Soil Bacterial Biodiversity in Drylands Is Dependent on Groundcover Under Increased Temperature
ABSTRACT
Introduction
Drylands are a major terrestrial biome, supporting much of the earth's population. Soil microbial communities maintain drylands’ ecosystem functions but are threatened by increasing temperature. Groundcover, such as vegetation or biocrust, drives the patchiness of drylands' soil microbial communities, reflected in fertile islands and rhizosphere soil microbial associations. Groundcover may shelter soil microbial communities from increasingly harsh temperatures under climate change, mitigating effects on microclimate, but few data on the microbial response exists. Understanding the fine-scale interactions between plants and soil is crucial to improving conservation and management of drylands under climate change.
Materials and Methods
We used open-top chambers to experimentally increase the temperature on five key groundcover species found in arid Australia, and are commonly present in drylands worldwide; bareground (controls), biocrust, perennial grass, Maireana sp. shrub, Acacia aneura trees, testing soil bacterial diversity and community composition response to the effects of increased temperatures.
Results
We found that groundcover was a stronger driver of soil bacterial composition than increased temperature, but this response varied with groundcover type. Larger groundcover types (Acacia and Maireana) buffered the impact of heat stress on the soil bacterial community. Bacterial diversity and species richness declined with heat stress affecting the bacterial communities associated with perennial grass, Maireana and Acacia. We identified 16 bacterial phyla significantly associated with groundcover types in ambient treatment. But, under heat stress, only three phyla, Verrumicrobiota, Patescibacteria, and Abditibacteriota, had significantly different relative abundance under groundcovers, Acacia and Maireana, compared to bareground controls. The soil bacterial community associated with perennial grass was most affected by increased temperature.
Conclusion
Our findings suggest soil communities may become more homogeneous under climate change, with compositional change, rather than diversity, tracking soil response to heat stress.
1 Introduction
Arid, semi-arid, and sub-humid regions (drylands) make up 47% of the world's land area (UNEP 1992). Drylands form the second largest terrestrial biome, supporting a third of global diversity hotspots and a quarter of plant diversity hotspots (Maestre et al. 2012; Millenium Ecosystem Assessment 2005). They have variable low rainfall and high temperatures, making them highly vulnerable to the increasing temperature and rainfall variability of climate change, with increasing intensity and duration of extreme dry periods (Maestre, Salguero-Gómez, and Quero 2012; Trenberth et al. 2014). Under increased climate change, warming in drylands is predicted to double that of temperate regions (Huang 2016). Despite this, dryland response to warming is underrepresented in global climate research (Haaf, Six, and Doetterl 2021).
Groundcover shelters soil, buffering temperature effects (Lortie et al. 2022). Under climate change, microclimates buffered by groundcover will become increasingly valuable in drylands (Beugnon et al. 2024). Fertile islands form beneath plants, where nutrients and soil moisture concentrate (Garner and Steinberger 1989; Ward et al. 2018), with open bare spaces between, often dominated by biocrusts (Rodriguez-Caballero et al. 2018; Valentin, d'Herbès, and Poesen 1999). Foundation plants such as shrubs and trees in drylands mediate impacts of drought on soil moisture (Lortie et al. 2022), most strongly the trees (Lozano-Parra et al. 2018). The relationship between groundcover and soil is integral to understanding future projected effects of climate change (Berdugo et al. 2019; Gilad et al. 2004). Groundcover type influences soil community composition and function through cover type-specific associations (Eldridge, Mallen-Cooper, and Ding 2021; Ferrenberg et al. 2018; Toledo et al. 2022; C. Trivedi et al. 2019). There is considerable complexity in arid landscapes, with different soil communities patchily distributed and varying in diversity and chemistry (Chamizo et al. 2022; Zuo et al. 2016). These dryland soil communities include complex adapted networks of invertebrates, fungi, bacteria and protists (Delgado-Baquerizo et al. 2020). Under global warming, groundcover is threatened, with changes to hydrology and temperature shifting vegetation communities and affecting soil moisture (Tietjen et al. 2017), potentially changing the soil community and its functioning (Kardol et al. 2010).
