Linking Surface and Subsurface: The Biogeochemical Basis of Cave Microbial Ecosystem Services
1 The Functional Basis of Cave Microbial Biodiversity
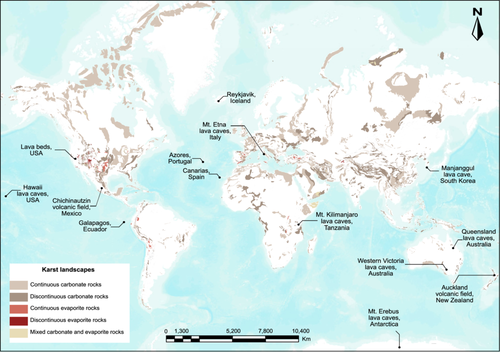
Most caves form by the dissolution of soluble rock (typically limestone or dolomite, but occasionally halite or gypsum), and occur within karst landscapes, where dissolution is the dominant geomorphic process (Ford and Williams 2007). Karst landscapes occupy approximately 20% of terrestrial ice-free areas globally and are major geomorphological features in North America, Europe, the Middle East, Asia and Australia (Figure 1) (Palmer 1991; Goldscheider et al. 2020; Chen et al. 2017). Caves also occur within insoluble rocks, where they form by a variety of processes. Caves within basalt lava flows form as internal conduits (tubes) (White, Culver, and Pipan 2019). Lava tubes are much less common than limestone caves but are found worldwide, scattered within basalt lava fields in every continent and on volcanic islands such as New Zealand, Hawaii, the Azores, Galapagos and the Canary Islands (Figure 1) (Espinasa-Pereña 2006; Greeley and Hyde 1972; Middleton et al. 2023; Webb 2023).
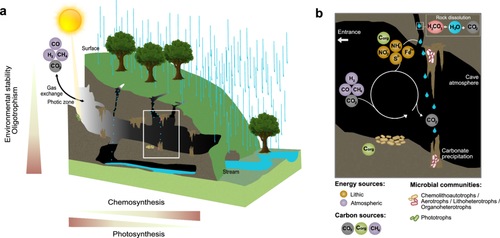
Cave environments are typically classified as oligotrophic ecosystems, where traces of surface-derived organic carbon and nutrients enter the cave via sinking streams or water percolation (Simon, Pipan, and Culver 2007; Ravn, Michelsen, and Reboleira 2020; Jones and Macalady 2016). Yet, despite this energy limitation, caves harbour diverse microbial communities which live on cave walls and speleothems (particularly, flowstone and rimstone dams) as biofilms and in allochthonous sediments on the cave floor (Figure 2). Dominant bacterial phyla frequently described in cave surveys include Pseudomonadota, Actinobacteriota, Acidobacteriota, Chloroflexota and Bacteroidota, while the more prevalent archaeon is Thermoproteota (Engel 2010; Zhu et al. 2019; Luis-Vargas et al. 2019). Recent studies have also identified fungi, especially those from the phylum Basidiomycota, which play a significant role in organic matter degradation and nutrient cycling (Martin-Pozas et al. 2022). Most microbial communities depend on the heterotrophic breakdown of allochthonous carbon sources for energy (Engel 2010; Stevens 1997). However, chemolithoautotrophs, bacteria and archaea, which couple the oxidation of inorganic compounds to CO2 fixation, have also been reported. As cave primary producers, these microorganisms play key roles in subterranean carbon and nutrient cycles (Zhu, Jiang, and Liu 2022). Conventional chemolithoautotrophs that are commonly reported include nitrifying microorganisms, such as ammonia-oxidising bacteria and archaea, as well as sulphide and iron oxidisers (Tetu et al. 2013; Ortiz et al. 2014; Chen et al. 2009; Jones and Northup 2021).
Caves provide microbial life with a relatively stable climate, which is characterised by relatively small variations in temperature and moisture content of the cave air (de Freitas and Littlejohn 1987). In the absence of atmospheric exchange with the surface, cave climates reflect the temperature and moisture content of their host rock. However, most caves frequently exchange air with the surface in response to changes in atmospheric pressure and air movements (Wigley 1967). This realisation has led to the discovery that, besides reduced nitrogen, sulphur, and iron compounds, cave bacteria also use reduced atmospheric gases as an energy source, such as hydrogen (H2), carbon monoxide (CO) and methane (CH4). At the surface, soil bacteria are a major biogeochemical sink for these trace gases, accounting for net losses of approximately 75%, 15% and 4%, respectively (Greening and Grinter 2022). Indeed, trace gas oxidisers are widespread across various grassland, forest, wetland and dryland ecosystems (Bay, Dong, et al. 2021), but little is known about their prevalence and ecological role in caves.
