Single-Molecule FRET Imaging for Enzymatic Reactions at High Ligand Concentrations†
Mitsuhiro Sugawa
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan)
Search for more papers by this authorSo Nishikawa
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan)
Search for more papers by this authorAtsuko Hikikoshi Iwane
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan)
Search for more papers by this authorVasudevanpillai Biju
Nano-Bioanalysis team, Health Technology Research Center National Institute of Advanced Industrial Science and Technology 2217-14 Hayashi-cho, Takamatsu, Kagawa, 761-0395 (Japan)
Search for more papers by this authorCorresponding Author
Toshio Yanagida
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan)
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan).Search for more papers by this authorMitsuhiro Sugawa
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan)
Search for more papers by this authorSo Nishikawa
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan)
Search for more papers by this authorAtsuko Hikikoshi Iwane
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan)
Search for more papers by this authorVasudevanpillai Biju
Nano-Bioanalysis team, Health Technology Research Center National Institute of Advanced Industrial Science and Technology 2217-14 Hayashi-cho, Takamatsu, Kagawa, 761-0395 (Japan)
Search for more papers by this authorCorresponding Author
Toshio Yanagida
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan)
Graduate School of Frontier Biosciences, Osaka University 7F Nanobiology Building, 1-3 Yamadaoka, Suita, Osaka, 565-0871 (Japan).Search for more papers by this authorWe thank K. Oiwa for the kindly gift of Cy3-ATP, T. Komori for helpful discussions, P. Kareginius for carefully reading the manuscript, and colleagues of the Yanagida Lab for suggestions and comments. This work is supported by a Grant-in-Aid for Scientific Research (to S.N.) and the “Yuragi Project” from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Graphical Abstract
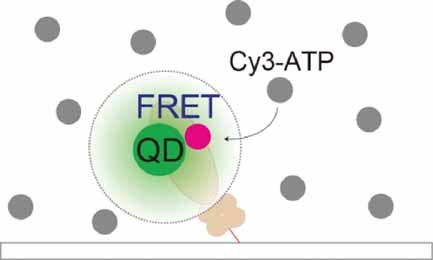
Single-molecule Förster resonance energy transfer (FRET) imaging at micromolar concentrations of an analyte is demonstrated. A quantum dot (QD)–dye donor–acceptor pair permits the approach of physiological levels of fluorescently labeled analytes when imaging molecular dynamics. Single-molecule FRET imaging of myosin ATP hydrolysis is performed using QDs attached to myosin as a donor enzyme and Cy3-labeled ATP as an acceptor ligand at micromolar levels (see image).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| smll_200901827_sm_supplfigs.pdf445.9 KB | supplfigs |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 C. Joo, H. Balci, Y. Ishitsuka, C. Buranachai, T. Ha, Annu. Rev. Biochem. 2008, 77, 51– 76.
- 2 Single-Molecule Techniques: A Laboratory Manual, (Eds: P. R. Selvin, T. Ha), Cold Spring Harbor Laboratory Press, New York 2007.
- 3 T. Funatsu, Y. Harada, M. Tokunaga, K. Saito, T. Yanagida, Nature 1995, 374, 555– 559.
- 4 M. Tokunaga, K. Kitamura, K. Saito, A. H. Iwane, T. Yanagida, Biochem. Biophys. Res. Commun. 1997, 235, 47– 53.
- 5 A. Ishijima, H. Kojima, T. Funatsu, M. Tokunaga, H. Higuchi, H. Tanaka, T. Yanagida, Cell 1998, 92, 161– 171.
- 6 K. Oiwa, J. F. Eccleston, M. Anson, M. Kikumoto, C. T. Davis, G. P. Reid, M. A. Ferenczi, J. E. Corrie, A. Yamada, H. Nakayama, D. R. Trentham, Biophys. J. 2000, 78, 3048– 3071.
- 7 T. Komori, S. Nishikawa, T. Ariga, A. H. Iwane, T. Yanagida, Biophys. J. 2009, 96, L4– L6.
