The Interplay of Growth Mechanism and Properties of ZnO Nanostructures for Different Applications
Dedicated to all human rights activists in the world such as those in Ukraine and in Iran that have been killed by unjust wars or only for pursuing human rights, and to all university students in Iran that have been imprisoned or expelled from universities, to all the young people and women that have been risking their lives and are being killed, injured, tortured, imprisoned or being raped in prisons, on almost a daily basis for such human rights quests
Abstract
This review provides a background on the structure and properties of ZnO nanostructures. ZnO nanostructures are advantageous for many applications in sensing, photocatalysis, functional textiles, and cosmetic industries, which are described in this review. Previous work using UV Visible (UV–vis) spectroscopy and scanning electron microscopy (SEM) for ZnO nanorod growth analysis in-solution and on a substrate for determination of optical properties and morphology is discussed, as well as their results in determining the kinetics and growth mechanisms. From this literature review, it is understood that the synthesis process greatly affects nanostructures and properties; and hence, their applications. In addition, in this review, the mechanism of ZnO nanostructure growth is unveiled, and it is shown that by having greater control over their morphology and size through such mechanistic understanding, the above-mentioned applications can be affected. The contradictions and gaps in knowledge are summarized in order to highlight the variations in results, followed by suggestions for how to answer these gaps and future outlooks for ZnO nanostructure research.
1 Background
The properties of nanoparticles and their vast variety of applications have resulted in substantial progress in the field of nanotechnology. Nanomaterials have sparked particular interest in biosensing and medical applications.[1, 2] Nanotechnology, in general, entails the use of nanoscale materials; typical sizes range from 1 to 100 nm at least in 1D, and are classified from 0D to 3D.[3] Recent studies have also described certain nanomaterials as 4D, in which the fourth dimension has referred to time.[4, 5] Nanostructures have different features than their bulk material due to their size and have been found to have unique electrical conductivity, compatibility with biological molecules, and optical absorption properties from their bulk counterparts, which are attributed to quantum effects and their high surface-to-volume ratios.[6-9] Decreasing nanostructure size results in increased band gap due to quantum confinement of the electrons, altering the conductivity of the nanomaterial. In addition, the greater surface-to-volume ratio allows for a larger area for biomolecules to attach on to the surface.[8]
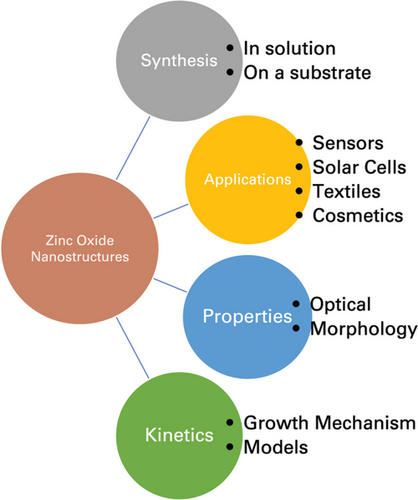
The characterization of nanomaterials is essential for understanding their properties, as well as for the control of synthesis and application. The characterization methods are used to evaluate many different properties such as size, morphology, and solubility, as well as to help determine their growth pattern and mechanisms.[10] Some of the more common nanomaterials include magnetic, metal, and metal-oxides. Metal-oxide nanomaterials have been shown to exhibit unique electronic structures and chemical properties, which determines their semiconducting properties. Metal oxide nanostructures tend to have wide band gaps and large dielectric constants, and those of transition metals are reactive as the transition metal ions generally have unfilled d-orbitals.[11-13] Altering the size of the nanostructures alters the chemical and electronic properties of the metal-oxide structures, which is beneficial for use in several applications. The upcoming sections will discuss the properties, applications, and kinetic studies of zinc oxide (ZnO) nanomaterials, outlined in Figure 1.
1.1 ZnO Nanostructures
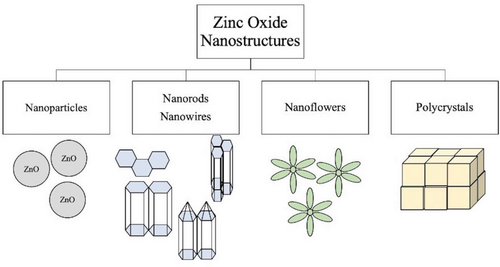
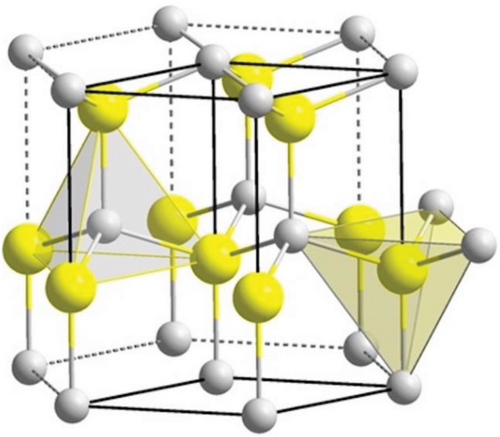
In recent years, the study of zinc oxide (ZnO) nanostructures has become very popular due to their wide range of electrical, piezoelectrical, chemical sensing, and optical properties that are suitable for many applications. ZnO is a II–VI semiconductor with a wide band gap of 3.37 eV and an exciton binding energy of 60 meV.[14] ZnO has many different growth morphologies such as nanowires, nanobelts, nanoflowers, and nanorods, shown in Figure 2.[15] They have shown a high thermal and mechanical stability, as well as the ability to endure large electric fields and breakdown voltages.[16] ZnO structures are polar crystals with a polar (001) zinc-terminated plane, a polar (00) oxygen-terminated plane, and a nonpolar (100) plane.[17] Under ambient temperature and pressure, the most thermodynamically stable structure of ZnO is the hexagonal wurtzite form.[18] This structure consists of alternating stacks of Zn2+ and O2- ions in a tetrahedral conformation that lacks central symmetry (see Figure 3), inducing strong piezoelectric properties.[19] The layered stacking of positively and negatively charged ions leads to the presence of polar surfaces that can be manipulated to utilize the resulting electrical properties for different functions such as biosensors.[20] In general, ZnO nanostructures have natural n-type electrical conductivity due to the presence of intrinsic defects.[21] These defects include: oxygen vacancies, where an oxygen is missing from the crystal structure, and zinc interstitials, where an extra zinc atom is found in the crystal lattice.[22] However, some studies suggest that the involuntary absorption of impurities present in the environment that act as shallow donors, such as hydrogen, provides the n-type conductivity found in ZnO nanostructures.[23, 24]
ZnO nanostructures are relatively inexpensive to produce, non-toxic, and biocompatible, making them an attractive material for many applications.[26, 27] Other nanostructured semiconductors such as TiO2 and GaN have been used for similar applications, including sunscreens and light-emitting diodes.[28, 29] The synthesis of ZnO nanostructures and the crystal growth process are relatively simpler, and the raw materials for making them are less expensive, and in turn, allow for lower cost production of ZnO-based devices. Commercially available GaN nanopowders can cost over $2500 for 100 g, while their starting materials, commonly Ga(NO3)3, can cost over $1000 for 100 g making them an unattractive material for large-scale synthesis. Although TiO2 nanoparticles and their starting materials are closer in price to ZnO materials, ZnO is still slightly less expensive and much less toxic for various applications.[30] The availability of high-quality ZnO crystals and large exciton binding energy of them are other advantages of ZnO nanostructures.[31]
Although ZnO is generally diamagnetic, the ZnO nanorods grown from these experiments displayed magnetic properties. The magnetic susceptibility results showed increasing susceptibility with decreasing temperature, typical of paramagnetic materials. As well, the B–H curve showed no hysteresis, which would otherwise have been indicative of ferromagnetism. Therefore, it was determined that the synthesized nanorods were exhibiting super-paramagnetic properties.[32] This is important for various potential medical applications as the magnetic nature of the nanorods can be beneficial for drug delivery or magnetic-field induced hyperthermia in cancer treatments.[33] Therefore, further understanding of the effect of each reaction parameter on the size and properties of the ZnO nanorods is significant for potential applications.
1.1.1 Factors Affecting Synthesis
- Seeding of substrate with a zinc source (mostly ZnO nanoparticles or zinc acetate) to promote nucleation.[34]
- A mixture of a basic reagent (e.g., OH− or an amine) and zinc salt (e.g., zinc nitrate or zinc acetate) is used as the precursor solution.
- The seeded substrate remains in solution at varying temperatures over a period.
- Substrate with newly formed ZnO nanorods is then washed and dried.
