Aridity Structures the Microbial Potential for Carbon Cycling and Mediates the Impact of Mammal Bioturbation at the Continental Scale
ABSTRACT
Introduction
In Australia, the historical loss of native digging mammals has profoundly changed ecosystems and their functioning. However, little is known about how the decline in digging mammal presence alters microbes and their functional potential and how aridity affects these relationships.
Materials and Methods
We used metagenomic sequencing to explore changes in genes encoding enzymes for carbon cycling (CAZymes) in five sites along a continent-wide aridity gradient, with and without digging mammals.
Results
The diversity of CAZy genes was reduced with increases in aridity, which also affected their structure and reduced the abundance of genes involved in both plant (cellulose and starch) and microbial (glucans, peptidoglycan and chitin) biomass degradation. Conversely, digging mammals had a limited effect on the structure and diversity of CAZy genes, indicating an overall resistance of the microbial carbon cycling potential to mammal disturbance at the whole community level. However, when considering individual functional groups, digging activity increased the abundance of genes involved in microbial biomass decomposition (i.e., glucanases), while reducing the abundance of genes associated with recalcitrant plant biomass degradation (i.e., cellulases). Notably, these effects were observed only in the most arid sites and was mostly mediated by increases in SOM content linked to mammal activity.
Conclusions
Overall, our study shows that aridity shapes the diversity and structure of CAZy genes, while also modulating the effect of mammal bioturbation on the microbial potential for carbon cycling. This suggests that the loss of digging mammals throughout much of Australia's arid zone, in particular, is likely to have important repercussions on the microbial capacity to carry decomposition processes and the turnover of organic matter in soils.
1 Introduction
Global change is causing biodiversity losses at an alarming rate (Johnson et al. 2017), with important consequences on ecosystem functioning. Of particular concern is the loss of digging mammals, because of their disproportionate role in maintaining complex ecological processes (Losapio et al. 2024). Digging mammals act as ecosystem engineers by creating microhabitats and altering biophysical conditions, which influence other species through the modification of niche and resource availability; such changes initiate ecosystem cascades, shaping biodiversity, ecological networks and overall ecosystem functioning (Coggan, Hayward, and Gibb 2018; Sanders and Frago 2024).
While much attention has been given to the effects of digging mammals on aboveground communities, far less is understood about how digging activity—and its loss—affects belowground communities and their functioning (Andriuzzi and Wall 2018). Yet, digging mammals have a potentially disproportionate role in driving soil structure, nutrient cycling and microbial community dynamics, which are essential for maintaining belowground biodiversity and ecosystem processes. Their burrowing, tunnelling and foraging activities, along with the deposition of faeces and urine, significantly alter the soil chemical composition (Beca et al. 2022; Coggan, Hayward, and Gibb 2018). These activities improve the physical characteristics of soil (e.g., structure and composition), such as aeration, water infiltration and water-holding capacity (Platt et al. 2016; Wilkinson, Richards, and Humphreys 2009) and facilitate the dispersal of plant litter and propagules in the deep soil layers. Collectively, such disturbance results in higher amounts of available carbon and soil organic matter (SOM) content and quality in diggings (Coggan, Hayward, and Gibb 2018; Travers and Eldridge 2016) and across the landscape (Decker, Leonard, and Gibb 2019), which have been linked to altered microbial community composition and enzymatic activity (e.g., cellobiosidase and phosphatase activity) (Decker, Eldridge, and Gibb 2019). These changes suggest that digging may have profound impacts on the microbial potential to support essential ecosystem processes, such as decomposition and nutrient cycling (Yang 2021). However, the extent of these functional shifts remains largely unquantified, highlighting a critical gap in our understanding of how digging mammals shape the functional potential of belowground communities. Addressing this knowledge gap is crucial for predicting how the loss of these ecosystem engineers might affect ecosystem resilience and functioning under global change.
A deeper understanding of how digging mammals influence belowground communities requires considering the environmental context in which these interactions occur. The level of aridity in an ecosystem, in particular, can profoundly affect the outcomes of soil disturbance on microbial communities (Xu et al. 2020), ultimately shaping the contribution of digging activity to key ecosystem processes. For example, in arid systems, higher abundance in the landscape of organic matter and available nitrogen mediated by digging mammals can significantly enhance microbial activity and enzyme production (Decker, Eldridge, and Gibb 2019; Travers and Eldridge 2015). This influx of readily available resources can stimulate microbial communities that carry a broader range of enzymes, particularly those involved in the degradation of simple carbon compounds (Dal Bello et al. 2021). Such effects, however, might be less evident in mesic systems, where the consistent availability of moisture and organic matter could support a stable microbial community with less pronounced functional diversification (Decker, Eldridge, and Gibb 2019).
