Artificial Humic Acid Diminishes the Effect of Drought on the Soil Microbiome
Abstract
Humic substances have an enormous potential for regenerative agriculture to improve soil quality and plant growth. Recently developed technologies called hydrothermal humification enabled the conversion of waste into artificial humic acids, that would allow for sustainable and large-scale applications. However, not much is known about the effect of artificially produced humic acid on the soil microbiome and its effect on drought-exposed soil. Therefore, we studied the effect of drought stress and artificial humic acid on the soil microbiota in sandy soil in a controlled experimental design. Analyses of 16S rDNA amplicon libraries by bioinformatics and statistics revealed that both drought and artificial humic acid application influenced bacterial community composition significantly, but only artificial humic acid affected bacterial diversity. Bacterial families like Pseudomonadaceae, Peptostreptococcaceae and Moraxellaceae enriched under artificial humic acid conditions, suggest an adaptation and selection of the soil bacterial microbiome. Under drought stress, artificial humic acid treatment kept bacterial diversity stable in the changed bacterial community composition. We propose that artificial humic acid application in sandy soil can improve the soil bacterial community, diminish drought stress, favour plant growth-promoting taxa, and bring enormous potential to sequestrate carbon in the soil.
1 Introduction
Anthropologic climate change is expected to increase extreme weather events like severe and prolonged droughts, creating substantial challenges for ecosystems worldwide (Lee et al. 2023). Increasing temperatures and erratic rain fall patters have the utmost influence on ecosystem functioning as well as global biodiversity loss (García et al. 2018; Richardson et al. 2023). Healthy soils are crucial for ecosystem resilience and stability, providing services such as nutrient cycling, water retention and filtering, and a habitat for microbial diversity (Lehman et al. 2015; Wall 2004; Falkowski, Fenchel, and Delong 2008; Kowalchuk, Jones, and Blackall 2008; Pritchard 2011; Singh et al. 2023). Healthy soils are crucial for ecosystem resilience and stability, including services such as nutrient cycling, water retention and filtering, and a habitat for microbial diversity (Lehman et al. 2015; Wall 2004; Falkowski, Fenchel, and Delong 2008; Kowalchuk, Jones, and Blackall 2008; Pritchard 2011; Singh et al. 2023). As soils serve as complex habitats for diverse microbial communities, drought stress can lead to significant shifts in microbial composition, impacting bacteria and fungi differently.
Drought stress significantly impacts soil health, altering its physical structure, nutrient availability, and microbial communities. While moderate droughts may promote adaptive responses such as increase tolerance to drought, severe droughts can cause more lasting damages, including reduced microbial activity and diversity (Geng et al. 2015). Further, the impact of drought stress on the soil microbiome is heavily influenced by soil quality (Kundel et al. 2020). One hypothesis is that physiological stress from drought may result in microbial mortality, which could result in reduced biomass and diversity. However, recent studies have shown contradicting responses regarding microbial diversity, community composition as well as the response of certain microbial species to drought conditions. For instance, Fuchslueger et al. (2014) observed that drought increased microbial biomass, and the effect of drought has been linked to changes in the relative abundance of certain taxa, particularly in increasing the abundance of drought-tolerant microorganisms. For example, Ochoa-Hueso et al. (2018) found an increased abundance of Actinobacteria in arid ecosystems, but also an increase of Chloroflexi. Additionally, drought increased the relative abundance of Acidobacteria but also decreased Proteobacteria (Sun et al. 2020).
The effect of abiotic stress like drought is usually accociated with a decrease in microbial diversity and a changed bacterial and fungal community composition (Hoefle et al. 2024). Since water is the driver of microbial growth its absence is detrimental and linked to diversity loss. Specifically, sandy soils are influenced more severely by drought since nutrient- and water-holding capacity is reduced due to a lack of small pore space. Further, bacteria and fungi seem to react differently to drought. Here, too, literature shows contradicting tendencies of drought effects. For example, Ochoa-Hueso et al. (2018) showed a higher sensitivity of fungi to severe drought, whereas Preece et al. (2019) state that bacteria were more affected than fungi. Additionally, drought altered bacterial and fungal community composition at more mesic sites and decreased bacterial diversity. Interestingly, bacterial diversity increased in soil with a history of drought (Ochoa-Hueso et al. 2018; Preece et al. 2019). Tóth et al. (2017) did not observe significant differences in bacterial alpha diversity between experimental drought and control treatment suggesting variability in the findings across the literature.