The nature of the substrate can affect both orientation and aspect ratio; therefore, it has a significant role in the growth process of ZnO nanorods. Seeding of the substrate is necessary as it increases the number of nucleation sites available on the substrate, allowing for the growth of a larger number of ZnO structures. In addition, selection of the precursors and concentrations is important for growth of the desired morphologies. The formation of ZnO nanoparticles commonly arises from the use of a direct hydroxide source, such as NaOH, LiOH, KOH, and NH4OH.[35-37] ZnO nanorods and nanoflowers are more commonly produced through the use of an amine, generally hexamethylenetetramine (HMTA), ammonia (NH3) or ammonium chloride (NH4Cl), as they tend to hinder lateral growth.[38-40] As ZnO nanorods grow, their side facets are negatively charged, which allows for preferential attachment to amines, owing to their positively-charged nature in solution.[41] As a result, formation of ZnO favors growth along the c-axis to form nanorods.
Using ammonia and HMTA at varying concentrations as precursors has allowed for the control of the size and shape of ZnO nanorods and their resulting aspect ratios.[42, 43] In addition, several studies have used nonpolar polymers such as polyethyleneimine (PEI) in tandem with HMTA or ammonia to further increase anisotropic growth of ZnO nanorods.[44, 45] PEI has numerous side amino groups which can be readily protonated in solution; the positively charged molecule then adsorbs onto the negatively charged side facets of ZnO nanorods, increasing epitaxial growth. One study employed HMTA and PEI during ZnO nanowire growth for use as a photoanode in dye sensitized solar cells.[44] When using HMTA alone, the aspect ratio was found to be ≈20, while the addition of PEI increased the aspect ratio to over 125. Other studies have compared the effect of alkylamine chain-length on the growth of ZnO nanorods, using alkylamines such as octylamine (C8) up to oleylamine (C18).[46, 47] They found that the alkylamines adsorb onto the nonpolar side facets of ZnO promoting oriented growth along the c-axis, similar to the behavior of other amines, with increased growth occurring more as chain length increased. This is a result of complex formation between Zn2+ and the alkylamine, which showed increasing stability with longer carbon chains. Shorter chain complexes are more easily hydrolyzed; and thus, the nonpolar ZnO surfaces are more exposed, leading to shorter and more disordered nanorods. Therefore, the use of amines is essential for anisotropic growth of ZnO nanostructures.
The concentration of the precursors affects morphology as lower starting concentrations lead to nanoflower growth on the substrate, while higher concentrations form well-aligned nanorods with larger diameters. This is proposed to be caused from rapid depletion of the molecules in solution at lower concentrations, which results in incomplete nanorod formation with less ordered orientations.[48] In addition, when the precursors are not of equimolar concentrations (higher zinc salt concentration than basic reagent), the morphology has been shown to become sphere-like at lower temperatures and the reaction takes longer for nanorods to grow.[49] Further investigations are necessary for complete understanding of why this occurs.
1.1.2 Methods of Synthesis
The more common methods of synthesis include solution-based synthesis as they are low temperature, inexpensive, and relatively easier, that allows for a wider range of substrates to be used, when compared to gas-phase synthesis.[50] Common solution-based synthesis methods include hydrothermal method, sol–gel method, chemical bath deposition, electrochemical deposition, and various others.[27]
Due to the above-mentioned properties of ZnO nanostructures and the relatively low-cost of production, this allows for the fabrication of readily available and easy to use biosensors. Previously, our group investigated the effects of gravity and an external magnetic field on the hydrothermal synthesis of ZnO nanorods for biosensing applications.[51, 52] ZnO nanorods were grown on an indium tin-oxide (ITO) substrate, both against and with gravity, as well as in the presence of various strengths of external magnetic fields. From this, it was shown that the size and morphology of the ZnO nanorods could be altered by the effects of the combined fields, which in turn affected the sensor response to detect bacteria and fungi. The ZnO biosensors were non-solution-based and were able to detect various bacteria strains by changes in the electrical conductivity. The synthesis carried out at 90 °C showed that the with gravity (WG) results produced much smaller but well-aligned nanorods, while the against gravity (AG) results produced much larger nanorod-flowers. The presence of the magnetic field also aided in aligning the nanorods into bundles; however, the bundles did not align with each other. Increasing the precursor concentration greatly increased the length of the nanorods when the magnetic field was aligned parallel. In contrast, when the magnetic field was perpendicular to the nanorod growth, the width greatly increased. This was significant for the sensor response as the differences in size and morphology produced different results for various bacteria and fungi samples. The WG nanorods grown in the presence of 150 Gauss magnetic field produced nanorods with the smallest surface area-to-volume ratio but surprisingly showed the best sensitivity to the presence of E. coli over 51 h.
While conventional synthetic methods have many advantages for industrial applications, especially hydrothermal synthesis, ZnO growth by bulk heating can often be time-consuming with low growth efficiency and requires multiple steps for device fabrication.[53] In recent years, there has been much interest in mitigating these drawbacks through use of laser-induced hydrothermal growth. Yeo et al. developed a novel method in which ZnO nanowires were hydrothermally grown using a laser as a local heat source, which allowed for growth site placement on the substrate and simultaneous growth in one step.[53] Through this method, the laser was focused at a desired spot to raise the local temperature for nanowires to grow, which led to faster growth rates and increased lengths (and aspect ratios) than conventional hydrothermal methods. It has also been reported that the addition of a metal laser light absorbing layer on the substrate can shrink the local temperature produced by the laser, allowing for growth of ZnO nanowire arrays smaller than the focused laser spot and versatility in the chosen substrate.[54] Other studies have shown the applicability of this low temperature method for inexpensive fabrication of UV sensors without use of vacuum.[55, 56]
An alternative method for creating a localized temperature rise that has been reported apart from using a laser includes the use of a single nanowire resistive nano-heater.[57] Hierarchical heterojunction ZnO nanowires were hydrothermally grown on this nano-heater, and similarly, at low temperature without vacuum. However, this method has shown some limitations as it is not as easily scalable as the above-mentioned methods and due to the difficulty of required number of auxiliary electrical pads needed for the heater's wiring to the external power source. Another study used a nanowire network structure functionalized with ZnO nanoparticles as a localized heating source by which hierarchically branched ZnO nanowires were hydrothermally synthesized.[58] This provides another method in which the area of nanowire growth can be manipulated for where it is needed by use of a fixed bias voltage. Therefore, slight alterations to the conventional synthesis method enables these processes to allow for targeted growth and tunability of nanostructure size as well as to be much more beneficial and readily available for device fabrication at low costs and shorter times.
2 Applications of ZnO Nanostructures
The various morphologies and properties of ZnO nanostructures that can be synthesized allow for numerous applications. For the synthesis of ZnO nanorods or nanoflowers grown on a substrate, the most common application has been for sensing purposes. The basic concept of sensors is their ability to transmit an output signal response when a selective material interacts with the signal transducer.[59] These instruments can be categorized in the way they exhibit measurable optical, electrochemical, or electrical output signals convertible to digital signals. ZnO nanostructures are commonly used for gas sensing, UV sensing, as well as biological sensing.
The optical properties of ZnO nanostructures, namely exciton binding energy and bandgap, have shown ZnO to be an attractive material for use in solar cells. In addition, their UV absorption capacity and antibacterial activity has provided applications in both the textile and cosmetic industries.
2.1 Sensors
2.1.1 Gas Sensors
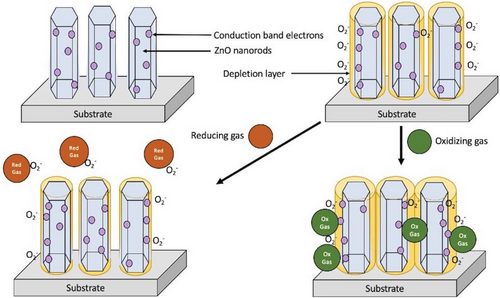
ZnO nanorods are an attractive material for gas sensors due to their high surface-to-volume ratio, which can aid in overcoming fundamental limitations.[60, 61] Typically, these sensors are resistance-based, where the variations in the resistance on the surface of the ZnO electrode is measured at a set voltage.[25] The adsorption of oxygen present in the atmosphere onto the surface of the ZnO nanorods forms an electron-depleted surface layer, shown in Figure 4 below, which increases the surface resistivity in the sensor.[62] A study done by Han et al.[63] determined that the structural defects on ZnO nanostructures affect sensing abilities as excess local zinc from oxygen vacancies and/or zinc interstitials increases chemisorption of oxygen on the surface, enhancing response of the sensor. Our group has observed similar effects but in biosensors.[32, 51, 64] In addition, gas sensing abilities are reliant on the size of morphology of the nanostructures as decreased width of the nanorods heightens the surface depletion effect which increases sensitivity.[65] Other studies have shown the greater surface area and surface defects of ZnO nanoflowers enhance the response as there is increased adsorption of the target gas molecule.[66] Therefore, tunability of the synthesis of ZnO nanorods is important.