In this study, we investigated the impact of digging activity on microbial functional potential for decomposition and how this effect is modulated by aridity. We focussed on decomposition because the primary source of organic matter released into disturbed soils is represented by plant litter, which is the main substrate for microbial decomposers (Frouz 2018; Wardle et al. 2006). Specifically, using a metagenomics approach we surveyed the diversity and composition of genes encoding for soil microbial CAZymes (Lombard et al. 2014) from five reserves with reintroduced digging mammals and adjacent unmanipulated areas over an aridity gradient ranging from 0.077 to 0.88 (1-aridity index; 166–900 mm average annual rainfall), with site ranging over 3300 km of the Australian continent. CAZymes consist of five classes of enzymes (glycosyl hydrolases [GHs], auxiliary activities [AAs], carbohydrate-binding modules, polysaccharide lyases [PLs], glycosyl transferases [GTs] and carbohydrate esterase [CEs]), which are further grouped into functional families involved in various aspects of decomposition. Consequently, characterizing genetic changes in this group of enzymes could allow us to predict microbial functional responses to digging activity and their effects on carbon turnover (Allison 2012; Huang et al. 2024). Australia, one of the most biodiverse regions of the world, is particularly affected by the losses of digging mammals: more than 10% of the 273 endemic terrestrial mammal species have gone extinct since the European settlement (∼200 years), and a further 21% are considered threatened (Woinarski, Burbidge, and Harrison 2015). These trends involve important ecosystem engineers such as bettongs (Bettongia lesueur and Bettongia penicillata) and the greater bilby (Macrotis lagotis), which have experienced dramatic population declines and high rates of local extinction due to predation and habitat degradation (Burbidge and McKenzie 1989; Johnson and Isaac 2009). Previous work has shown that the reintroduction of digging mammals had a pronounced effect on nutrient availability in arid systems, while also affecting the enzymatic activity and taxonomic composition of microbial communities in these systems (Decker, Eldridge, and Gibb 2019; Decker et al. 2023; Decker, Leonard, and Gibb 2019).
We hypothesized that (H1) digging mammals would increase CAZy genetic diversity, and the abundance of genes related to plant-biomass decomposition and microbial turnover due to the higher abundance of nutrients and litter in disturbed sites. We also predicted that (H2) the effect of bioturbation on CAZy diversity and abundance would be more pronounced in the most arid sites, where resource limitations and harsher conditions make bioturbation a key driver of microbial community dynamics. In contrast, in mesic environments, the higher rainfall and greater ecosystem productivity create more stable conditions, effectively buffering microbial communities from the full impact of bioturbation by providing sufficient moisture and nutrients independent of soil disturbance. Characterizing shifts in the distribution of functionally important genes within soil microbial communities in response to the combined effects of aridity and bioturbation offers a powerful approach to fully elucidate the ecological impact of digging mammals on key functional traits that underpin the ecosystem carbon storage potential (Schmitz et al. 2023). This understanding supports our ability to better estimate changes in soil health and ecosystem properties attributable to the loss of key digging mammals from Australian ecosystems.
2 Materials and Methods
2.1 Sites Description and Sampling Design
Soil samples were collected from five predator–proof reintroduction reserves in southern Australia, from driest to wettest: Arid Recovery (independent incorporated charity, Kokatha country, South Australia), Scotia Wildlife Sanctuary (Australian Wildlife Conservancy—AWC, Barkinji country, New South Wales), Yookamurra Wildlife Sanctuary (AWC, Ngarrindjeri country, South Australia), Mt Rothwell Conservation and Research Centre, (privately owned, Wurundjeri country, Victoria) and Karakamia Wildlife Sanctuary (AWC, Noongar country, Western Australia; Supporting Information S1: Figure S1a). Study sites covered a > 3000 km rainfall gradient. For each site, the aridity index (the ratio of mean annual precipitation to mean annual potential evapotranspiration) was retrieved from the Atlas of Living Australia (https://www.ala.org.au/). Invasive predators (foxes and cats) were absent from all reserves, and control of feral animals is ongoing. The reserves differed in both biotic and abiotic attributes; for details see Supporting Information S1: Table S1. Native digging mammal species were largely locally extinct from the reintroduction sites and surroundings before the reintroductions However, echidnas (Tachyglossus aculeatus) occur throughout Australia at varying densities (Griffiths 2012) and quenda (Isoodon fusciventer) persisted outside the fence at Karakamia (Western Australia) in low densities (Valentine et al. 2012).