Organic matter like humic and fulvic acids are mainly associated with peat soils and are able to remain in the soil for long periods (Swaby and Ladd 1963). They are known for their positive effect on plant growth and fitness (Jin et al. 2024) and are also used to improve soil quality and increase agricultural productivity. Recently, Yang et al. (2019) introduced the hydrothermal humification process, which brings immense potential to produce fast and cheap artificial humic acid from crude, complex bio-waste at a large scale. The product is distinguishable, but very similar to natural humic acids. By slowly releasing essential nutrients to the surrounding area, natural humic acid supports the fitness of the soil's microbiome and diversity.
The effects of natural and artificial humic acid on the soil bacterial community are manifold, but all improve soil and plant health. Naturally occurring humic substances extracted, for example, from soils or sediments, have shown to improve soil fertility, increase biomass and microbial diversity and change the microbial community composition. For instance, Li et al. (2019) found that humic acid treatment showed a higher turnover rate in rare taxa compared to common taxa and was significantly correlated with copiotrophic and significantly negative correlated with oligotrophic taxa. However, recent literature shows contrasting effects; natural humic acid preparation of winter wheat increased the relative abundance of Gram-negative bacteria (Bezuglova et al. 2019), contradictingly, Li et al. (2022) found increased Gram-positive Mycobacterium and Crossiella and decreased Gram-negative Acidothermus, Bryobacter, and Acidibacter. Moreover, an enrichment of Oxalobacteriaceae due to applied Lignohumate, a conventional product containing humic and fulvic acids, resulted in an threefold increase in nitrogen fixation. By uncovering significant changes in specific taxa caused by natural humic acid also microbial community composition is affected. Additionally, in bayberry trees, humic acid showed a significant effect by increasing the diversity of microbial communities in rhizosphere soil (Ren et al. 2022).
By applying artificial humic substances in agriculture, expectations are high to accomplish similar or superior results as the ones derived from natural environments. However, literature and research are scarce about the effects of artificial humic acid on soil health and its microbiome. Artificial humic acid treatment was shown to significantly influence and increase fungal community richness and diversity in rhizosphere and non-rhizosphere soil (Ai et al. 2023). Thereby, Schizothecium and Arthrobotrys accounted for a high proportion in artificial humic acid treatment and fungal community composition was significantly changed due to artificial humic acid addition. Most recently, Wang et al. (2024) found that the bacterial composition in soil was significantly influenced by artificial humic acid and increased bacteria like Glutamicibacter, Enterobacteriaceae and Pseudomonas as well as growth-promoting fungi such as Mortierella.
In this study, we aim to tackle the following research questions: (i) What is the effect of drought on the soil microbiome? (ii) What is the effect of artificial humic acid on the soil microbiome? (iii) What is the effect of artificial humic acid on the soil microbiome in drought-stressed soil? We hypothesize that drought stress will impact the soil microbiome by decreasing bacterial diversity and changing community composition severely due to substantial changes in habitat. Artificial humic acid-treated soil is expected to increase bacterial diversity and affect bacterial community composition (Figure 1). We hypothesize that the soil microbiome is capable of using certain components of artificial humic acid as a carbon source and furthermore, artificial humic acid facilitates greater accessibility to nutrients. Under drought stress artificial humic acid should increase bacterial diversity. This expectation is based on our previous hypothesis that drought stress reduces bacterial diversity. We propose that the strong water retention properties of artificial humic acid can counteract this adverse effect. To obtain a comprehensive understanding of bacterial community changes under different conditions, we performed a cultivation-independent (16S rDNA amplicon barcoding) analysis of the bacterial microbiome and analysed the data by state-of-the art bioinformatic methods.