2.1.2 Biosensors
Biosensors are sensors where the receptor is based on biomolecules, cells, or pathogens. The bioreceptor refers to a molecule capable of specifically detecting the analyte.[64] The signal production processes involve changes in light, heat, pH, charge or mass through the interaction between the bioreceptor and the analyte. ZnO nanostructures are stable, biocompatible, and have a high isoelectric point, making them advantageous for biological detection. The first use of ZnO nanorods as a biosensor was by Zhang et al.[67] in 2004, where they developed a uric acid biosensor based on the immobilization of uricase. Since then, many ZnO biosensors have been developed that are able to detect various analytes including the urea,[68, 69] DNA,[70, 71] glucose,[72] cholesterol,[73] proteins,[74] and pathogens.[51, 52] ZnO nanorods have been found to stabilize the biological activity of the target molecule and provide an agreeable environment for immobilization due to large surface area and electron communication features.[75] In addition, the conductivity of individual well-aligned nanorods provides numerous transport channels that promote electron transfer between biomolecule and electrode.[76]
2.1.3 UV Sensors
Ultraviolet (UV) sensors are utilized in a variety of applications, such as fire detection, pollution and ozone monitoring, and industrial manufacturing.[77] ZnO nanorods can be used for this application due to their many previously mentioned properties, including an optical band gap of 3.37 eV and exciton binding energy of 60 meV, allowing for the excitons to remain stable at room temperature.[78] When stimulated by a UV-source, ZnO nanorods exhibit enhanced electrical properties due to increased generation of electron–hole pairs which can recombine directly or by trap states.[79] The intrinsic defects found in ZnO nanostructures are crucial as they favor adsorption of oxygen molecules, which produces these trap states in the forbidden gap and creates oxygen ions, resulting in the conductance change of the ZnO.[80] Exposure to UV radiation with photon energy higher than the optical band gap results in absorption of a photon and generation of the hole and electron carriers.[81] The positive-charged holes will recombine with the oxygen ions to release the electrons back to ZnO at the same time. This results in a change in conductivity of ZnO nanostructures equivalent to the UV radiation.[82] The surface area of nanostructures is important for UV sensors as increase in diameters and surface-to-volume ratios increases response.[83] Therefore, simple, low-cost synthesis methods that allow for modification of surface defects and optical bandgaps are necessary for the wide range of UV sensor applications.
2.2 Solar Cells
The primary purpose of solar cell applications is to provide an efficient method to convert sunlight into electricity. This is achieved by photon absorption and formation of an exciton in a light-absorbing material, which is then followed by separation of charges to conductive materials where the charges are transferred.[11] The donor and acceptor materials used in this process can be both organic or inorganic semiconductors. Although TiO2 is the most common semiconductor-oxide for fabrication of solar cells, there has been much investigation into ZnO-based solar cells as it has similar properties to TiO2 but also has a more diverse range of morphologies that can be synthesized by a variety of techniques. In addition, ZnO nanostructures have been shown to have a higher electron mobility and longer electron lifetime, which allows for lower charge recombination that is advantageous in solar cell efficiency.[84] As ZnO is an inorganic semiconductor oxide, it can be used as the electron transport material in both hybrid solar cells and dye sensitized solar cells, where at least one phase in the device is inorganic.
2.2.1 Hybrid Solar Cells (HSC)
In hybrid solar cells, the organic polymer/semiconductor acts as the hole transporting material (donor), while the inorganic semiconductor acts as the electron transport material (acceptor).[85] Both materials are in direct contact, increasing the efficiency of exciton dissociation at the interface. As a result, one of the semiconducting materials performs the dual task of light-harvesting and transport of the charge carrier, either hole or electron, while the other material has the function of transporting the opposing charge carrier.[86]
The performance efficiency of hybrid solar cells is generally lower than that of dye sensitized solar cells, and that depends greatly on composition as well as on film quality, which can be more affected by synthesis conditions such as the nature of the solvent.[87] Common organic components used for ZnO-based HSCs include: poly(2-methoxy-5-(3′,7′-dimethyloctyloxy)-p-phenylene vinylene) (MDMO-PPV), and poly (3-hexyl thiophene) (P3HT) alone or combined with either TiO2 or phenyl-C61-butyric acid methyl ester (PCBM). ZnO nanoparticles are grown hydrothermally with only the use of P3HT produced HSCs with conversion efficiencies between 0.9% and 1.4%.[88, 89] The use of MDMO-PPV as the organic component with ZnO nanoparticles has produced slightly better efficiency, as some have been reported to be 1.47%[90] and 1.6%,[91] with the higher efficiency corresponding to the smaller diameter. One of the higher conversion efficiencies was produced from ZnO nanorods with P3HT/PCBM mixture as this allows for improved exciton dissociation and charge transport, relative to the use of pure P3HT.[92]
2.2.2 Dye Sensitized Solar Cells (DSSC)
Dye sensitized solar cells are similar to HSCs in the sense that both can use organic and inorganic materials and follow comparable mechanisms for exciton dissociation.[93] However, a significant distinction between these solar cells includes the presence of a dye and liquid redox electrolyte. Due to this, the functions of the active materials in DSSCs differ: a dye is absorbed onto the surface of the semiconductor (ZnO) nanostructures and is used for light-harvesting. This results in excitation of an electron into the conduction band of the semiconductor which is moved through the nanostructured-network and diffuses into the anode.[94] The liquid electrolyte provides electrons to the dye molecules that have previously provided electron to the nanostructures after excitation. A diagram of the mechanism is shown in Figure 5.
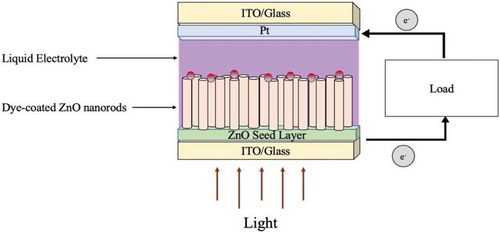
ZnO-based DSSCs have been extensively researched over the years as the performance efficiency of DSSCs is heavily influenced by the varying morphologies and resulting surface areas of ZnO nanostructures. DSSCs based on hydrothermally grown ZnO nanowires have been reported to exhibit increased electron transport and collection compared to ZnO nanowires.[44] Nanowire arrays from this study showed a power conversion efficiency of 1.5% for nanowires of lengths 18–24 µm. In addition, the electron transport in these ZnO nanowire array electrodes was found to be over two orders of magnitude faster than the electrodes with deposited nanoparticles. However, 1D ZnO nanostructures tended to have lower surface areas, resulting in decreased dye adsorption that leads to a poorer performance. Another study comparing the efficiency of various morphologies (flowers, sheet-spheres, and plates) demonstrated that the DSSC with the highest conversion efficiency resulted from the nanostructures with the largest surface area and most uniform film morphology.[95] In this study, the most efficient ZnO-based DSSC was from the sheet-sphere morphology with an efficiency of 2.61%. In addition, DSSCs fabricated with ZnO tetrapod-shaped nanofilms with nanoparticles interspersed,[96] as well as ZnO nano-porous nanosheets doped with boron[97] greatly increased the surface area, which resulted in efficiencies of 3.27% and 6.75%, respectively.
Intrinsic defects found in ZnO nanostructures also play an important role in DSSC performance as they can affect charge transport and dye adsorption.[98] Defects occur naturally in ZnO; however, various synthesis conditions can increase their concentration. The number of electron charge carriers increases from the presence of both oxygen vacancies and zinc interstitials, which in theory should increase dye loading and in turn improve conversion efficiencies. However in a study done by Hsu et al., the samples with the highest dye loading were hydrothermally grown and had slightly lower efficiencies than those with smaller dye loading that were synthesized by vapor deposition.[99] This demonstrates that the surface conditions and film quality of the solar cell also affect the conversion efficiencies as the hydrothermally grown ZnO nanorods have a larger number of OH groups present and affecting charge collection. Another study demonstrated that increasing dye concentration can lead to overexposure in an acidic environment which deteriorates the top layers of the ZnO nanostructured-films, decreasing efficiency.[100] For these reasons, synthesis of ZnO nanostructures and selection of dye is very significant for DSSC applications. There have also been reports of using inorganic quantum dots as sensitizers in DSSCs that have great potential.[98, 101] Therefore, synthesis and morphology of ZnO nanostructures is significant in solar cell performance, and future investigations into the development of ZnO-specific sensitizers and electrolytes are essential for improved efficiency and performance of ZnO-based DSSCs.
2.3 Textiles
The use of ZnO nanostructures in the textile industry has gained much traction as it has been demonstrated in various studies that treatment of the fabrics improves the antibacterial and self-cleaning qualities, as well as the UV absorption capacity.[102] While several types of nanostructures have been investigated for their antimicrobial effects, ZnO nanostructures remain one of the preferred materials as they have various advantages. For example, ZnO nanostructures have antimicrobial properties that are light-independent, compared to TiO2, which needs light irradiation to have effective antibacterial properties.[103, 104] In addition, in relation to nanostructures such as silver, ZnO offers a much lower cost. In addition to being biocompatible, coatings nanoscale-ZnO have demonstrated far better air-permeability and UV-blocking ability than their bulk counterparts.[105] ZnO nanostructures have therefore gained a lot of appeal and have been used in a variety of ways as UV-protective textile coatings. For example, cotton fabric coated with SiO2 and hydrothermally produced ZnO nanoparticles show exceptional UV-blocking capabilities.[106, 107] A considerable increase in UV-absorbing activity was obtained by synthesizing ZnO nanoparticles elsewhere through a homogeneous phase reaction at high temperatures, which were then deposited on cotton and wool fabrics.[108] Several studies have investigated the synthesis of ZnO nanoparticles for antibacterial and UV-protection properties on various textiles.[109, 110] However, detailed investigations of the above-mentioned properties for ZnO nanostructures with varying morphology and sizes are lacking.