In each site, paired plots of 20 m by 20 m were established inside (‘reintroduction’) and outside (‘control’) the fence line of each reserve (Supporting Information S1: Figure S1b). Reserves differ in size, hence we selected 10 paired plots in the larger reserves (Arid Recovery, Scotia, Yookamurra), and 8 pairs in the smaller reserves (Karakamia and Mt Rothwell). Plots were selected to represent the dominant vegetation types in each reserve (Supporting Information S1: Table S1), with similar vegetation, ground cover, fire and grazing histories. Study plots were located at least 50 m from any road and at least 60 m from the predator-proof fence and were separated by a minimum of 60 m, except for Yookamurra, where three paired plots were only 20 m from one another and 5 m from roads. At each plot, 2 m wide band-transects were set up running east–west across the 20 m wide plot: one transect was 6 m to the centre from the south, and another one was 6 m to the centre from the north edge of the plot. This covered a total area of 80 m2 of the 400 m2 study plot. Mammal-produced pits were counted and measured (width, length and depth), within each band-transect. The two transects were used to estimate ground cover as point-measures by determining ground cover at every metre (litter, wood, grass or bare), resulting in 40 point measurements at each plot. Soil sampling on all reserves was conducted in August 2016 (winter). From each study plot, we established four sampling points along two transects situated 6 m from the edge lines of the plot. We took four subsamples from each sampling point, and samples were pooled together per plot for later analysis (Supporting Information S1: Figure S1c), resulting in 5 sites × 2 digging mammal treatments × 10 replicates (plots): 100 samples in total. All samples were taken from unvegetated, undisturbed soil. After collection, the samples were immediately frozen and kept at −20°C until further analysis. We chose four paired (reintroduction and control) replicates from each site. In total, we analyzed 40 soil samples (representing 640 pooled subsamples) for this study.
2.2 DNA Extraction and Sequencing
DNA was extracted from 1 g homogenized soil using bead beating and chemical lysis (Zymo Quick DNA Fecal/Soil Microbe MiniPrep) following the manufacturer's protocol. Genomic DNA concentration was quantified using a Qubit 2.0 fluorometer (Invitrogen). A shotgun metagenomic library was generated and sequenced using Illumina HiSeq at the Hawkesbury Institute for Environment NGS research center utilizing TruSeq library preparation.
2.3 Bioinformatics Analysis and Genes Annotation
The metashot/mag-illumina v2.0.0 Nextflow-based (Di Tommaso et al. 2017) workflow (https://github.com/metashot/mag-illumina, parameters: --metaspades_k 21,33,55,77,99) was used to perform raw reads quality trimming and filtering, assembly and contings binning. In brief, adapter trimming, contaminant (artifacts and and spike-ins) and quality filtering were performed using BBDuk (BBMap/BBTools v38.79, https://sourceforge.net/projects/bbmap/).
Functional annotation was performed using the workflow metashot/prok-annotate (https://github.com/metashot/prok-annotate, commit da2d0bb, parameters: --run_eggnog --eggnog_db emapperdb-5.0.2). Translated coding DNA sequences (CDSs) were predicted using Prokka (Seemann 2014) v1.14.5 which in turn wraps the gene predictor Prodigal (Hyatt et al. 2010) and (ii) functionally annotated using EggNOG-mapper (Cantalapiedra et al. 2021; v2.1.4, parameters -m diamond --itype protein) against the eggNOG Orthologous Groups (OGs) database (Huerta-Cepas et al. 2019) v5.0.2. The eggNOG database integrates functional annotations collected from several sources, including CAZy database (Cantarel et al. 2009).
2.4 Statistical Analysis
To account for uneven sequencing depths, we rarefied CAZymes sequences in each sample to the lowest number of total sequences in a given sample (4072) across the 39 sites using the ‘phyloseq’ package in R (McMurdie and Holmes 2013). Thereafter, all analyzes were conducted using rarefied data. All statistical analyzes were performed in R (R Development Core Team 2016) and we used a significance level of 0.05 for all statistical tests.