Methane has a global impact as a powerful greenhouse gas and its oxidation by cave microbes has received increasing attention. Indeed, high-affinity aerobic methanotrophs, bacteria which use atmospheric methane as both carbon and energy source, have been shown to rapidly consume this gas across diverse cave ecosystems (Cheng et al. 2022; Mattey et al. 2013; Nguyễn-Thuỳ et al. 2017; McDonough et al. 2016; Allenby et al. 2022; Waring et al. 2017; Fernandez-Cortes et al. 2015). These findings are supported by a preprint study showing that both aerobic high-affinity methanotrophs and hydrogenotrophs, bacteria which can couple the oxidation of atmospheric H2 to CO2 fixation, act as major primary producers (Bay et al. 2024). Reflecting the fact that the atmospheric energy source is used for carbon assimilation and fixation, the study termed these microbes as ‘aerotrophs’. The study further shows that cave aerotrophs are supported by other groups, such as nitrifiers and to a lesser extend sulphide oxidisers, to support energy requirements independent from surface-derived organic carbon sources (Figure 2).
2 Cave Microbial Ecosystem Services Drive Nutrient Cycling and Climate Control
Caves provide numerous provisioning, regulating, supporting and cultural ecosystem services (Goldscheider 2019). Most of these services are mediated by microbial communities which drive the biogeochemical processes underlying them (Table 1). In turn, these processes affect the air, water and soil of surface ecosystems through groundwater flows, aeolian transport and atmospheric exchange (Goldscheider 2019; Bennett, Peterson, and Gordon 2009). Supporting services, such as the microbial degradation of surface-derived organic matter, are relatively well understood (Simon, Pipan, and Culver 2007; Ravn, Michelsen, and Reboleira 2020; Dong et al. 2024). This process converts nitrogen and phosphorous from dissolved and particulate organic carbon into more usable forms, such as ammonium and phosphorus. These compounds can serve as the energetic basis for other processes, such as nitrification, or benefit surface ecosystems, which are supplied by groundwater flow (Ravn, Michelsen, and Reboleira 2020). However, services such as chemolithoautotrophic primary production, sustained by various lithic and atmospheric energy sources, are much less known. For example, bacteria and archaea involved in stepwise nitrification are important mediators of nitrogen cycling. They can use energy from the oxidation of ammonium to fix CO2 using various carbon fixation pathways (Tetu et al. 2013; Ortiz et al. 2014; Zhao et al. 2017; Silva Marques et al. 2018). Similarly, atmospheric trace gases can fuel hydrogenotrophic CO2 fixation and methanotrophic carbon assimilation, which provide a hidden energy source that can sustain primary production independent from sunlight. Overall, this suggests that cave ecosystems may be more productive than previously thought (Stevens 1997; Bay et al. 2024). While sulphur and iron oxidisers are less common in aerated caves, they are an important group of primary producers in caves where these substrates are abundant from either mineral or geothermal sources (Chen et al. 2009; Macalady et al. 2008).
| Type | Service | Main functional group | Cave | References |
|---|---|---|---|---|
| Supporting | Organic matter degradation | Organoheterotrophs/lithoheterotrophs | Karst and Basalt | Simon, Pipan, and Culver (2007); Ravn, Michelsen, and Reboleira (2020); Dong et al. (2024) |
| Chemosynthetic primary production/nutrient cycling/carbon sequestration | Chemolithoautotrophs (nitrifiers) | Karst and Basalt | Tetu et al. (2013); Ortiz et al. (2014); Zhao et al. (2017) | |
| Chemosynthetic primary production/nutrient cycling/carbon sequestration | Chemolithoautotrophs (sulphide and iron oxidisers) | Karst and Basalt | Chen et al. (2009); Macalady et al. (2008); Engel (2007) | |
| Chemosynthetic primary production/nutrient cycling/carbon sequestration | Aerotrophs (high-affinity aerobic methanotrophs and hydrogenotrophs) | Karst and Basalt | Bay et al. (2024) | |
| Regulating | Climate-active trace gas sink (CH4) | Aerotrophs (high-affinity aerobic methanotrophs) | Karst Caves | Cheng et al. (2022); Mattey et al. (2013); Nguyễn-Thuỳ et al. (2017); McDonough et al. (2016); Allenby et al. (2022); Waring et al. (2017); Fernandez-Cortes et al. (2015) |
| Climate-active trace gas sink (H2, CO) | Aerotrophs (high-affinity aerobic hydrogenotrophs); lithoheterotrophs (H2 and CO oxidisers) | Karst and Basalt | Bay et al. (2024) | |
| Climate-active trace gas sink (CO2) | Organoheterotrophs; aerotrophs (high-affinity aerobic hydrogenotrophs) | Karst and Basalt | Ortiz et al. (2014); Bay et al. (2024); Goldscheider (2019) | |
| Provisioning | Groundwater storage and filtration | Organoheterotrophs and lithoheterotrophs | Karst caves | Shepard and Gutiérrez (1999); Griebler and Lueders (2009) |
Among regulating services, caves are potentially a major overlooked biogeochemical sink for climate-active trace gases, including H2, CO, CH4 and CO2. Mediated by various lithoheterotrophic and chemotrophic processes, microorganisms use H2, CO and CH4 as ubiquitous and potentially unlimited energy sources to support both cellular maintenance and, in the case of ‘arotrophs’, primary production. Similar modes of energy conservation and primary production driven by trace gases have been described in other challenging ecosystems such as deserts (Ji et al. 2017; Bay, Waite, et al. 2021; Jordaan et al. 2020; Ortiz et al. 2021; Ray et al. 2022). However, caves appear unique in that they facilitate these processes continuously, reflecting the relatively stable cave climate. Caves also act as major sinks for CO2. The uptake is primarily driven by chemoautotrophic CO2 fixation and the interaction of atmospheric CO2 from various sources, including microbial respiration, with meteorological water, forming carbonic acid, which reacts with carbonate rock to form dissolved calcium cations and bicarbonate anions (Figure 2) (Goldscheider 2019). Moreover, karst systems are crucial in many regions as underground water reservoirs where microorganisms drive provisioning services such as the degradation of pollutants (Goldscheider 2019). Overall, these microbial ecosystem services form a connection between surface and subsurface ecosystems which are central to climatic regulation, agricultural productivity and biodiversity.
