of metal ions and organic molecules that is cumbersome and inefficient. Therefore, it is necessary to develop fast-to-use, high-precision composite sensors based on LC-CDs’ PL responses such as inductive test strips or films. In terms of information anti-counterfeiting, the weather resistance (water resistance, photobleaching resistance, etc.) of the fluorescent patterns printed by CDs should be considered. Combining multiple encryption technologies may improve reliability of CDs in information encryption. LC-CDs are still in their infancy for their potential applications in the field of catalysis and photoelectric conversion. There are still many scientific problems that needed to be solved in terms of catalytic efficiency, application form, and fluorescence performance. It is envisaged that a broad emission spectrum ranging from blue to red can be achieved under the excitation of a single UV light wavelength. This offers the possibility of realizing white light emission through UV conversion, which could revolutionize the next generation of lighting and display technologies. It is well known that long wavelength emission is very beneficial for biological imaging. If the structures required for the long-wavelength emission are identified, the emission wavelength of LC-CDs can ultimately be selectively enhanced using structural engineering. In addition, we should also note that the ideal model of a LC-CDs’ core structure is actually not much different from polycyclic aromatic hydrocarbons (PAHs, carcinogens). Therefore, more caution is required in declaring the CDs’ low toxicity, and more persistence and in-depth research investment should be emphasized in the entire field of biomedical science.
In conclusion, the development of LC-CDs opens a bright pathway for converting agricultural and forestry materials into high-value added products. LC-CDs, as a new type of carbon-based nanomaterials, have shown significant potential in a wide range of science and engineering applications because of their unique properties such as optical, excellent biocompatibility, low cost, ease of modification and functionalization. These properties of LC-CDs are not inferior to other CDs fabricated using other materials or even SQDs. Finally, although LC-CDs have rapidly been developed into luminescence, catalysis, and biology research areas, this does not mask their shortcomings in the synthesis of high-quality products and the difficulties in mechanistic studies which are currently one of the biggest obstacles to the development of LC-CDs.
Acknowledgements
J.G. and L.C. contributed equally to this work. This work was supported by the Natural Science Foundation of Jiangsu Province (BK20220106) and the National Natural Science Foundation of China (32071687). In addition, the authors thank the Jiangsu Qing Lan Project and the Young Elite Scientists Sponsorship Program by CAST (for Dr. Caoxing Huang).
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Caoxing Huang is a young professor at Nanjing Forestry University in China. He got his Ph.D. degree in 2017 in the field of Chemical Processing Engineering of Forest Products from Nanjing Forestry University, China. In 2022, he got the Humboldt Research Fellowship in Germany and has worked as the postdoctoral researcher in University of Göttingen. His research focuses on isolating/characterizing the components from lignocellulosic materials and bio-application of different lignocellulosic components. He has published over 120 peer-reviewed papers with 44 H-index. From 2017 to 2023, his contributions to bio-application of different lignocellulosic components were supported by Foundation of the National Natural Science Foundation of China, Jiangsu Provincial Natural Science Foundation Project, Outstanding Young fund of Jiangsu, and Young talents of China Association for Science and Technology.

Yan Wu is an outstanding young professor of “Qinglan Project” in Jiangsu Province. She is mainly engaged in functional wood coatings, wood-derived photoluminescent of carbon dots and transparent wood materials. She obtained her BSc in 2002 from Beihua University in China, Ph.D. in 2009 from the Nanjing Forestry University in China. From 2016 to 2017, she was a visiting scholar at the Center for Renewable Carbon Materials at the University of Tennessee. Over the last decade, she has presided over and participated in more than 10 projects at the national, provincial, and ministerial levels, including the Youth Foundation of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the 948 Project of the State Forestry Administration, the Jiangsu Provincial Natural Science Foundation Project, the China Postdoctoral Special Grant. She has been authorized 13 national invention patents and published more than 100 research papers.

Kai Zhang is the Head of the Department for Wood Technology and Wood-based Composites, and Executive Director of Biotechnology Center of Faculty of Forest Sciences and Forest Ecology, University of Göttingen, Germany. He serves currently as editorial board member of “Smart Materials in Medicine” and ‘Scientific Reports’. He was Associated Editor of ‘Hydrogels’ and editorial board member of ‘Journal of Semiconductors’. He carried out a number of projects, such as from EU, German Research Foundation (DFG), Federal Ministry of Food and Agriculture, Federal Ministry of Economics and Energy. Alexander von Humboldt Foundation, German Chemical Industry Association and so on. He published more than 130 papers, such as in Nature Sustainability, Angewandte Chemie Int Ed, Advanced Materials, ACS Energy Letters, ACS Nano and Advanced Functional Materials.