To describe patterns of CAZy genetic diversity and distributions at community level as well as at individual family scales of resolution, we used a combination of multivariate and univariate statistical analyses and structural equation models. Alpha diversity for each site was estimated using the Shannon index (Phyloseq package). We used generalized linear mixed-effects models (lmer function in the lme4 package in R; Bates et al. 2015) to investigate the effects of digging mammal presence and aridity, and their interaction, on the diversity of microbial CAZymes in soil. We included paired plots as random effects in the models to account for non-independence of observations within sites, and the significance of the effects was further evaluated using the ANOVA test.
To visualize differences in gene composition across sites and among Reintroduction and Control plots for all CAZy families, we first produced a principal coordinates analysis (PCoA) plot in the R package vegan (Oksanen et al. 2013). Plots were generated from Bray–Curtis dissimilarity matrices based on the relative abundances of individual families. To quantify the influence of aridity, reintroduction and their interaction on the CAZyme composition, we conducted a PERMANOVA from the Bray–Curtis similarity matrices using the ‘adonis’ function in vegan. To further assess the relationship between contextual edaphic and vegetation data and CAZyme composition, we performed the envfit test in vegan (999 permutations) based on Bray–Curtis distances. Environmental variables were assessed for correlation and the following variables were used: time since grazing ceased, litter cover (%), disturbance (number of foraging pits), wood cover (%), grass cover (%), soil total carbon content (mg/kg of soil), fine SOM content (%), and nitrogen content (mL/kg of soil).
We then investigated the changes in the relative abundance of genes involved in the different steps of carbon cycling along the aridity gradient and in response to digging activity. First, we assigned each CAZyme family to a class, that is, GHs, GTs, PLs, CEs, AAs and carbohydrates binding modules (CBMs). Subsequently, we classified GH and AAs encoding the enzymatic activities involved in the plant and microbial compounds degradation according to (Xiong et al. 2023). We identified 44 GH and AA genes that targeted the plant cell wall (starch, hemicellulose and cellulose), fungal dead biomass (chitin and glucans) and bacterial dead biomass (peptidoglycans), which collectively accounted for ~50% of the total CAZymes abundance (Supporting Information S1: Figure S2). We then investigated the effects of digging mammal presence and aridity, and their interaction, on the abundance of the CAZyme classes and CAZymes grouped by their plant and microbial biomass degrading potential following the same procedure described above for the Shannon index. Response variables were log and square-root transformed where appropriate to meet the assumptions of normal error distribution.
To determine the causal pathways through which aridity, mammal reintroduction and their interactions influenced the abundance of plant and microbial degrading CAZymes, we constructed a Structural Equation Model (SEM; Lefcheck 2016). We designed the SEM model based on hypothesized causal pathways (Supporting Information S1: Figure S3) and performed them using the ‘piecewiseSEM’ package in R (Lefcheck 2016). Our model included multiple linear mixed-effects models to account for the hierarchical structure of the data. Specifically, to construct each SEM pathway, we ran independent generalized linear models for each response variable using the ‘lmer’ function in the lme4 package (Bates et al. 2015). CAZyme response variables were the abundance of genes encoding for: (a) plant-degrading enzymes (starch-, hemicellulose-, and cellulose-degrading), (b) bacterial-degrading enzymes (peptidoglycan-degrading), (c) fungal-degrading enzymes (glucans- and chitin-degrading). Plot number was set as a random variable, while the predictor variables were litter cover (%), disturbance (number of foraging pits), wood cover (%), grass cover (%), soil total carbon content (mg/kg of soil), fine SOM content (%) and nitrogen content (mL/kg of soil). The investigated pathways also included the effects of aridity and reintroduction on these soil and vegetation properties (Supporting Information S1: Figure S3). This approach allowed us to simultaneously evaluate direct and indirect effects of aridity and mammal activity, and their interactions, on the ecological variables. We performed log and square-root transformations on response variables where appropriate to meet the assumptions of normal error distribution for all pathways. For the final model, we further calculated the indirect effects of reintroduction using multiplicative path tracing (by multiplying significant coefficients of interacting response variables; Lefcheck 2016).