Increased atmospheric temperature of 1.5°C on average is projected to disrupt biogeochemical cycles (Intergovernmental Panel on Climate Change IPCC, 2022; Rockström et al. 2009), such as carbon, nitrogen and hydrology (Campbell et al. 2009; Chen et al. 2015; Finzi et al. 2011). This temperature does not account for soil temperature changes. Soil temperature can differ by 10°C, compared to atmospheric temperature, around the world (Lembrechts et al. 2022), with groundcover vegetation type creating microclimates buffering temperature differences (De Frenne et al. 2019; Huang et al. 2024; Xiao, Ma, and Hu 2019). Under extreme dry conditions, such vegetation reduces high air temperatures and soil moisture loss (Lortie et al. 2022), which is linked to evapotranspiration and rainfall in drylands (Zhou et al. 2021). Importantly, high plant diversity can increase buffering and stabilisation of soil temperature (Beugnon et al. 2024; Huang et al. 2024). Current understanding is predominantly confined to shelter effects of shrubs or trees on ambient temperature in temperate climates, with less understanding of other groundcover types, including perennial grasses and biocrust. While specific microbial associations with groundcover types are known to occur (Toledo et al. 2022), how and the degree to which they vary between groundcover types remains poorly known.
Variation in groundcover drives functional variation across landscapes, driven by its associated soil communities (Eisenhauer et al. 2018). Increasing aridity from climate change is known to change soil communities at a landscape scale (Delgado-Baquerizo et al. 2020), but there is a need for fine-scale experimental analyses of the effects of temperatures on soil biodiversity, testing effects of groundcover types. Dryland soils are dominated by key bacterial phyla, such as Proteobacteria, Actinobacteria, Acidobacteria (Delgado-Baquerizo et al. 2018; Eldridge et al. 2018) but how heat stress might change dominant phyla or shift composition remains unknown.
We tested the effects of increased temperatures on soil bacterial community composition and diversity, using a field experiment in semi-arid Australia with five different groundcover types. To our knowledge, this is one of the first experimental field studies in drylands examining how groundcover type may influence soil bacterial community in the face of climate change. We increased ambient temperature of different groundcover types, using open top chambers (OTCs). The five groundcover types were typical and common in dryland communities in Australia and around the world, including bareground (as a control for no groundcover), biocrust, perennial grass, Maireana sp. (shrub), Acacia aneura (tree). Using a paired design, we compared the soil bacterial diversity and community composition with and without increased temperatures. While soil microbial communities contain bacteria, archaea and fungi we focused our study on bacteria which is the dominant part of the community and faster to respond to changes (Delgado-Baquerizo et al. 2017). Previous studies have focused on phylum level changes and here we wanted to examine if there were specific families within phylum that responded to increased temperature and could be potential indicator taxa. Specifically, we had three objectives: (1) to differentiate whether temperature or groundcover was a greater driver of soil biodiversity; (2) to test whether soil bacterial heterogeneity was affected by temperature increase and (3) to identify which of the five groundcover types most effectively buffered the impacts of increased temperature on the soil bacterial community.
2 Materials and Methods
2.1 Site Description
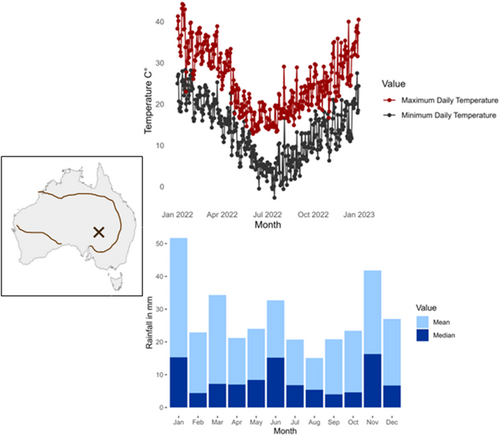
We conducted a field experiment at Fowlers Gap Arid Zone Research Station (−31.0871923, 141.7051727, 400 km2) in semi-arid Australia (Figure 1), a landscape of grass plains, rocky slopes, and woodlands and vegetation, dominated by shrubs in the Chenopodiaceae family and driven by rainfall with long dry periods (Beadle 1948). Rainfall is low and variable, averaging 241.9 ± 72.7 mm of annual rainfall (Fowlers Gap AWS 2004–2023, Bureau of Meteorology), falling mainly in the summer (November to February). Temperature varies from 14°C in winter (June to August) to 40°C in summer (Figure 1).