2 Methods
2.1 Experimental Outlines
2.1.1 Artificial Humic Acid Synthesis
The artificial humic acid synthesized in our previous study using the hydrothermal humification method (Marzban et al. 2024) was utilized in this work. Briefly, digestate manure and KOH were placed in a high-pressure hydrothermal reactor and processed at 240°C for 4 h. KOH was added as an alkaline agent and catalyst to facilitate the conversion of biomass to artificial humic substances, according to the carbohydrate content of the digestate, as described in detail in our previous work (Marzban et al. 2024). After the reaction, the reactor was cooled to room temperature, and the solid and liquid products were separated using flat filter paper (ROTH Type 113A-110, 5–8 µm). The liquid phase was then used for the extraction of artificial humic acid through an acid-extraction method, yielding pure artificial humic acid. More details on the production, extraction, characterization, and analysis of the artificial humic acid were reported in our previous publications (Ghaslani et al. 2024; Volikov et al. 2024).
2.1.2 Experimental Design
To investigate the effect on the soil of drought, artificial humic acid and the effect of artificial humic acid on drought-stressed soil we designed an experiment including artificial humic acid treated and untreated soil each exposed to drought and without drought (Supporting Information S1: Figure S1). Therefore, we included four treatments in the experiment: (a) control, (b) drought, (c) artificial humic acid and (d) artificial humic acid drought. All treatments consisted of the same sandy soil which was sampled in 2022 at our Field Research Station in Marquardt (Potsdam, Germany). This soil type is representative of the Brandenburg region, Germany; it is characterized as loamy sand (76% sand, 13% clay and 11% silt) and has a pH value of 6.75 as already described in Storch et al. (2023). For the experiment, we used column-like containers, including a sampling hole on the side and a water drainage hole with a sieve on the bottom. For each treatment we had nine replicates, each column-like container comprised 65 g of soil. Soil sampling was performed every 5 days for 20 days sampling on Days 1, 5, 10, 15, and 20, collecting 200 mg of soil from each column. The soil samples were stored at −20°C until further processing.
2.1.3 Soil Preparation and Artificial Humic Acid Application
Artificial humic acid treated soil contained in addition 0.01% artificial humic acid calculated on the soil dry weight. For application, we dissolved artificial humic acid in water by adding 1 M KOH until complete dissolution of artificial humic acid. Afterwards, we adjusted pH again to neutral with 1 M NaOH.
2.1.4 Irrigation and Drought Simulation
All soils were irrigated with 15 mL of sterile water in the beginning of the experiment and only the control treatments were irrigated again on Days 10, 15 and 20 (irrigation management, Supporting Information S1: Figure S2).
2.2 Dna Extraction, PCR and Amplicon Sequencing
To analyse the bacterial community, soil samples were mechanically lysed in a FastPrep (MP Biomedicals, California, USA) for 30 s and 6.5 m/s, and DNA was extracted with the FastPrep DNA Spin Kit for Soil (MP Biomedicals, California, USA) following the manufacturers manual. Thereafter, extracted DNA was used to generate an amplicon library of the 16S V4 rRNA gene using 515f and 806r standard primers according to the Earth Microbiome Project protocol (www.earthmicrobiome.org, Thompson et al. (2022)) with modifications which include Illumina adapters (www.illumina.com). The PCR reactions were performed in a total of 25 µL containing 12.5 µL KAPA HiFi HotStart Ready Mix (Roche, Basel, Switzerland), 1 µL of each primer (5 µM), 0.375 µL of mPNA and pPNA (50 µM), 8.75 µL of PCR grade water and 1 µL of DNA template. PCRs were performed with the following program: 3 min at 98°C, 35 cycles of 30 s at 95°C, 5 s at 78°C, 30 s at 53°C, 30 s at 72°C and the reactions ended with a final extension of 5 min at 72°C. A Biometra TProfessional Standard Gradient Thermocycler (Analytik Jena, Jena, Germany) was used to perform amplifications. Afterwards, all PCR products were purified with AMPure XP magnetic beads (Beckman Coulter, California, USA) following the instructions provided by the manufacturer. The second step PCR was performed with the Nextera XT DNA Library Preparation Kit (Illumina, California, USA). The total volume of each reaction was 50 µL containing 25 µL KAPA HiFi HotStart Ready Mix, 5 µL Nextera XT Index primers and 5 µL of DNA template of the first PCR. The PCR was incubated for 3 min at 95°C followed by eight cycles of 30 s at 95°C, 30 s at 55°C and 30 s at 72°C and ended with a final extension of 5 min at 72°C. PCR products were purified with AMPure XP magnetic beads (Beckman Coulter, California, USA) following the instructions provided by the manufacturer. DNA concentration was measured with a NanoPhotometer NP80 Mobile (Implen GmbH, Munich, Germany), and the samples were normalized and pooled together. Afterwards, all samples, including negative controls, were sent to Novogene UK Ltd (Cambridge, UK) and sequenced with an Illumina NovaSeq. 6000 (2 × 150 bp).