2.1 Linking Surface and Subsurface Ecosystems
Over five decades ago, Thomas Poulsen and William White first asked in their visionary paper entitled ‘The Cave Environment’ how limestone caves can serve as unique ecosystems to observe the interaction between biological and geochemical processes (Poulson and White 1969). Since then, the study of the microbial ecology of cave ecosystems has rapidly expanded, showcasing the taxonomic and metabolic diversity of these unique ecosystems. However, there are still significant research gaps, in particular, understanding how microbes utilise various organic and inorganic energy sources, how these metabolic processes change according to substrate availability and how this energy is allocated towards maintenance and growth (Mammola et al. 2020). Furthermore, it is unclear to what extent cave microbes assert their metabolic influence on surface processes and how these processes can be integrated into nutrient and carbon balances on an ecosystem or global scale. One major challenge of drawing generalities is the complexity involved in conducting systematic ecological surveys and metabolic activity measurements of cave microbiota. This mainly reflects the unique physical formation, structure and landscape features of each cave, as well as climatic influences, surface vegetation and hydrological patterns.
Accounting for this variation requires a systematic sampling approach, linking the downstream analysis with microbial taxonomy, metabolic function and environmental parameters to understand caves as essential biological systems. Microbial ecology studies in caves need to integrate culture-independent omics analysis (i.e., metagenomics, metatranscriptomics and metaproteomics), the physicochemical analysis of the cave's matrices (i.e., sediments, mineral surfaces and water) and the measurement of atmospheric exchange rates and nutrient fluxes to upscale metabolic processes. To capture environmental and biological spatial variation, studies should measure the three-dimensional spatial structure of cave environments with laser-based surveying tools. Together, such insights could reveal the biogeochemical and ecological basis governing cave microbial communities, their metabolism and contribution to ecosystem function.
Hidden below ground, cave ecosystems are mostly overlooked in biodiversity, climate and nutrient cycling assessments. Understanding the biogeochemical basis of cave microbiota is an important step towards quantifying metabolic processes and tracking the connection between surface and subsurface ecosystem services. By linking cave microbial diversity and activity to surface processes, we gain a more holistic understanding of ecosystem connectivity, stability and resilience in the face of environmental challenges, such as climate change. Filling gaps in carbon and various nutrient budgets would enhance the robustness of future climate predictions. Importantly, studying these processes also offers unique opportunities for interdisciplinary insights, blending approaches from atmospheric chemistry, geochemistry, geomorphology, ecology and microbiology. Various methodological innovations from these disciplines would provide a comprehensive framework to not only capture biodiversity information, but to establish linkages to specific processes occurring at various interfaces between sediments, rocks and the atmosphere. In doing so, we can improve our system-based understanding of both subterranean and surface realms and protect these critical ecosystems which are essential to safeguarding global environmental and human health.
Author Contributions
S.K.B. conceptualised the article, writing, reviewing and editing. M.N.L.V. writing of original draft, writing, reviewing and editing, visualising main figures. J.W. and S.W. reviewing and editing.
Acknowledgements
We thank the reviewers and Professor Chris Greening and Dr. Pok Man Leung for helpful discussion. M.N.L.V is supported by a La Trobe PhD Scholarship and S.K.B. is supported by an Australien Research Council Discovery Early Career Research award (DE230101346). Open access publishing facilitated by La Trobe University, as part of the Wiley - La Trobe University agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.