2.4 Cosmetics
Zinc oxide is frequently employed in the production of many raw materials used in medicine such as disinfection agents, and for dermatological applications due to its antibacterial properties and effective antifungal activity.[111] ZnO nanostructures are most commonly utilized in sunblock products to absorb UVA and UVB rays and provide defense against sunburns.[112] A recent study of nano/micro-composite made of nanosized TiO2 dispersed over ZnO micro-particles showed a greater sun protection factor (SPF) than individual TiO2 and ZnO particles.[113] Zinc oxide nanostructures have also been employed in the manufacturing of eye makeup products such as eye shadow.[114, 115] However, there has been some concern about the ecotoxicity of ZnO nanostructure use in sunscreens as their antimicrobial properties make them harmful in aquatic environments.[116] In addition, the long-term effects of inhalation of ZnO nanomaterials during the manufacturing phase of these products requires further investigation.[112] Therefore, while there is strong potential for ZnO nanostructures to be employed in many cosmetic products, further studies into the long-term toxicity are necessary for use in everyday life.
3 Optical Properties
Ultraviolet–visible (UV–vis) spectroscopy is a well-known, inexpensive instrumental method that allows for the determination of nanomaterial size, shape, and concentration. UV–vis spectroscopy is a simple quantitative technique to measure the transmittance or absorbance of light passing through the sample; the intensity of light through the reference is referred to as I0, and the intensity of the light through the sample is referred to as I. The Beer–Lambert law expresses absorbance, A, as a function of concentration C, as well as extinction coefficient ε, and the two intensities.[117] Absorption measurements can be done at a single wavelength or over an extended spectral range; the measured light transmittance is transformed into an absorbance measurement by following the Beer–Lambert law equation.
where α is the absorption coefficient, hv is the photon energy, n equals ½ for a direct transition (direct band gap semiconductors), and B is a material-dependent constant. The Tauc method can be used for all semiconducting materials that have negligible absorbance below the band gap energy; otherwise, significant distortion of the results can occur.[120] This is generally what happens for bulk or surface-modified and doped materials which can introduce additional broad absorption bands in the absorbance spectra caused from intraband gap states, providing unreliable estimations of the band gap energy.[120]
Singh et al. grew ZnO nanorod thin films on a glass substrate using the hydrothermal method.[121] Their absorbance showed a broad peak across a wavelength range between 350 and 375 nm. This allowed them to calculate the optical bandgap of the ZnO nanorod thin films using the Equation (1.5). A curve of (αhv)2 versus hv gives the optical bandgap. For bulk ZnO, the optical bandgap is 3.37 eV; while in this study, they found the optical bandgap of their thin films to be 3.23 eV due to the optical confinement effects of the formation of the nanorods.[122]
Another study done by Mwankemwa et al. demonstrated the effects of growth process on UV–vis spectra for organic solar cell applications.[123] In their study, they grew one sample of ZnO nanorods on an FTO substrate for one hour at 70 °C, while another sample followed the same steps; however, after an hour, they then dipped the same substrate into another identical precipitating growth solution in order to form nanoparticles on top of the grown nanorods. They found that the magnitude of absorbance was higher for the nanorods sample; however, the absorption peaks were blue shifted in relation to the nanorod/nanoparticle sample. In addition, they found the absorption peaks of the nanorod/nanoparticle sample to be significantly sharper than the only-nanorod sample. They determined the bandgap for each sample using the cut-off wavelengths and found the nanorod sample to have a bandgap of 3.34 eV, while the nanorod/nanoparticle sample had a bandgap value of 3.28 eV.
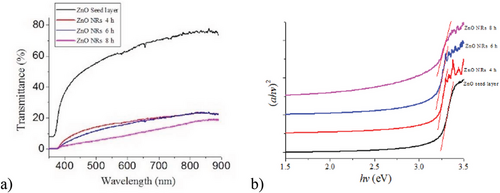
Idiawati et al. also used UV–vis to determine the effect of growth time on the optical bandgap of the prepared ZnO nanorods.[124] The characterization of the energy bandgap Eg was done using the equation mentioned above, determined from a plot between (αhv)2 versus hv, seen in Figure 6b. A thin energy bandgap was show in each film from the intersection between the line extrapolation and the x-axis, from interpretation of Figure 6b. The optical bandgap values of the ZnO nanorods were found to be 3.14, 3.12, and 3.05 eV, respectively. This is like the study by Mwankemwa et al., where the optical bandgap was lower than bulk ZnO. The bandgap also decreased with increased growth time due to reduction of surface area.[125]
4 Morphology and Crystal Structure
UV–vis spectroscopy can help researchers distinguish different concentrations and morphologies of ZnO nanomaterials. In a study done by Farhadi et al., they produced zinc oxide nanomaterials of different morphologies: nanoparticles, nanorods, and nanoflowers, using the sol–gel technique.[126] They used varying ratios of citric acid (CA) to ethylene glycol (EG), dissolved with zinc nitrate in water to produce the sol and then carry out the synthesis process. The EG:CA ratios were 2:1, 4:1, and 9:1. These concentrations produced different morphologies of ZnO nanomaterials that could be distinguished by the UV-vis. The product with the 2:1 ratio produced the nanoparticles (a) with a peak around 355 nm, while the nanomaterials produced from the 4:1 ratio solution formed the nanorods (b) with a peak around 368 nm. The highest concentration of ethylene glycol (9:1) produced flower-like nanorods (c) and the largest peak around 374 nm, which is comparable to the maximum absorbance ZnO bulk material at 375 nm.[127] The shift in peaks shown from the UV–vis can be a result of the quantum confinement which is caused from the change in mean particle size. Decreasing the size of the nanostructures increases the band gap; thus, the optical differences between the bulk and nano materials show great potential for photocatalysis and optical sensing. In addition, the shape of the peaks differs between morphologies. The peak associated with nanoparticles has a sharp edge and decrease, while the peaks associated with both nanorods and nanoflowers are broader and absorb across larger wavelengths. This is attributed to the size distribution of the nanostructures as the nanoparticles are of uniform size, while the nanorod and nanoflowers vary in length and width.
Scanning electron microscopy (SEM) is also used for determination of nanostructure morphology as images are produced by focusing a high-energy electron beam onto the surface of a sample, which then detects signals from the interaction between the incident electrons with the sample surface.[128] SEM images generate a characteristic 3D appearance that is beneficial for understanding the surface structure and morphology of a sample. During SEM analysis, the electron beam scans the specimen across its surface. The interaction between the electron bean and sample results in emission of various signals which are collected and processed by the detector.[129] SEM instruments detect three signals: secondary electrons for characterization of a sample, backscattered electrons for atomic number, and Auger electrons for the luminescent properties of a sample.[130, 131] SEM is beneficial for its ability to image a relatively larger area of samples (compared to TEM) and to image bulk materials such as nanorod arrays.
SEM is very useful when studying the effect different experimental parameters have on the morphology of ZnO nanorods. In a study done by Landry et al., they prepared ZnO nanorods on ITO substrates by hydrothermal synthesis, and the effects of an external magnetic field were studied.[32] The presence of the external magnetic field allowed for control over the morphology and resulting magnetic properties. In most cases, a Gauss meter could be used to determine the exact magnitude and direction of relatively small (smaller than 1 Tesla) applied magnetic fields. The Gauss meter worked by using a probe that contained a small conductor element on the tip where a small electric current was passed through.[132] The force of the magnetic field would push the electrons to one side of the conductor, producing a voltage with a magnitude directly proportional to the strength of the magnetic field.
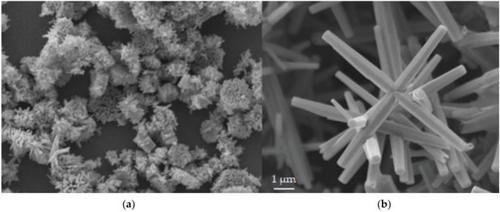
Shown below in Figure 7a, the application of a magnetic field of 850 Gauss caused significant bundling of the nanorod structures. The nanorods were well-aligned within each bundle; however, the bundles were not aligned with each other. The ZnO nanorods grown without a magnetic field are seen in Figure 7b, which appear more nanoflower-like. In relation to the rods grown with magnetic field, the 0G rods also appear significantly larger with an average length and width of 9.2 and 1.8 µm, respectively. The rods grown with a magnetic field had average dimensions of 0.66 µm in length and 0.17 µm in width. They attributed this large variation in size to the magnetic properties that seem to develop when the size of the ZnO structures is in the nanometer range. The magnetic behavior is thought to be developed from the large defects present when at nanoscale. Thus, the effects of varying the magnitude of the magnetic field can be seen directly using SEM.