2.2 Experimental Design
Using a stratified approach, we selected five sites with similar habitat (Figure 2A). At each site, a 25 × 25 m plot was established, with the five groundcover types—bareground, biocrust, perennial grass (hereafter grass), Maireana sp., Acacia sp. To test the buffering potential of groundcover types on soil temperature, we implemented a paired design, with a ‘heat stress’ treatment using an OTC (Figure 2A), placed over the groundcover types, except for the Acacia treatment, where it was placed at the base (Figure 2B). A matching ‘ambient’ treatment, with no OTC within 2 m of the heat treatments and marked with a flag (Figure 2B).
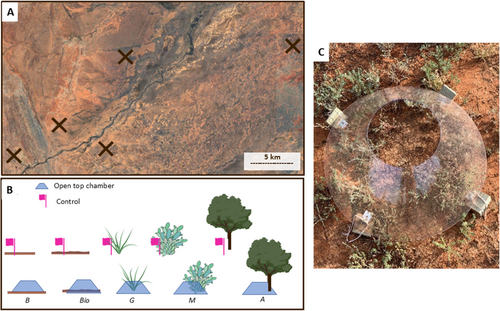
OTCs made of clear plastic were designed to increase the ambient temperature inside by 1°C–3°C, based on similar experiments (Escolar et al. 2012; Maphangwa et al. 2012) (Figure 2). Dimensions were 75 cm in diameter and 45 cm high with a 30 cm opening for rainfall and direct sunlight (Figure 2) and were installed with a 5 cm space between the chamber and the ground, allowing air flow. These were installed in November 2021 and maintained for a year, at the five sample sites, after which we collected 200 g of soil from the top 5 cm of each sample which was placed into a zip lock bag (n = 50, 5 × groundcover types, 2 × treatments, 5 × sites). Only soil directly below the OTC opening was sampled, where rainfall could enter. Ambient treatment soil samples were taken directly at the base of the groundcover or over a similar 30 cm area for the biocrust cover. Samples were aseptically collected and refrigerated during transport and in a Sydney laboratory, until DNA extraction, which was performed within 4 weeks of collection.
We measured soil surface temperature on bareground, using ibuttons (model DS1921G#F50), for both heat stress and ambient treatments for the first 3 months. The ibuttons measured soil surface temperature every 4 h, of which we took the minimum and maximum temperature of each day. We compared these data to daily minimum and maximum ambient temperature (1.2 m off the ground), measured by the weather station at Fowlers Gap (BOM 41268, Figure 1). To compare surface temperature variation between groundcover types, we also randomly measured across all sites, soil surface temperature of all groundcover types (bareground, biocrust, perennial grass, Maireana, Acacia, n = 8 replicates × 5 groundcover types = 40) at ambient treatment sites for a single timepoint (mid-late morning), using a handheld digital thermometer to a depth of 1 cm.
2.3 DNA Extraction and Sequencing
DNA from the soil samples collected at the end of the trial was extracted using the DNeasy PowerSoil Kit (Qiagen), according to manufacturer instructions. This required selection of 2.5 g of soil from each sample (n = 50), avoiding plant and rock material. Extracted DNA concentration was checked via NanoDrop (Thermo Fisher). We targeted the 16S ribosomal RNA V3-V4 region for bacteria (341f-805r) (Klindworth et al. 2013), by preparing samples and using PCR, and amplicon sequencing, using the Illumina MiSeq. 2 × 300 bp platform. For each sample, paired-end reads were then quality checked, filtered, and amplicon sequence variants (ASVs) produced, using the DADA2 pipeline (Callahan et al. 2016). We used the SILVA 138.1 database (Quast et al. 2012) to assign the taxonomy of bacteria, cyanobacteria, and archaea.
2.4 Statistical Analysis
A one-way analysis of variance (ANOVA) was used to compare soil temperature between groundcover and bareground, with a Tukeys post-hoc analysis identifying which groundcover types were significantly cooler than bareground. A two-way ANOVA with pairwise analysis was also used to compare the bareground ambient and heat treatment soil temperature taken over 3 months to the ambient air temperature. After checking normality of distribution, we compared the minimum and maximum of each category to assess if the heat treatment was significantly greater than the ambient soil temperature and if this was significantly greater than ambient air temperature. To identify whether groundcover type or heat stress had the strongest influence on the soil bacterial community, we used PERMANOVA to assess drivers of the differences in ASV's between samples. To visualise separation, we used Bray-Curtis dissimilarity for relative abundance of ASV's and plotted these using nMDS with the ellipse function to highlight clustering in ‘vegan’ (Oksanen et al. 2007).