2.3 Bioinformatic Analysis and Statistics
Sequences were imported and processed in QIIME2 (Bolyen et al. 2019) as single-end sequences. DADA2 (Callahan et al. 2016) was used to filter, truncate reads and discarding chimeric reads. Taxonomy was assigned to the representative sequences using BLAST classifier (Camacho et al. 2009) and the SILVA v138 database for bacteria (Quast et al. 2013). Downstream analyses were done in R Version 4.4.0 in R studio (Posit Team 2024). We used negative controls and utilized the decontam v1.22.0 package (Davis et al. 2018) with the function isContaminant (method ‘prevalence’) to identify any contaminant sequences arising from reagents or introduced during the amplicon library preparation. Contaminant taxa, mitochondrial, chloroplast, archaea and eukaryotic were removed using phyloseq's v1.46.0 functions prune_taxa and prune_samples (McMurdie and Holmes 2013). We rarefied the data set to 10,000 reads with the phyloseq's function rarefy_even_depth. Bacterial diversity was calculated using estimate_richness function, ANOVA analysis was performed using the aov function from stats v4.4.0 package (Supporting Information S1: Table S1), the dplyr's summarise function was used to calculate diversity values and standard deviation (Supporting Information S1: Table S2), and the figures were plotted with ggplot v3.5.1 (Hadley 2016). To analyse bacterial community composition phyloseq_transform_css function from metagMisc package v0.5.0 was used to normalize with cumulative sum scaling and accounting for uneven sequencing depth. Bray-Curtis dissimilarity matrices (Bray and Curtis 1957) were calculated using distance function. Pairwise comparisons between the community composition of different soil treatments were done using the function adonis2 from the package vegan v2.6-4 package (Oksanen et al. 2023) (Supporting Information S1: Table S1). When we observed a significant effect, we tested this result for homogeneity between groups using the vegan's function betadisper followed by an ANOVA test on the dispersion result (Supporting Information S1: Table S3). To plot community composition RDA metrices were calculated using the vegan's rda function and then plotted using ggplot. To discover microbiome markers, we used edgeR (Robinson, McCarthy, and Smyth 2010) as implemented in the package microbiomeMarker (Cao 2021). Samples were normalized with cumulative sum scaling to adjust the calculated p-values p_adjust = ‘none’ was used, and bacterial families were defined as significantly different if the padjusted value was less than 0.001. Functional differences between control and drought stress, as well as artificial humic acid treated and untreated soil, were analysed using FAPROTAX (Functional Annotation of Procaryotic Taxa) and FAPROTAX v. 1.2.10 was used for analysis (Louca, Parfrey, and Doebeli 2016). The highest gene score was used as 100% to normalize calculated scores, and the bubble plot was plotted using ggplot2. We want to emphasize here on the limitations of FAPTROTAX, since it only uses current literature on cultured strains. Nevertheless, we aimed to interpret our 16S data and gain an additional layer of analysis regarding the functional potential.