Another study done by Yang et al., explored the different morphologies of ZnO nanostructures grown by varying the substrate.[133] In their study, they synthesized ZnO nanorods via hydrothermal method on Si wafer, Cu substrate, and Si wafer coated with a ZnO seed thin film layer by pulsed laser deposition. The SEM images showed that the nanorods grown on the bare Si substrate were pencil-like with a fine tip and grew in a disordered manner. On the Cu substrate, it was seen that the nanorods were dense and highly oriented with lengths of 1.2 µm and 300 nm in diameter. Last, from the SEM images, it was shown that the nanorods grown on the seeded Si wafer had the most densely aligned and oriented ZnO nanorod arrays with lengths of 1.4 µm and were closely packed together. It was demonstrated that the nucleation density was greatly increased when seeded initially, as opposed to growth on a bare substrate, and it promoted anisotropic growth and formation of aligned nanostructures.
Transmission electron microscopy (TEM) depends on the penetration of electron beam into the crystal sample instead of absorption as in SEM.[134, 135] In TEM, the incident electron beam from an electron gun is made to penetrate through the sample. Before reaching the sample, the electrons enter the condenser lens, which focuses the electrons into a controlled diameter.[136, 137] The focused beam interacts with the sample, and the X-ray emitted can be used to determine elemental composition. The beam that penetrates through the sample goes to the objective lens which delivers it to the projector lens, which is detected by the detector providing imaging. This instrument gives structural and morphological composition in addition to crystallographic data of the nanomaterial from the difference between the contrast of the sample and the background.[138, 139] The main difference between SEM and TEM is that SEM creates an image by detecting reflected electrons, while TEM uses transmitted electrons to produce an image. The samples must be very thin, mostly less than 150 nm, in order for the electrons to pass through.[140]
TEM imaging can be utilized to study the growth process of ZnO nanorods, as done in a study by Zhu et al.[141] In this study, ZnO nanorods were prepared via sol–gel method on lime glass substrates, with the annealing time varied. It had previously been postulated that the growth of ZnO nanorods happens out-of-plane, and the Ehrlich–Schwoebel (ES) barrier model explained the existence of an activation barrier on the (002) Zn surface.[142, 143] It was suggested that this barrier was overcome at higher temperatures and was weak, which would lead to layer-by-layer growth, and “whiskers” with a uniform diameter would form during the growth process. However, it was seen from their TEM images of a single ZnO nanorod microstructure from this method, that growth occurs in the (002) direction and the nanorod is smooth with no whiskers observed. The selected-area electron diffraction (SAED) which can be performed using the TEM, confirms the presence of single crystal. Therefore, this indicates that the nanorod is a perfect crystal with no dislocation, and this growth process cannot be explained by the previous theories. They found that at higher temperatures, more atoms have enough energy to overcome the barrier, and a multilayer growth process occurs to form mound-like structures.
In another study by Shabannia, the TEM was used to probe the effects of the heating time on the properties and quality of aligned ZnO nanorods.[144] Vertically aligned ZnO nanorods were grown on porous silicon substrates by chemical bath deposition and placed in an oven from 2 to 8 h at 95 °C. It was seen that the nanorods grown 5 h were most aligned and had uniform diameter, as well as the smoothest surfaces. Therefore, longer growth times do not necessarily allow for proper growth of nanorods than are required for certain applications.
X-ray diffraction (XRD) is one of the more significant characterization techniques which is used to reveal the crystal structure of nanomaterials based on their diffraction pattern. The number and type of atoms in the unit cell, as well as the size of the unit cell and the crystallite size, all typically contribute to the intensity of the XRD pattern. The diffraction pattern directly relates to the morphology of the crystal.[145-147] The morphology directly influences the intensity as both shape and orientation affect the measure of diffracted X-ray radiation dispersed from the crystal lattice; and therefore, the XRD peaks. In addition, the size of the crystal determines the XRD diffraction as smaller crystals tend to scatter the X-rays more than larger crystals.[148] The position of the patterns also provides information about the structural composition and lattice properties of nanostructures.
A study done by Kumar et al. demonstrates the effects increasing concentrations of the precursors (zinc nitrate and hexamine) has on the growth and morphology of ZnO nanorods.[149] In their studies, ZnO nanorods were hydrothermally grown on a stainless steel substrate at 100 °C for 3 h with increasing concentrations of 10, 20, and 30 mm. The XRD spectra taken for these experiments show an increase in peak size as well as becoming increasingly narrower, with increasing concentration. This shows more favourable growth conditions and higher crystal quality with increasing concentration. However, in a study done by Li et al., ZnO nanorods were also grown hydrothermally at increasing molar concentrations of 20, 40, and 60 mm, at 95 °C for 4 h.[150] Their XRD spectra showed that the reaction using 40 mm produced the highest intensity diffraction peak for (002) crystal orientation, as well as the narrowest peak, suggesting these nanorods had the highest crystallinity. Therefore, this implies the slightest change in reaction condition can have a major impact on nanorod growth.
5 Growth Mechanisms
One of the major aspects necessary for the actual realization and development of these applications is the ability to synthesize nanostructures of the required size with a controlled size distribution. There are many synthesis routes, with a great degree of variability in the conditions and parameters. The fundamental drawback of this is that the dependence of the average size and morphology of the produced particles on reaction parameters is not fully understood; therefore, the ideal reaction conditions are essentially determined empirically and instinctively. For that reason, tunability of various physical and chemical properties of materials by varying the size in the region of nanometers is essential for progression in several fields of current research.[151] This is done by understanding the various growth mechanisms and associated kinetics by which ZnO nanostructures grow in solution and on a substrate.
In general, the growth of nanostructures occurs following two processes: nucleation and crystal growth. The nucleation is when nuclei (seeds) are used as a template for nanocrystal growth.[152] Nucleation initially occurs in solution or on a substrate with a constant precursor concentration. Consumption of the precursor over time results in the growth of nanostructures. When this concentration diminishes past the level necessary for nucleation (supersaturation level), nucleation ceases and crystal growth is the dominant reaction.[153] The following sections describe the most commonly accepted ZnO nanostructure growth mechanisms.
5.1 Ostwald Ripening – LSW Model
In smaller particles, the surface-to-volume ratio is generally quite large. Due to this large surface area, the excess of surface energy plays an increasingly significant role in nanoparticles, contributing to a non-negligible portion of the total energy.[154] As a result, a mechanism of growth in which larger particles are formed at the expense of smaller particles reduces the surface energy for a solution, following the Gibbs–Thomson relation,[155] and would accordingly, play an important role in the growth of nanostructures.
The growth of nanoparticles is mostly controlled by the diffusion of “monomers” to the surface when diffusion is the slowest step in the growth process. The energy associated with an interface is related to changes in the chemical potential between molecules or atoms in an interfacial area and those in nearby bulk phases.[153] Based on the Gibbs–Thomson relation, the chemical potential of a solid particle increases with decreasing particle size at a solid–liquid interface. In addition, the smaller particles have a higher surface energy and area as well as solubility than the larger particles, which increases the solubility. Thus, at the interface of the smaller particles, the concentration of the molecules is larger than the average concentration in bulk solution; the result is a net flow of molecules from the smaller particle dissolving back into the solution phase, further shrinking the smaller particles and lowering the overall energy of the system. Conversely, for larger particles, the local concentration at the interface is lower than the concentration in the bulk solution, leading to a transfer of molecules from solution to the larger particles. Therefore, concentration gradients are created from variations in local equilibrium and act as a driving factor for larger particles to grow at the expense of smaller particles.[156]
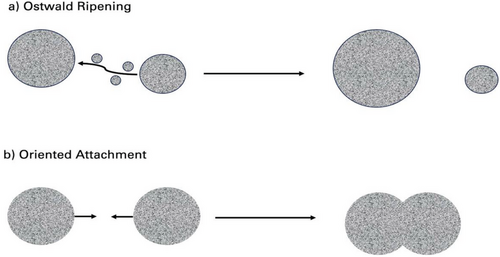
Coarsening effects that are limited by mass transport are often called the Ostwald ripening process, first described in 1900[157] and shown in Figure 8a below. The diffusion-limited Ostwald ripening process is the most prevalent mechanism of growth and was first quantified by Lifshitz and Slyozov,[158] followed by a related work by Wagner,[159] known as the LSW theory. The LSW theory was a major advancement in the Ostwald ripening theory and is based on a few assumptions.[160] First, the theory is based on the formation of spherical nanoparticles grown in a supersaturated solution. Second, there is an assumption that particles are dependent on the average concentration and the overall mass of the solute remains constant. Last, processes such as nucleation or aggregation that further introduce new particles can be considered negligible.