3 Results
3.1 Shifts in CAZymes Diversity and Structure
The overall diversity of CAZy families (Shannon index) decreased significantly with aridity but was not affected by mammal reintroduction or their interaction (Figure 1A). PERMANOVA analysis showed that the gene composition was significantly structured by aridity (p < 0.05, Figure 1B), with clear differentiation between wet and arid sites along the first axis of the PCoA plot (Supporting Information S1: Figure S4), while digging mammal reintroduction (Reintroduction vs. Control) and its interaction with aridity were non-significant. Results from the envfit procedure indicate that grazing disturbance (ungrazed years) and the amount of SOM had the highest level of correlation with the environmental variables, followed by soil properties (carbon and available nitrogen content), and litter content (Figure 1C).
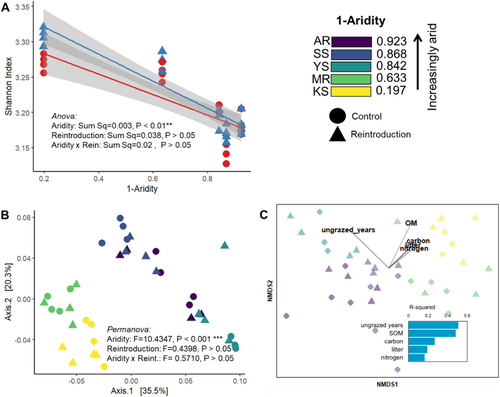
3.2 Changes in Carbon Cycling Genes Abundance
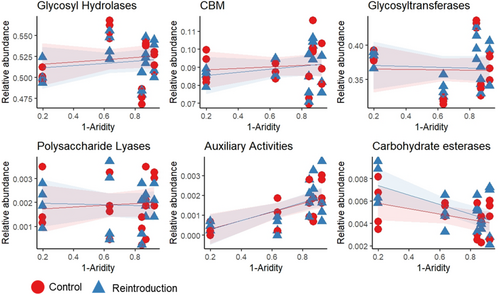
We found significant differentiation among the different CAZy classes in response to the aridity gradient, with AAs increasing and CEs decreasing with aridity (Figure 2, Table 1a). However, none of the CAZy classes showed changes in abundance with mammal reintroduction or its interaction with aridity.
| Group | Term | NumDF | DenDF F | value | Pr (> F) |
|---|---|---|---|---|---|
| Glycosyl hydrolases | Treatment | 1 | 13.168 | 0.120 | 0.735 |
| Aridity | 1 | 20.038 | 0.462 | 0.504 | |
| Treatment:Aridity | 1 | 13.760 | 0.002 | 0.967 | |
| Glycosyl transferases | Treatment | 1 | 12.842 | 0.150 | 0.705 |
| Aridity | 1 | 20.003 | 0.032 | 0.860 | |
| Treatment:Aridity | 1 | 13.362 | 0.031 | 0.863 | |
| Carbohydrate-binding Modules | Treatment | 1 | 13.245 | 0.246 | 0.628 |
| Aridity | 1 | 16.541 | 0.748 | 0.399 | |
| Treatment:Aridity | 1 | 14.299 | 0.165 | 0.691 | |
| Polysaccharide lyases | Treatment | 1 | 11.292 | 1.712 | 0.217 |
| Aridity | 1 | 16.812 | 1.398 | 0.254 | |
| Treatment:Aridity | 1 | 12.042 | 1.531 | 0.240 | |
| Auxiliary activities | Treatment | 1 | 12.955 | 0.011 | 0.918 |
| Aridity | 1 | 16.278 | 16.456 | 0.001 | |
| Treatment:Aridity | 1 | 13.998 | 0.033 | 0.859 | |
| Carbohydrate esterases | Treatment | 1 | 35.000 | 1.859 | 0.181 |
| Aridity | 1 | 35.000 | 12.280 | 0.001 | |
| Treatment:Aridity | 1 | 35.000 | 0.904 | 0.348 |
When grouped according to the type of carbohydrate substrate they target, the relative abundance of the CAZy genes involved in the degradation of plant and microbial biomass changed in the presence of digging mammals, but the magnitude and direction of this effect varied among the different groups and along the aridity gradient (Figure 3, Table 1b). Specifically, the effect of digging mammals depended on aridity when considering the relative abundance of cellulose- and glucans-degrading genes, with glucans-degrading genes increasing in the reserve plots in arid sites, but decreasing in mesic sites, and cellulose-degrading genes having the opposite trend. The other groups did not respond to digging mammal presence or absence, but aridity had a significant impact on their abundance, whereby peptidoglycan- and chitin-degrading genes decreased with aridity, while hemicellulose-degrading genes increased. Starch-degrading genes did not respond to either aridity or digging mammals.