3 Results
3.1 The Effect of Drought Stress on the Soil Microbiome
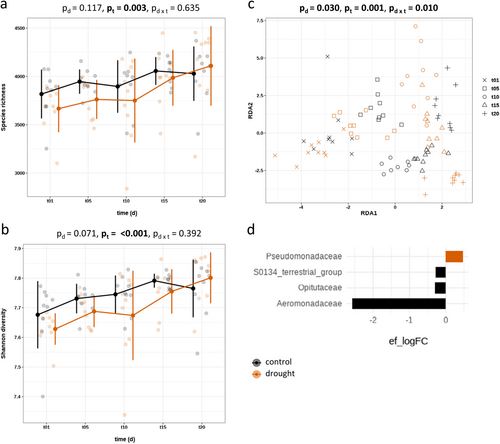
Drought stress had no effect on bacterial diversity, neither in species richness nor in Shannon diversity (Figure 2a,b). Nevertheless, bacterial diversity increased over time, regardless of irrigation management practices, and Shannon diversity increased 1.01-fold and species richness 1.06-fold in drought compared to control (Supporting Information S1: Table S2). Interestingly, drought stress increased bacterial diversity above the control treatment. It is important to note that drought stress had a significant effect on the bacterial community composition (p = 0.030, df = 1, F = 1.15) (Figure 2c). Linear discriminant analysis identified one bacterial marker in the family of Pseudomonadaceae which was enriched drought stress. Control treatment, on the other hand, enriched Aeromonadaceae, Optituaceae, and one unknown family level belonging to the S0134 terrestrial group (Figure 2d and Supporting Information S1: Table S4).
3.2 The Effect of Artificial Humic Acid on the Soil Microbiome
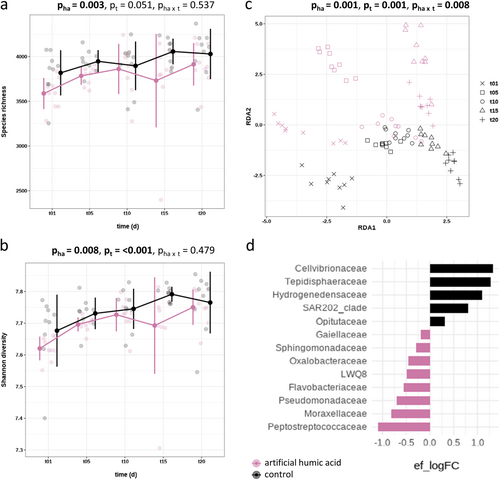
Artificial humic acid treatment in soil affected bacterial diversity in both species richness (p = 0.003, df = 1, F = 9.67) and in Shannon diversity (p = 0.008, df = 1, F = 7.41) (Figure 3a,b). Essential to note that the overall bacterial diversity was lower in the presence of artificial humic acid compared to untreated soil. Similar to drought treatment bacterial diversity increased with time, regardless of the application of artificial humic acid. Further, artificial humic acid influenced the bacterial community composition significantly (p = 0.001, df = 1, F = 1.50) (Figure 3c), and linear discriminant analysis showed an enrichment of 13 bacterial families (Figure 3d and Supporting Information S1: Table S4). Artificial humic acid-treated soil enriched Peptostreptococcaceae, Moraxellaceae, Pseudomonadaceae, Flavobacteriaceae, LWQ8 family, Oxalobacteraceae, Sphingomonadaceae and Gaiellaceae. On the other hand, untreated soil was found to be enriched by Cellvibrionaceae, Tepidisphaeraceae, Hydrogenedensaceae, SAR202_clade family and Opitutaceae.