5.2 Oriented Attachment
Another important mechanism of nanocrystal growth was first proposed in 1998 by Penn et al.,[165] called “oriented attachment,” (OA) and can also be referred to as oriented assembly. Oriented attachment occurs where nanoparticles with shared crystallographic orientations spontaneously align their atomic lattices with adjacent nanoparticles and grow together.[166] Nanocrystal development frequently occurs simultaneously through several different methods, such as oriented attachment and coarsening, which can complicate the study of this process.[167]
The process of nanoparticle growth through oriented attachment occurs where nanoparticles initially collide to form loosely bound crystals that are randomly oriented. These nanostructures self-organize and align along their shared crystallographic orientation, seen in Figure 8b. Therefore, these aligned crystals become one larger crystal by epitaxy of two specific planar interfaces of each crystal.[168] This can lead to a variety of morphologies including wires, rods, prisms, and many irregular shapes.[169-172] The alignment of the planes eliminates high-energy facets, reducing the surface energy and leading to an overall reduction in the free energy of the system.[167] After completion of the nanostructure growth process, the original structural properties of the primary units as well as the porosity and interfacial defects on the surface can be observed.[173] This can allow for investigation into the details of the oriented attachment growth mechanism for specific types of nanostructures.
Previous studies have shown the oriented attachment process is the dominant mechanism in the early growth stages, when nanocrystal sizes are ≈10 nm.[174-176] At this time, crystallographic alignments with respect to each crystal are nearly perfect, which can be achieved through Brownian motion or short-range interaction. Generally, high temperature and pressure conditions are used by way of hydrothermal and solvothermal methods to produce sufficient energy for Brownian motion.[177] However, some studies have shown growth of nanowires through oriented attachment of nanodots at low temperatures (60 °C) as well.[178, 179] The oriented attachment growth occurring at the beginning of the growth process has a significant influence on the final morphology and properties of the materials; therefore, further understanding is required.[180]
5.3 Oriented Growth of the ZnO Nanocrystals
As mentioned above, the growth of nanostructures relies on nucleation and crystal growth. Understanding the mechanisms of growth that result from the synthetic methods and their various reaction conditions allows control over size and morphology, beginning with crystal structure. Morphology and crystallite size affect ZnO nanostructure applications as both have a large influence on their properties such as photocatalytic and antimicrobial activity.[181] As briefly mentioned above, reaction conditions play a large role in controlling nanostructure growth. One of these conditions includes the nature of the solvent as the structure and polarity of the solvent affect the growing crystals and the growth rates of each plane.[182] As mentioned previously, the zinc-terminated surface is the polar (001) plane which is positively charged; studies have shown that negatively charged species can adsorb onto this plane.[183] Thus, adsorption of these molecules inhibits crystal growth in this direction and along the c-axis. In addition, in polar solvents, the (001) surface strongly interacts with the solvent, leading to increased crystal growth perpendicular to that surface.[184]
One study showed that ZnO crystal size increased with decreasing polarity of the solvent, in which 1-hexanol had a higher growth rate along the c-axis (002) direction due to weaker interface–solvent interactions than ethylene glycol and water.[185] When water was used as a solvent, there were strong interface–solvent interactions with the polar (002) face, which led to branched nanorod formation. When using ethylene glycol as a solvent, the ZnO structures had a spherical morphology; attributed to the ZnO wurtzite structure which has a top tetrahedron polar zinc (001) face and a polar oxygen (00) face. In addition, the two hydroxyl groups on the ethylene glycol molecule could attach to the (002) face, further inhibiting growth in that direction. Nanoflowers with large surface areas were grown from use of water as a solvent. The morphology and crystal faces of the ZnO nanostructures had a large effect on the photocatalytic and antibacterial activities too. The ZnO nanoflowers grown in water showed the highest photocatalytic activity due to the large surface area and increase in the structural defects. These defects, oxygen vacancies, and zinc interstitials,[32] can serve as centers to trap electrons, thereby inhibiting recombination of electrons and holes. Furthermore, preferential crystal growth is regulated by the surface energy of each plane. In ZnO nanostructures, the polar (002) surface has a higher surface energy, resulting in increased oxygen vacancy formation, and in turn, higher catalytic activity. This result was also found to be the same for the antibacterial activity as larger surface areas increased the activity.
6 Kinetic Studies
In effective mass approximation, the mass of electron and hole are replaced with effective masses and an exciton wavefunction is considered to be confined to a spherical volume of the nanocrystal.[187] In Equation (1.6), the second additive term on the right-hand side refers to the additional energy caused by quantum confinement of the exciton based on “particle in a box” model, where the overall band gap energy depends on the size of the radius (r−2). The third term in the equation refers to the columbic interaction energy of the electron–hole pair (exciton), which is often negligible when the material has a high dielectric constant. The last term on the right-hand side of Equation (1.6) refers to the electron–hole spatial correlation effect that is independent of particle radius.[188] This term is also referred to as the surface polarization energy, which is caused from the difference between the dielectric constant of the material (ε) and permittivity of free space; ( ε0), thus, it is only a factor when materials have low dielectric constants.[189]
In this effective mass model, there are two limiting cases for the quantum confinement regime which are dependent on the ratio between the nanoparticle radius (r) and the effective Bohr radius of the exciton (aB), as demonstrated by Efros and Efros.[190] When the ratio r/aB is less than 1, this is considered the strong confinement limit, while when r/aB is greater than 1, it is considered the weak confinement limit. However, it has been found that Equation (1.6) does not provide an accurate estimation for very small nanoparticles (radius smaller than 1 nm) near the strong confinement limit as it overestimates the band gap.[191] This is a result from the assumption that in an ideal spherical nanoparticle, the excitons are confined to an infinite square-well. In nanoparticles with smaller radii, it has been found that the ground state energy of the excitons is considered to be confined by finite potential barriers, which has a significant impact on the resulting calculation and must be considered.[188, 189] In addition, due to the assumption of a spherical morphology, this approximation can be unreliable for nanostructures with large aspect ratios.
One of the first studies on the kinetics of ZnO nanoparticle growth was done by Wong et al., where they prepared nanocrystals from colloidal suspensions.[192] This was done using zinc acetate and sodium hydroxide in 2-propanol at varying temperatures for 2 h. Based on their UV–vis measurements and subsequent calculations using the Brus equation to find the radii of the nanoparticles, they used Equation (1.4) above: r3 – r03 = kt, and the Stokes-Einstein equation, . In the first equation, k is the experimentally found rate constant. From Equation (1.5) mentioned above, the rate constant can then be used to determine the diffusion coefficient D. In the latter equation, η is the viscosity of the solvent, a is the hydrodynamic radius of the solute, and kBT is the Boltzmann constant and temperature. The diffusion coefficients calculated from both equations were in good agreement; thus, the growth kinetics were found to be consistent with the Ostwald ripening mechanism and the particle distribution to follow the LSW model.
Likewise, Pesika et al. synthesized ZnO nanoparticles in the same manner, using octanethiol as a capping agent to quench growth after 15 min.[193] They first measured the absorbance spectra of ZnO nanoparticles without the use of a capping molecule. Their spectra were taken immediately after mixing zinc acetate and sodium hydroxide solutions and showed a well-defined characteristic ZnO peak indicating that nucleation and crystal growth occur quickly. The absorption onset shifts to a larger wavelength with time showing that the average particle size is in the quantum confinement regime. The growth of the particle radius with time is calculated. In solution phase synthesis, mechanisms such as aggregation and coarsening can compete with nucleation and growth in altering the particle size distribution. In their results, they show the time dependence of the particle size replotted as r3 versus time. After ≈30 min, the results show that over longer times, the increase in the size of the particles is determined only by diffusion-limited coarsening, according to the rate law determined by the LSW model, where larger particles grow at the expense of smaller particles. Furthermore, these results demonstrate that the supersaturation reaction is depleted, and growth is concluded after 30 min.
In 2007, Viswanatha et al. found the growth kinetics of ZnO nanocrystals differed from other ZnO semiconductor nanocrystals kinetic studies.[35] In their studies, they also synthesized ZnO nanoparticles using zinc acetate and sodium hydroxide in 2-propanol. At the time of their study, it was known that the growth of nanostructures in the absence of any capping agent would produce an exponent (n) between 2 and 3, where 2 is the limiting value where the surface reaction controls the growth and 3 is the limiting value when the growth is only diffusion limited. However, when they applied the rate law for Ostwald ripening, Equation (1.4), it was found the exponent of this equation did not follow the standard LSW model where n = 2–3; and in fact, their studies showed a range of exponent values from 4 to 8. They also observed that increasing the NaOH concentration decreased the average ZnO nanoparticle size. Based on the diffusion-limited Ostwald ripening process, this result was qualitatively different from the expected behaviors of the nanoparticles, where previously, the diameter was a function of time and temperature because diffusion rates are independent of reactant concentration at such small amounts. Thus, the growth of ZnO nanoparticles is actually hindered by increasing basicity of the reaction solution.