- 8 K. T. Samiee, M. Foquet, L. Guo, E. C. Cox, H. G. Craighead, Biophys. J. 2005, 88, 2145– 2153.
- 9 P. R. Selvin, Nat. Struct. Biol. 2000, 7, 730– 734.
- 10 T. Ha, Methods 2001, 25, 78– 86.
- 11 A. N. Kapanidis, S. Weiss, J. Chem. Phys. 2002, 117, 10953– 10964.
- 12 U. Resch-Genger, M. Grabolle, S. C. Cavaliere-Jaricot, R. Nitschke, T. Nann, Nat. Methods 2008, 5, 763– 775.
- 13 I. L. Medintz, H. T. Uyeda, E. R. Goldman, H. Mattoussi, Nat. Mater. 2005, 4, 435– 446.
- 14 S. Hohng, T. Ha, ChemPhysChem 2005, 6, 956– 960.
- 15 T. Pons, I. L. Medintz, X. Wang, D. S. English, H. Mattoussi, J. Am. Chem. Soc. 2006, 128, 15324– 15331.
- 16 C. S. Xu, H. Kim, H. Yang, C. C. Hayden, J. Am. Chem. Soc. 2007, 129, 11008– 11009.
- 17 E. M. Galvez, B. Zimmermann, V. Rombach-Riegraf, R. Bienert, P. Graber, Eur. Biophys. J. 2008, 37, 1367– 1371.
- 18 F. Pinaud, D. King, H. P. Moore, S. Weiss, J. Am. Chem. Soc. 2004, 126, 6115– 6123.
- 19 M. Zhou, I. Ghosh, Pept. Sci. 2006, 88, 325– 339.
- 20 K. Boeneman, B. C. Mei, A. M. Dennis, G. Bao, J. R. Deschamps, H. Mattoussi, I. L. Medintz, J. Am. Chem. Soc. 2009, 131, 3828– 3829.
- 21 M. Nirmal, B. O. Dabbousi, M. G. Bawendi, J. J. Macklin, J. K. Trautman, T. D. Harris, L. E. Brus, Nature 1996, 383, 802– 804.
- 22 P. Frantsuzov, M. Kuno, B. Janko, R. A. Marcus, Nat. Phys. 2008, 4, 519– 522.
- 23 L. R. Rabiner, Proc. IEEE 1989, 77, 257– 286.
- 24 S. A. McKinney, C. Joo, T. Ha, Biophys. J. 2006, 91, 1941– 1951.
- 25 S. Hohng, T. Ha, J. Am. Chem. Soc. 2004, 126, 1324– 1325.
- 26 G. Luo, M. Wang, W. H. Konigsberg, X. S. Xie, Proc. Natl. Acad. Sci. USA 2007, 104, 12610– 12615.
- 27 M. J. Levene, J. Korlach, S. W. Turner, M. Foquet, H. G. Craighead, W. W. Webb, Science 2003, 299, 682– 686.
- 28 T. Miyake, T. Tanii, H. Sonobe, R. Akahori, N. Shimamoto, T. Ueno, T. Funatsu, I. Ohdomari, Anal Chem. 2008, 80, 6018– 6022.
- 29 J. Eid, A. Fehr, J. Gray, K. Luong, J. Lyle, G. Otto, P. Peluso, D. Rank, P. Baybayan, B. Bettman, A. Bibillo, K. Bjornson, B. Chaudhuri, F. Christians, R. Cicero, S. Clark, R. Dalal, A. deWinter, J. Dixon, M. Foquet, A. Gaertner, P. Hardenbol, C. Heiner, K. Hester, D. Holden, G. Kearns, X. Kong, R. Kuse, Y. Lacroix, S. Lin, P. Lundquist, C. Ma, P. Marks, M. Maxham, D. Murphy, I. Park, T. Pham, M. Phillips, J. Roy, R. Sebra, G. Shen, J. Sorenson, A. Tomaney, K. Travers, M. Trulson, J. Vieceli, J. Wegener, D. Wu, A. Yang, D. Zaccarin, P. Zhao, F. Zhong, J. Korlach, S. Turner, Science 2009, 323, 133– 138.