3.3 The Effect of Artificial Humic Acid on the Soil Microbiome Undergoing Drought Stress
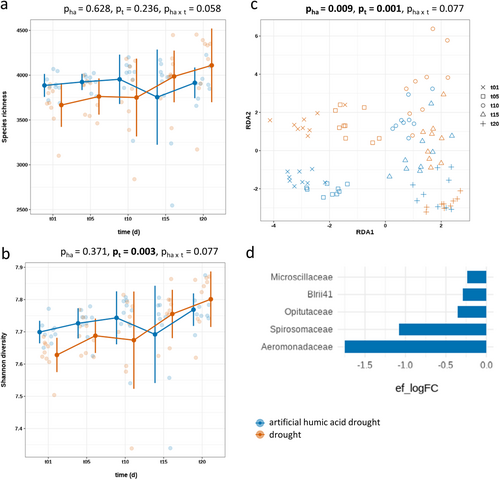
Under drought stress artificial humic acid did not affect the bacterial diversity in soil (Figure 4a,b). Over time, artificial humic acid-treated soil initially showed a higher bacterial diversity compared to untreated drought-stressed soil. However, between Days 10 and 15 the trend reversed and the bacterial diversity of untreated soil exceeded the bacterial diversity of artificial humic acid-treated soil. Further, under drought stress artificial humic acid treatment changed the bacterial community composition (p = 0.009, df = 1, F = 1.24) (Figure 4c). In artificial humic acid-treated soil under drought stress enriched various bacterial families like Aeromonadaceae, Spirosomaceae, Optitutaceae, Blrii41 family and Microscillaceae (Figure 4d and Supporting Information S1: Table S4). Untreated soil exposed to drought stress did not enrich any bacterial taxa.
3.4 Functional Prediction of the Soil Microbiome
After mapping the amplicon sequences to established metabolic/ecological relevant functions using FAPROTAX we found that drought or artificial humic acid treatment showed differences only in six bacterial functions. Artificial humic acid facilitated independent of drought thiosulfate and sulfur respiration, plastic degradation, nitrous oxide denitrification and dark sulfur oxidation (Supporting Information S1: Figure S3). On the other hand, drought showed functional differences compared to the other treatments in chlorate reducers and dark sulfur oxidation.
4 Discussion
In this study, we provide new insights about the effect of drought, artificial humic acid, and drought-stressed soil treated with artificial humic acid on the soil microbiome. We found that although drought did not affect bacterial diversity, it had a significant effect on the community composition. Artificial humic acid, on the other hand, only had a significant effect on the bacterial community composition of the treated soil. Artificial humic acid application to drought-stressed soil resulted in a changed community composition and an inverting effect of bacterial diversity, where artificial humic acid-treated soil kept bacterial diversity on a stable level compared to untreated soil.
Contrary to our expectation, drought only showed a significant effect on bacterial community composition but not on bacterial diversity. Although diversity was not affected, drought showed a slight increase in bacterial diversity over time. We postulate that this slight increase in bacterial diversity originates from drought stress induced changes in the bacterial community composition and, subsequently, an increase of rare, slow-growing taxa. Drought could inhibit fast-growing bacteria, giving new niches to the slow-growers. We support this hypothesis by the fact that drought changes soil physical properties by decreased soil aggregate stability and soil aggregated OC and N concentrations (Zhang et al. 2019) and, therefore, changes the habitat for microorganisms. Additionally, it was previously shown by Fuchslueger et al. (2014) that drought weakened the link between plant and bacterial C turnover, facilitating the growth of potentially slow-growing, drought-resilient soil bacteria. Further, we showed that functional capacity changed under drought stress, facilitating functions of chlorate reduction and dark sulfur oxidation. Microbial taxa, which are incapable of drought adaptation, may be have been replaced by better-adapted taxa, which become more dominant. Moreover, an increasing soil bacterial diversity correlates to soil with a history of drought compared to untreated soil, whereas short-term drought decreases bacterial diversity (Wang et al. 2021; Preece et al. 2019). Referring to the weather data of summer 2022 in Potsdam, Germany, we suggest that the soil we used had a history of drought, as 2022 was one of the warmest and driest years recorded in Europe (Martins et al. 2024). Differential abundance analysis showed an enrichment of Pseudomonadaceae in drought-stressed soil. We consider this accumulation of the Pseudomonadaceae family in drought-stressed soil as an adaption of the soil microbiota to drought and a potential beneficial effect for agricultural land usage. Earlier, Maestro-Gaitán et al. (2023) already showed an enrichment of Pseudomonadaceae in quinoa rhizosphere due to drought. Pseudomonas is one of the largest genera in the Pseudomonadaceae family and is widely known for being versatile and having beneficial traits like drought resistance and other plant growth-promoting characteristics such as increasing shoot and leaf biomass or photosynthetic activity (Roquigny et al. 2017; Rolli et al. 2015). Conversely, Metze et al. (2023) found Streptomycetaceae, Nocardiaceae, Nocardioidaceae, and Intrasporangiaceae enriched and positively responding to drought and further, reporting an inactivation of Proteobacteria of over 70%. Nevertheless, our results of enriched Pseudomonadaceae confirms previous literature, isolating Pseudomonas from drought-stressed (rhizospheric) soil (Nishu, No, and Lee 2022; Chandra et al. 2018; Kour et al. 2020).