To assess how soil bacterial heterogeneity changed with the heat treatment, we compared the composition and diversity associated with each groundcover type to the bareground community. We used a multi-variate additive general linear model with ‘Maaslin2’ (Mallick et al. 2021) to identify phyla, which were significantly associated with each groundcover type in ambient conditions compared to the bareground. We then repeated this for the heat treatment samples to see which phyla were most sensitive to heat stress for each groundcover. For those phyla identified as significantly associated with groundcover (p = < 0.05), we created a heatmap using ‘-log(coefficient) × sign(qvalue)’ of the linear model results to show the relationship between groundcover, soil bacterial community, and heat stress.
To identify soil bacterial families associated with each groundcover potentially vulnerable to temperature increase, we compared ambient treatment samples to the heat treatment samples, for each groundcover type. We used multivariate general linear and mixed models to identify those ASVs which were significantly different for each groundcover. Filtering the dataset to only contain significant ASVs, we then combined at family level and plotted relative abundance of only those families significantly affected within each groundcover.
We also used overall alpha diversity metrics (Chao1 species richness, Shannon diversity index) to assess differences in the bacterial diversity among treatments, using functions within Bioconductor (Lahti et al. 2017). ANOVA was used to identify significant variation in alpha diversity of ambient and heat treatments for each groundcover.
3 Results
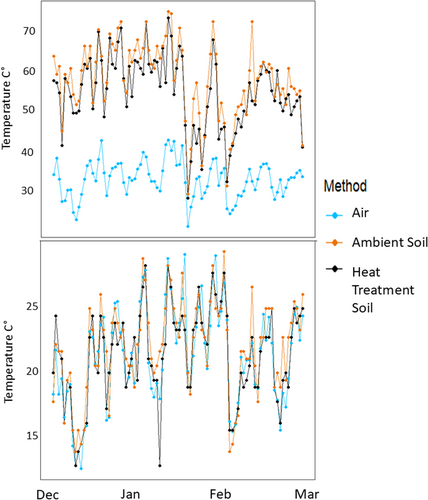
Our Type 2 ANOVA results comparing air temperature to the ambient soil surface temperature showed the heat treatment bareground temperatures were significantly greater than both ambient bareground and air temperatures (p =< 0.0001) (Figure 3).
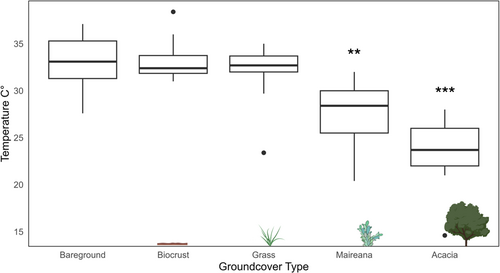
Soil temperature beneath groundcover types also varied, with bareground being the hottest and Acacia sp. the coolest (Figure 4); temperatures under Acacia and Maireana were significantly cooler than bareground (Acacia p =< 0.001, Maireana p = 0.0139).
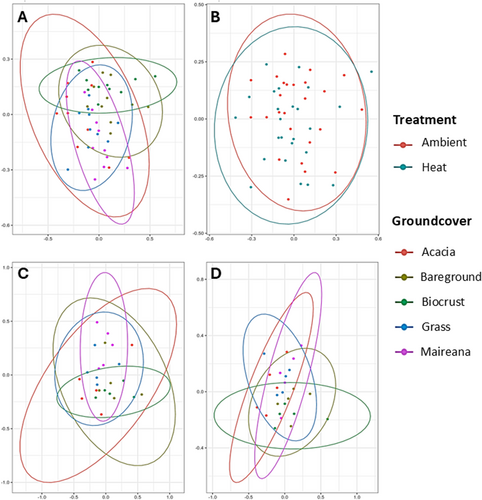
Groundcover type significantly influenced the soil bacterial community composition (PERMANOVA, p = 0.001), while temperature did not have a significant effect (PERMANOVA, p = 0.546) (Figure 5A,B). The distribution of soil communities overlapped more in samples under the heat treatment compared to the ambient treatment (Figure 5C,D).