A study done by Hu et al. observed the influence of alcohol solvent on the growth of ZnO nanoparticles.[194] ZnO nanoparticles were synthesized by precipitation in five different n-alkanols from ethanol to 1-hexanol alcohols, which resulted in stable colloids of nanoparticles. UV–vis spectra were taken from 2 to 120 min for each, and were used once again to determine the absorption edge for each in order to determine particle size. From the graphs of r3 versus time, as per the LSW model, the reaction rate constant could be determined for each. For all alcohols, the kinetics of coarsening were consistent with this model. For ethanol and 1-propanol, nucleation and growth were much slower compared to longer chain length alcohols where nucleation and growth are fast. After the supersaturation had been depleted and nucleation and growth concluded, the continuous increase in average particle size was due to diffusion-limited coarsening. The coarsening rate constant for each synthesis increased with increasing temperature and longer solvent chain length due to the effects of surface energy, solvent viscosity, and the bulk solubility of ZnO. These results demonstrate that the solvent is a valuable factor in controlling particle size.
A similar study was done by Sikora et al. that also observed the effects different alcohol solvents have on ZnO nanoparticle growth using both water and NaOH as a reactant.[195] They applied particle size determination found from the absorption edge of the UV–vis spectra from 0–100 min and at temperatures from 25 °C to 65 °C. The absorption spectra showed a well-defined peak from absorbance onset that is characteristic for bulk ZnO, and in all cases, the absorbance onset was shifted toward shorter wavelength compared with bulk ZnO, demonstrating that the sizes of particles are in the quantum confinement regime. The absorbance onset shifts toward longer wavelength over time; and thus, with particle growth, for each of the applied solvents. They found that the ZnO nanoparticles were formed within 2 min and the size control of ZnO nanoparticles was difficult. At longer times, the apparent linear regions of r3 versus time plots were consistent with the LSW model for both reactants. This implies that a relatively fast nucleation and nucleation growth was followed by a slower period of nanoparticle growth determined by diffusion limited coarsening. Thus, the initial nucleation and growth were kinetically controlled, while the subsequent ZnO nanocrystals growth was thermodynamically controlled through the diffusion limited Ostwald coarsening. They found the rates of ZnO coarsening increased with number of alkyl group carbons on the alcohols and with temperature increase, similarly showing the importance of solvent viscosity, dielectric constants, surface energy, and the bulk solubility. They also found for all alcohols in the NaOH reaction, the observed activation energy was lower when compared to the reactions using water. These results are in good agreement, confirm the previous studies done by Hu et al., and demonstrate the importance of selecting a reactant in terms of controlling the kinetics of the nanostructure formation and size.
In a study done by Bell et al., UV–vis spectroscopy and transmission electron microscopy were combined to study kinetics of ZnO nanorod growth.[196] In this study, they first synthesized ZnO nanodots using zinc acetate and tetramethylammonium hydroxide (TMAOH) in 1,4 butanediol at 30 °C via sol–gel method. This process was considered a surface reaction-limited ripening based on the condensation of precursors. UV–vis spectra were taken periodically up to 1000 min, and the absorption onset data was plotted as a function of time. As well, assuming the LSW model for growth of nanoparticles through coarsening the rate, was determined from Equation (1.4). From this data, it was observed that the graph shows a rapid increase in particle volume up to 100 min, followed by a more gradual growth rate period. The initial increase was thought to be the period of nucleation and reaction of the zinc oxide–acetate intermediate phase and not part of the coarsening reaction. The nanodots were then diluted by solvent to a third of the initial concentration and annealed in an oil bath at both 90 °C and 120 °C for up to 196 h. The annealed ZnO nanodots transitioned to form nanorod morphologies where either end of the c-axis was non-symmetric, which was observed from TEM images. They also found the nanorod growth rates to be interesting, in that the rate was lower at 120 °C than at 90 °C, while the initial size was larger for the 120 °C study. The growth rate appeared to deviate from the normal model of activation energy-controlled growth. This indicated that the annealing process was affected more by the dissolution of the unstable nanodots and growth of nanorods as faceted particles. Therefore, it was found that the initial nanodot growth followed diffusion-limited coarsening, while the annealing process at elevated temperature exhibited a log(time) dependence, which also did not follow the usual mechanism of traditional diffusion-controlled Ostwald ripening.
Pacholski et al.[178] observed the growth of ZnO nanorods in methanol solution at 60 °C to follow oriented attachment mechanism. Nanoparticles were first grown from precursor concentration of 0.01 m, followed by dropwise addition of increasing precursor concentrations up to 0.1 m. They found the nanoparticles were aligning on the c-axis with minimal stacking faults, to produce nanorods with high aspect ratios. Similarly, Caetano et al. observed the growth of ZnO nanoparticles using NaOH, KOH, and LiOH at a temperature of 40 °C, in an absolute ethanol solvent.[36, 197, 198] The LSW model could fit the experimental data at the advanced stage. This indicates that diffusion-limited coarsening is the dominant growth mechanism and heavily influences the kinetics of the ZnO nanoparticle growth at a longer time. There was a deviation from the LSW mechanism under 30 min, where aggregate growth was observed, and this was associated to nanoparticle growth via a coalescence process (oriented attachment). Like the study by Bell et al., it seems ZnO nanoparticle growth does not just follow the LSW model of diffusion-limited Ostwald ripening but rather also has a more complex growth. It is also interesting to note that the various synthesis conditions and parameters have a huge role in the reaction kinetics, as each study, even when almost the same had different results. Therefore, the conclusions made from the previous studies on ZnO growth cannot be applied to every system as the growth mechanisms are truly dependent on reaction conditions and synthesis methods.
Studies have also been done to determine the reaction rates and activation energies of ZnO nanorod growth. Zhou et al.[199] analyzed ZnO growth in solution for the kinetic studies of ZnO nanostructure growth by using ICP to measure the Zn2+ concentration as a function of time. In their synthesis, zinc nitrate and hexamine were used as reactants in aqueous solution at varying temperatures from 55–85 °C for 3 h. At the time, the role of hexamine was known to serve as a buffer, providing a continuous supply of hydroxide ions and maintaining a low supersaturation level for nanorod growth.[200] A quasi-steady state assumption was made as they assumed the reaction between Zn2+ and OH- takes place rapidly, and the decomposition of the intermediate product (Zn(OH)2) to form ZnO was considered the control reaction. These assumptions allowed for them to fit a model to their experimental data, based on a previous model used for “aluminogermanate” nanotubes.[201] They found the concentration of Zn2+ to decrease following a first-order reaction model. As well, using Arrhenius law, the activation energy was determined to be 80.52 kJ⋅mol−1. However, the results from this study show that the concentration of Zn2+ can be fitted to a first-order reaction model if the nucleation can be ignored compared to crystal growth but only at low temperatures. This is because hexamine decomposes faster at high temperatures and results in a high supersaturation level in solution, beneficial for nucleation. Contrarily, at low temperatures, the hexamine hydrolyzes slowly, resulting in a low supersaturation level.
Ashfold et al. also did a study on the kinetics of the hydrothermal growth of ZnO nanorods.[202] In their work, nanorods were grown on a Si wafer hydrothermally using hexamine and zinc nitrate at a temperature of 90 °C for up to 10 h. The concentrations of Zn2+ and hexamine were determined by periodically taking aliquots of the solution for atomic absorption spectroscopy and proton NMR analysis. The Zn2+ concentration rapidly decreased for the first 2 h followed by a more gradual decrease up to 8 h. From their results, they determined the zinc ion concentration to follow a zero-order reaction model at t > 2 h, with a rate constant of 7.4 × 10−4 mol⋅L−1⋅s−1. Consequently, their results directly contradict the results mentioned in the previous study by Zhou et al., leaving the kinetic model of Zn2+ concentration in ZnO nanorod synthesis undetermined. However, they found the consumption of hexamine to follow a first order reaction model, with the first-order rate constant determined to be 0.059 h−1. From previous data, the rate constant for the decomposition of hexamine in a solution at 90 °C without zinc is 0.055 h−1.[203] The strong agreement between this value and that found experimentally for time above 2 h demonstrates that the rate of decomposition of hexamine is independent of the reaction that produces zinc oxide, implying that hexamine acts as a kinetic pH buffer.