Against our hypothesis, soil treated with artificial humic acid showed a lower bacterial diversity than untreated soil. Independent of artificial humic acid treatment, bacterial diversity increased with time, but was overall lower in artificial humic acid-treated soil. Additionally, the bacterial community was affected by artificial humic acid, and predicted functions increased in thiosulfate and sulfur respiration, plastic degradation, nitrous oxide denitrification and dark sulfur oxidation. Artificial humic acid application in soil probably initially releases harmful substances and has an initial negative effect on the soil microbiome by lowering the bacterial diversity, changing the bacterial community composition and metabolic functions. During the production process of artificial humic acid it is exposed to harsh chemical conditions like potassium hydroxide, temperatures of 240°C and hydrochloric acid (Marzban et al. 2024). We link the significant change in bacterial community composition to various enriched taxa, namely Peptostreptococcaceae, Moraxellaceae, Pseudomonadaceae, Flavobaceriaceae, Oxalobacteraceae, Sphingomonadaceae and Gaiellaceae. Peptostreptococcaceae is known for living in soils and was found in higher abundance in pen soils (Mills et al. 2021). It probably is able to use artificial humic acid as a carbon source for anaerobic fermentation since the bacterial family has been shown to be typically anaerobic with a fermentative metabolism (Slobodkin 2014). Moraxellaceae is a family characterized as aerobic or facultative anaerobic and chemoorganotrophic and found in soil (Huang et al. 2013; Fernández-Garayzába, Miguela-Villoldo, and Vela 2022). By enriching Moraxellaceae we propose that the artificial humic acid applied in the soil is similarly used and processed as natural humic substances since Moraxellaceae was previously identified as humic-reducing microorganisms during composting (Xi et al. 2016).
Artificial humic acid in drought-stressed soil did not affect bacterial diversity but significantly changed community composition. Bacterial diversity pattern showed a higher diversity in artificial humic acid-treated soil until Day 10, and afterwards, the pattern inverted, showing a higher bacterial diversity in untreated soil. Linking this pattern to the measured soil moisture (Supporting Information S1: Figure S2) we propose that artificial humic acid diminishes the effect of drought regarding bacterial diversity, even though affecting bacterial community composition. We suggest that the chemical and structural properties of artificial humic acid are responsible for that, showing similar characteristics as natural humic substances like mitigating the effects of drought, buffers pH and improving soil health and plant growth (Piccolo, Pietramellara, and Mbagwu 1996; Kıran et al. 2019; Pertusatti and Prado 2007; Gama-Rodrigues 2011). Regarding enriched taxa, drought stress enriched only taxa such as Aeromonadaceae, Spirosomaceae, Opitutaceae and Microscillaceae in artificial humic acid-treated soil. Rhamnose is a sugar occurring in bogs and we suggest also a part of the humic acid structure. Drought stress-exposed soil probably facilitates Aeromonadaceae to utilize sugars as a source of carbon, specifically rhamnose in artificial humic acid. Aeromonadaceae was shown to be the dominant family in a rhamnose-supplemented fermentation process using the gut-microbiome of the earth-worm Lumbricus terrestris (Meier, Hunger, and Drake 2018). Moreover, we hypothesize recruitment of Aeromonadaceae on account of artificial humic acid, potentially leading to an increase of drought-resistant Aeromonas sp. during drought stress. The molecular structure of artificial and natural humic acids consists, among others, of phenols like flavonoids (Burges et al. 1963) It was recently shown that the Arabidopsis root microbiome attracted preferentially Aeromonadaceae, which was linked to flavonoid-responsive taxa and flavonoid-induced chemotaxis as well as flagellum biogenesis. The identified Aeromonas sp. had plant-beneficial characteristics and was capable of enhancing plant drought resistance. As another enriched bacterial family, we found Opitutaceae, a proven antagonist towards Fusarium oxysporum, strongly correlated to cotton health and probably plant growth promoting (Li et al. 2015; Zhang et al. 2022). Consequently, we correlate the bacterial family Opitutaceae drought resilient and tolerant to artificial humic acid since Opitutaceae was also enriched in untreated soil, broadening the functional set of drought resistance in soil.