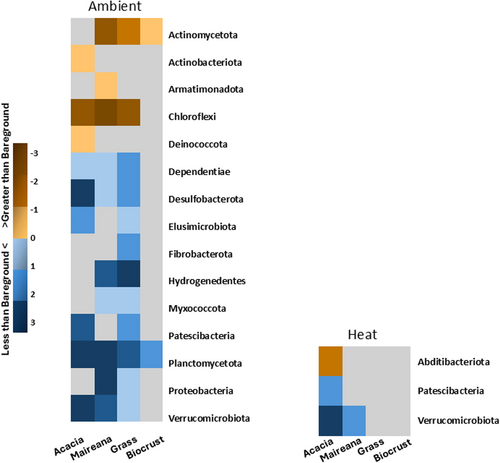
Using standard statistical cut-offs (p =< 0.05, qval =< 0.25), our multivariate association general linear and mixed models analyses showed 15 phyla significantly associated with groundcover type, compared to bareground type in the ambient treatment. This was considerably greater than in the heat treatment, with 3 phyla (Figure 6). Verrucomicrobiota and Patescibacteria were the only phyla that remained greater in groundcover types Acacia and Maireana compared to bareground in the heat treatment. Abditibacteriota was not significantly associated with groundcover under the ambient treatment but was significantly less than bareground in Acacia under the heat treatment (Figure 6).
Within each groundcover type, we identified ASVs significantly affected by the heat treatment, hereafter referred to as ‘significant taxa’. In total, there were 200 ASVs identified to be affected by the heat treatment, these were assigned to 49 taxa at family level (Figure 7). These varied among groundcover types: Acacia had 71 ASVs assigned to 25 taxa; biocrust had 47 ASVs assigned to 18 taxa; Maireana had 35 ASVs assigned to 18 taxa; perennial grass had 33 ASVs assigned to 18 taxa and; bareground had 23 ASVs assigned to 14 taxa (Figure 7).
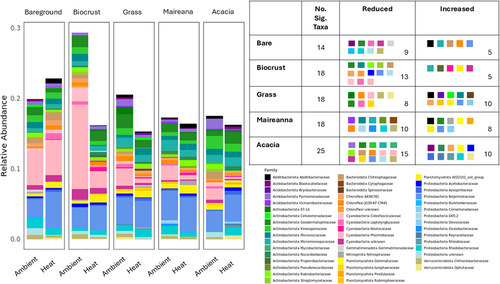
Relative abundances of significant taxa changed the least in bareground, Maireana and Acacia overall. Biocrust had the largest reductions in relative abundance with the heat treatment, reflected in Cyanobacteria families. Grass also had large reductions in relative abundance, mainly in Actinobacteria families. Composition of taxa significantly affected by heat varied compared to the ambient treatment, including within groundcover types. Within bareground, Bacteroidota Chitinophagaceae was not present in the ambient but was in the heat treatment, while Proteobacteria Rhodobacteraceae was present in ambient but absent in the heat treatment. Biocrust changed with Actinobacteria Propionibacteriaceae absent in the ambient but present in the heat treatment, and Proteobacteria Acetobacteraceae was present in ambient but not in the heat treatment. Bacteroidota Spirosomaceae was absent in Grass under the ambient but present under the heat treatment. Maireana had four families (Bacteroidota Cytophagaceae, Protobacteria D05-2, Proteobacteria Oxalobacteraceae, Verrumicrobiota Chthoniobacteracea) present in ambient but absent under the heat treatment. Planctomycetota Gemmataceae was absent in the ambient treatment samples for Acacia but present in the heat treatment samples, while Bacteroidota Chitinophagaceae was present in ambient but not in heat treatment samples.
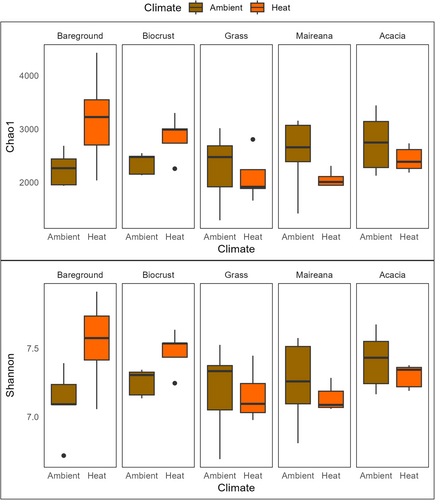
Overall, there was no difference in species richness and diversity between ambient and heat treatment for any of the groundcover types (Figure 8).