7 Conclusions and Gaps in Knowledge
Depending on their morphologies, ZnO nanostructures have been used in various applications. Several analytical techniques have been used to investigate synthetic mechanism and tune the synthetic conditions by probing the resulting morphologies. It is also important to understand the growth and reaction kinetics involved in their synthesis. The growth mechanisms that have been proposed for the growth of ZnO nanostructures include Ostwald ripening based on the LSW model, as well as oriented attachment. The previous sections described the different analytical techniques that have been used to answer the questions related to kinetics and growth mechanisms of ZnO nanostructures in solution; however, the answers to these questions have varied greatly. There seem to be several apparently contradictory answers to the same question depending on a few conditions: synthetic method, reaction conditions, starting materials, and morphologies. These conditions and results are summarized in Table 1. With regards to ZnO nanostructure growth where the reactants are often zinc acetate and some sort of hydroxide (mainly NaOH) in an alcohol, the resulting morphology tends to be nanoparticles. In this case, UV–vis spectroscopy is the most commonly used technique for kinetic studies and is also used for particle size analysis based on the effective mass approximation proposed by Brus.[186] The consensus based on the varying studies is that the ZnO nanoparticles grow by way of Ostwald ripening at one point or another; however, it cannot be said that this mechanism is followed throughout the whole growth process. The more recent studies have demonstrated that in the initial stages of synthesis, oriented attachment is responsible for the growth of the ZnO nucleus. In turn, the process obeys the diffusion-limited Ostwald ripening mechanism.
| ZnO morphology | Synthesis method | Reaction conditions | Kinetics | Reference |
|---|---|---|---|---|
| Nanoparticle | Sol–gel |
Precursors: zinc acetate, NaOH Temp: 25–65 °C Solvent: 2-propanol |
Diffusion-limited coarsening, LSW model Not diffusion limited, NaOH concentration dependent |
[35, 192, 193] |
| Nanoparticles | Hydrothermal |
Precursors: zinc acetate, NaOH Temp: 30–60 °C Solvent: ethanol, 1-propanol, 2-propanol 1-butanol, 2-butanol pentanol, hexanol |
All solvents consistent with diffusion-limited coarsening, LSW model | [194, 195] |
| Nanoparticles, nanorods | Sol–gel |
Precursors: zinc acetate, tetramethylammonium hydroxide Solvent: 1,4-butanediol Temp: 30 °C, annealed 90 °C |
Nanoparticles: diffusion-limited coarsening, LSW model Nanorods: not diffusion-limited, log(time) dependence | [196] |
| Nanoparticles, nanorods | Sol–gel |
Precursors: zinc acetate, KOH Solvent: methanol Temp: 60 °C |
Oriented attachment | [178] |
| Nanorods | Sol–gel |
Precursors: zinc acetate, KOH, LiOH, NaOH Solvent: ethanol Temp: 40 °C |
t < 30 min: oriented attachment, t > 30 min: diffusion-limited coarsening, LSW model | [36, 197, 198] |
| Nanorods | Hydrothermal |
Precursors: zinc nitrate, HMTA Solvent: water Temp: 55–90 °C |
Based on Zn2+ consumption: First-order reaction Zero-order reaction |
[199, 202] |
Another result to note is the effect the starting materials and solvents have on the reaction kinetics and growth mechanisms. The nature of the hydroxide source also matters in the beginning stages of the oriented attachment mechanism. There is a higher coalescence from oriented attachment efficiency when the radius is smaller (LiOH) versus when the cation is larger (Na+, K+) as there is a shielding effect caused by the adsorption of the alkali solvated cation around the nanoparticle. This results in a quicker reaction rate and a more efficient preparation of the nanostructure with controlled size. As well, the solvent in which the reaction takes place affects the kinetics. Based on UV–vis data, it was found that nanoparticles grown in shorter chain alcohols (ethanol, propanol) have a much slower nucleation and growth compared to longer chain alcohols such as hexanol. The diffusion-limited coarsening following the LSW model has higher rates with increased temperature and increased solvent chain length in alcohols. These results demonstrate that solvent and reactant are an important reaction parameter in controlling the nanoparticle size.
When the ZnO nanostructures are grown using zinc nitrate and hexamine as starting materials, the products tend to be of the nanorod morphology. In this case, the growth mechanism is difficult to determine in solution; however, the kinetics of the reaction still can be by means of reactant concentration. However once again, the results of these analyses have varied. While the decomposition of hexamine in aqueous solution has been well-studied and found to be a first-order reaction, the kinetic model of Zn2+ in solution has yet to be officially determined. Although both studies grew ZnO nanorods hydrothermally using zinc nitrate and hexamine, Zhou and Deng found the concentration of Zn2+ in solution to follow a first-order reaction, while Ashfold et al. found the concentration to follow a zero-order reaction. The only major difference between the synthesis processes of the reactions is that the former study grew nanorods in solution, while the latter was done on a silicon wafer. However, it is unclear whether the presence of a substrate would have such an effect on the concentration of zinc in solution, especially considering that hexamine followed the same decomposition as in literature regardless of substrate and other reactants. Therefore, further studies need to be conducted to fully grasp how zinc behaves in solution, and which reaction parameters are the cause of such contradictions.
In conclusion, there is still room for further studies on this topic as many contradictions and variations in results exist. While there are a number of studies on the kinetics nanoparticle growth and only a few on nanorods, studies on the growth of other morphologies are lacking. Due to the variety of synthesis methods and reaction conditions, it can be hard to predict how changing the parameters would affect the growth of ZnO nanostructures. Many studies have been done on ZnO nanostructures; however, the results vary depending on the method used, how long the reaction took place, or what starting materials were used. In addition, the reaction kinetics change depending on these conditions. Therefore, a better understanding of the effects of the reaction conditions on nanostructure growth and resulting properties is required based on synthesis method of choice. What's more, much literature describes using a variety of synthesis methods that can be expensive and difficult to carry out, especially for applications in which they need to be readily produced and available for use.
8 Proposed Future Directions in Developing ZnO Nanorods
The study of ZnO nanostructures is still being thoroughly explored due to the distinctive and complex properties they possess, as well as their use in various applications with potential for many more. While there has been much progress in these aspects, their efficiency and reproducibility for applications such as solar cells still requires future work as the use of other semiconductors such as TiO2 provides better sensitivity and efficiency. In regard to ZnO-based sensors, there has been an issue of long-term stability. For ZnO gas sensors, adsorption of the target gas and other molecules in air on the surface of the nanostructures can have great effect on the conductivity and resulting sensitivity. There has also been some issue with selectivity as ZnO nanostructures are synthesized to have large surface areas promoting high reactivity, which can cause other species in air or surrounding environment to adsorb on the sensor. Adjusting the temperature at which the sensor is responsive or synthesizing the ZnO nanostructures with integrated nano-heaters can allow for direct control of the temperature, improving selectivity. There have also been much recent progress for use of ZnO nanostructures as biosensors, that has allowed detection of bacteria and fungi through changes in electronic signals without need for prior coating or doping the nanostructures. This will allow readily available devices for industry use, that are potentially easier and cheaper to mass produce. Furthermore, as ZnO nanostructures become increasingly prevalent in sunscreen and makeup uses, the toxicity and long-term effects of ZnO are necessary to understand for both the environment and personal health.
Use of ZnO nanostructures in these industry applications requires the development of a simple, cost-effective method for synthesis of ZnO nanorods on a substrate, that allows for tunability of morphology and properties and control over defects formed. Adjusting certain parameters can result in different morphologies with various lengths and diameters, as well as altering of the surface-to-volume ratio on the substrate. A detailed investigation into the effects of varying orientation, precursor concentration, reaction time, and application of external magnetic field should be performed. The goal should be to answer the following questions: How do each of these parameters specifically affect the morphology and size of the ZnO nanostructures grown on the substrate? How do these parameters affect the electrical and optical properties, and in turn, their utilization for the described applications? In addition, detailed kinetic studies should be performed using a variety of synthetic methods to determine why there has been a variation in results regarding reaction kinetics and growth mechanisms of seemingly similar processes.
For improvement of the current applications, it is necessary to have a full understanding of the relationship among synthesis conditions, concentrations of defects, and resulting properties. Answering these questions will help to achieve a clearer picture of ZnO nanostructure growth, which in turn, will allow for control over desired morphology and properties and further discovery of potential applications. A simple synthesis method of ZnO nanostructures with desired properties makes scaling up easier and more beneficial for industry use.
Acknowledgements
This research was funded by the Natural Sciences and Engineering Research Council of Canada to K.G.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Breanna Clarke is currently an M.Sc student at the University of Guelph. She received her B.Sc. degree at the Queen's University. Her research focuses on the growth kinetics and properties of zinc oxide nanostructures.

Khashayar Ghandi is a faculty member at the University of Guelph (Department of Chemistry) with research at the interface of material science, chemistry, and physics with applications in energy, health, and clean technologies. He is a council member of the Canadian Nuclear Society, co-chair of the Environment Waste Management and Decommissioning Division of the Canadian Nuclear Society, and the VP of Physical, Theoretical, and Computational Chemistry Division of Chemical Institute of Canada; he won the 2018-2019 Jean d'Alembert chair award. He has been the past elected president of the International Society for µSR Spectroscopy.