5 Conclusion
Our study provides first insights that artificial humic acid application in sandy soil can improve the soil bacterial community, diminish drought stress, and favour plant growth-promoting taxa. However, for a better understanding, we need to study microbiome functioning and interlinking in the long term. Considering the broader context of artificial humic acid application in agricultural soil we suggest a big potential for improving soil health and fitness. We showed that artificial humic acid affected the soil microbiome in a positive way, making the soil more resilient by favouring beneficial bacterial taxa. Furthermore, by bringing artificial humic acid into the soil without disturbing the soil's fitness and functions, it is possible to sequestrate enormous amounts of carbon from atmospheric CO2, mitigating anthropogenic climate change. Even more, there is a great potential to use artificial humic acid to restore bogs and wetlands and desert reclamation. Using artificial humic acid has the potential to massively improve agriculture, since carbon management in agriculture does not only boost crop yields but also promotes sustainable and resilient farming systems. Furthermore, artificial humic acid could replace peat and its exploitation, which has significant environmental drawbacks like habitat destruction and carbon emissions. Artificially produced humic acid offers a promising alternative to mimic peat's beneficial properties while mitigating environmental impacts. Given the main points, we summarize that the bacterial soil microbiome is capable of adapting to drought stress by changing bacterial community composition and favouring drought-resilient bacteria, even showing increasing patterns of bacterial diversity. Artificial humic acid treatment to soil results in an enrichment of various taxa, even selecting drought-resilient bacteria, some correlated to improved soil health. Considering artificial humic acid treatment during drought-stressed soil we propose a mitigating effect of increasing bacterial diversity due to drought. However, artificial humic acid could not avert the drought-induced change in bacterial community composition.
Author Contributions
Ahmed Abdelfattah, Gabriele Berg and Daniel Hoefle conceptualized the experiments. Nader Marzban produced the artificial humic acid. Daniel Hoefle and Sebastian Sperber performed the experiment and the sampling. Daniel Hoefle prepared amplicon libraries, performed bioinformatics analyses and wrote the first draft of the manuscript. Markus Antonietti, Gabriele Berg, Wisnu Adi Wicaksono, Markus Antonietti and Nader Marzban contributed to the interpretation of the results. All authors approved and contributed to the final version of the manuscript.
Acknowledgements
We want to thank Dr. Sebastian Vogel (ATB) and Dr. Benjamin Trost (ATB) for their helpful suggestions and advice when sampling at the Field Research Station in Marquardt, Potsdam. Further, we thank the technicians Beate-Kristin Kröck and Kerstin Mundt for their advice and help in the laboratory. We acknowledge that the graphic elements for Figure 1 were obtained from Freepik (www.freepik.com). This research was funded within the Project Save a tree in Park Sanssouci by Das Ministerium für Wissenschaft, Forschung und Kultur (MWFK).
Ethics Statement
The authors confirm that they have adhered to the ethical policies of the journal.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data set supporting the conclusions of this article will be available in the European Nucleotide Archive (ENA) repository with the accession number PRJNA1137280 upon acceptance of the manuscript. The code used in this study will be provided on Zenodo upon acceptance of the manuscript.