4 Discussion
Our field experiment is one of few initial attempts to test the effects of climate change on soil bacterial communities in drylands and the role that groundcover plays. Such analyses are more prevalent in mesic regions (Beugnon et al. 2023; Ma et al. 2022; Yang et al. 2022). We identified groundcover as a stronger driver of soil bacterial communities, compared with temperature (Figure 5a,b). In response to increased temperatures there were also major changes in the bacterial community composition, and also the relative abundance of soil bacteria, which generally declined (Figure 7). Most importantly our results highlight the loss of heterogeneity in drylands soil under increased temperatures (Figure 6).
Temperatures in drylands are predicted to double that of mesic regions under climate change and groundcover type will play an important role in facilitating microclimates (Lortie et al. 2022). Focus on microclimates created by tree canopies in forest systems have shown the buffering potential of vegetation to warming climates (De Frenne et al. 2019), potentially allowing various organisms a longer time to adapt to climate change than previously thought (Lenoir, Hattab, and Pierre 2017). While larger groundcover types, i.e., Acacia and Maireana, were significantly cooler than bareground, their soil bacterial communities were still impacted by the effects of increased temperature (Figures 6 and 7). Our 1-year field study demonstrates that soil microbial communities are sensitive to temperature increases even on short time scales.
Shorter groundcover types, such as grasses and biocrust, are often neglected in microclimate studies and conservation goals, but our results show distinct soil communities across all groundcover types (Figures 5 and 6). Biocrust and grass were most sensitive to the increased temperature. Large shifts in composition, reduced relative abundance, and a greater similarity to bareground soil communities (Figures 6 and 7), suggest that these groundcover types are particularly vulnerable to the impacts of climate change. In the case of biocrust, species richness and alpha diversity increased with temperature and this is may be due to reduced relative abundance of cyanobacteria which is dominant in biocrust allowing other bacterial taxa to establish, causing an increase in diversity but reduced functionality (Fernandes et al. 2018). Grasslands’ soil chemistry and moisture are sensitive to aridity changes and increased temperatures (Butterfield et al. 2017; Gremer et al. 2018; Jiao et al. 2016). Here we show there is also a microbial response. Microbial shifts in other grass studies led to changes in grass species (Bonkowski and Roy 2005), due to reduced plant fitness. Increased temperature disrupts biocrust at a similar level to physical disturbance over a 10-year period (Ferrenberg, Reed, and Belnap 2015), which has the potential to contribute to land degradation (Phillips et al. 2022). Our study shows that biocrust can be largely affected faster than previously thought, with the greatest response in the cyanobacteria composition (Figure 7), likely causing a reduction in functionality of these crusts (Fernandes et al. 2018).
Previous studies have found groundcover determines the differences in soil microbial communities within an ecosystem, but biotic factors responsible for fine scale differences (Wu et al. 2018). Our results build on this showing that groundcover drives the soil bacterial community but that pressure from climatic stress can alter it. Additionally, our results showed that the response of some bacterial taxa varied depending on groundcover type (Figure 7), increasing with some groundcover and decreasing in others. Suggesting that community composition associated with groundcover, rather than species specific reactions will drive the response of soil taxa to increased temperatures. These shifts in composition may represent weakening of plant-soil-microbe interactions (Ochoa-Hueso et al. 2018), and may be more valuable for monitoring stress response of drylands soil under climate change.
Considering plant-soil-microbe interactions underpin plant health and function (Meena et al. 2023; Ortiz and Sansinenea 2022; C. Trivedi et al. 2019), plant responses to stressors is correlated to these interactions. Many studies outline beneficial traits provided to plants by associated microbes such as resistance to drought, temperature, and also pathogens (Khan et al. 2016; Meijer, Holmgren, and Van der Putten 2011; Porter et al. 2020). In the context of climate change, extreme or prolonged stress can change the microbial community (Li et al. 2024). Uncoupling of these relations would likely impact plant resilience to climatic stress (Cesarano et al. 2017; Meijer, Holmgren, and Van der Putten 2011; Trivedi et al. 2022; Veresoglou et al. 2022), and may even change plant communities due to changes in plant-plant competition as a result of reduced fitness (Kaisermann et al. 2017). High level taxonomy does not provide an indication of microbial response to stress (Oliverio, Bradford, and Fierer 2017), as phyla contain taxa which both increase and decrease with heat. Our results support this also, with groundcover types showing changes within phylum of composition and relative abundance which were not detected in overall diversity or species richness metrics (Bent and Forney 2008).
Host plant type is known to drive microbial composition changes in response to drought stress (Naylor and Coleman-Derr 2018; Orwin and Wardle 2005) and this supports the differences seen in composition changes between the groundcover types in our study. Cyanobacteria families Coleofasciculaceae and Phormidiaceae and Leptolyngbyaceae were significantly reduced in all groundcovers except for Maireana. Cyanobacteria is important in drylands for stabilising soil, nitrogen fixation and improving water retention (M. C. M. C. Fernandes et al. 2022; Moreira et al. 2021; Sepehr et al. 2019), these bacterial families are common in dryland soil and are resistant to desiccation. Maireana, like most dryland shrubs, has dense foliage with fertile islands forming at the base of plants, supporting increased nutrients and soil moisture than the surrounding ground (Garner and Steinberger 1989). This may have potentially buffered the effect of the heat treatment for these cyanobacteria. Planctomycetota WD2101 a dominant soil bacteria, increased in all groundcover types except bareground. Planctomycetes are heat tolerant and WD2101 has been associated with arid soil formation (Rodríguez et al. 2024). Previous studies found a similar increase with temperature and it may be important for heat resistance to heat stress (Cuartero et al. 2024; Dedysh et al. 2021; Wang et al. 2019). Despite many studies on soil microbial communities, ecology of bacteria at lower taxonomic levels such as family remains scarce. These host specific responses highlight the need for fine-scale studies to better understand how climate change will impact drylands.
The majority of drylands are used for grazing and agricultural crops and mismanagement often causes degradation through loss of vegetation and soil erosion (Reynolds et al. 2007). Loss or reduction of groundcover in drylands reduces their functionality, with groundcover driving soil microbial functional niches (Delgado-Baquerizo et al. 2016; C. Trivedi et al. 2019). Microclimate diversity is modulated by groundcover diversity (Beugnon et al. 2024), and this further facilitates ecosystem function diversity (Feizabadi et al. 2021). Shifts in vegetation in response to increased temperatures could exacerbate drought conditions in drylands (Shriver et al. 2018). Soil temperature is predicted to increase at a greater intensity than air temperature (García-García et al. 2023), with soil temperature directly contributing to intensification of air temperature extremes (Miralles et al. 2014). Most concerning in this study is the soil communities associated with groundcover becoming more like bareground soil communities, which would result in less diversity across the landscape (Figure 6). Considering plant-soil-microbe interactions underpin plant health and function (Ortiz and Sansinenea 2022; Trivedi et al. 2020), uncoupling of these relations would likely impact plant resilience to climatic stress (Cesarano et al. 2017; Meijer, Holmgren, and Van der Putten 2011; Veresoglou et al. 2022). As climate change warms drylands at a rapid rate, better understanding of soil microbial taxa key to groundcover types may improve the response of these ecosystems and how we manage them. By incorporating the preservation of diverse groundcover types in drylands management, soil functional diversity may be conserved, having the potential to improve outcomes for these systems under climate change.
Author Contributions
Jana Stewart, Miriam Muñoz-Rojas and Richard Kingsford planned and designed the research. Jana Stewart collected and analysed the data with assistance from Nathali Machado de Lima. Jana Stewart wrote the first draft of the manuscript, and all authors contributed to subsequent edits of the manuscript. All authors on the manuscript have seen and approved the submitted version of the manuscript.
Acknowledgements
This research was supported by the Hermon Slade Foundation (HSF 1808), the Australian Research Council Discovery Early Career Research Award (DE180100570) and the New South Wales Government's Department of Planning and Environment University Grant Program. MMR is supported by the by the Spanish Ministry of Science and Innovation (RYC2020-029255-I, TED2021-132332A-C22 and PID2021-123097OA-I00). Field surveys were conducted at the Fowlers Gap Arid Zone Research Station with permission from the station. We acknowledge volunteers, Megan Suthers and Callum Fitzpatrick, who helped with field surveys, and Antony Miller for designing the Open Top Chambers to our requirements. Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in NCBI Sequence Read Archive at https://www.ncbi.nlm.nih.gov/sra, reference number PRJNA1178706. All raw sequence data has been deposited in NCBI Sequence Read Archive, project PRJNA1178706.