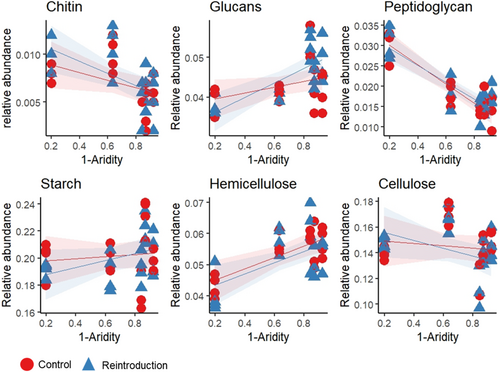
| Group | Term | NumDF | DenDF F | value | Pr (> F) |
|---|---|---|---|---|---|
| Chitin-degrading genes | Reintroduction | 1 | 12.522 | 2.091 | 0.173 |
| Aridity | 1 | 17.828 | 7.707 | 0.013 | |
| Reintroduction:Aridity | 1 | 13.357 | 1.406 | 0.256 | |
| Peptidoglycan-degrading genes | Reintroduction | 1 | 14.561 | 0.392 | 0.541 |
| Aridity | 1 | 16.725 | 69.742 | 0.000 | |
| Reintroduction:Aridity | 1 | 15.702 | 0.976 | 0.338 | |
| Glucans-degrading genes | Reintroduction | 1 | 16.095 | 3.145 | 0.095 |
| Aridity | 1 | 20.923 | 12.684 | 0.002 | |
| Reintroduction:Aridity | 1 | 17.062 | 6.516 | 0.021 | |
| Starch-degrading genes | Reintroduction | 1 | 11.292 | 1.712 | 0.217 |
| Aridity | 1 | 16.812 | 1.398 | 0.254 | |
| Reintroduction:Aridity | 1 | 12.042 | 1.531 | 0.240 | |
| Hemicellulose-degrading genes | Reintroduction | 1 | 10.327 | 0.136 | 0.720 |
| Aridity | 1 | 15.117 | 13.553 | 0.002 | |
| Reintroduction:Aridity | 1 | 11.115 | 0.002 | 0.962 | |
| Cellulose-degrading genes | Reintroduction | 1 | 12.704 | 2.546 | 0.135 |
| Aridity | 1 | 20.437 | 1.536 | 0.229 | |
| Reintroduction:Aridity | 1 | 13.082 | 4.960 | 0.054 |
3.3 Structural Equation Model
To further investigate the possible direct and indirect effects of environmental variables on the diversity and composition of the CAZy genes involved in dead plant and microbial biomass degradation, we generated a structural equation model (SEM) based on the known effects and relationships among aridity and other likely key drivers of the diversity and abundance of carbon cycling genes (e.g., plant cover, vegetation composition, soil nitrogen and organic carbon content, Supporting Information S1: Figure S2). Increases in aridity had direct influence on the abundance of CAZy genes involved in the degradation of microbial (peptidoglycan, glucans) and plant (cellulose, hemicellulose) biomass in our data set, while mammal reintroduction had a non-significant direct relationship with these response variables, in line with the results of the linear mixed models (Figure 4A). However, the reintroduction of digging mammals had an indirect effect on cellulose-degrading enzymes and overall CAZy gene diversity (Shannon index). Specifically, the presence of digging mammals indirectly increased the abundance of cellulose-degrading enzymes in arid sites through a higher number of pits created by the mammals compared to mesic sites. Among the soil variables, SOM had the strongest effect on cellulose-degrading enzymes, and its increase mediated by the interaction between aridity and reintroduction had an indirect negative effect on the abundance of these genes and overall CAZy gene diversity (Figure 4B). The SEM model also indicates that changes in site-level canopy attributes mediated shifts in CAZy diversity and structure. For example, the abundance of grasses had a positive influence on the abundance of genes encoding for starch-degrading genes while negatively relating to the overall CAZy diversity. Similarly, the proportion of woody plants negatively related to the CAZy gene diversity and abundance of genes for hemicellulose degrading enzymes. Overall, the structural equation model explained 65%–80% of the variation in diversity (Shannon index) and CAZy gene abundance (Figure 4B).
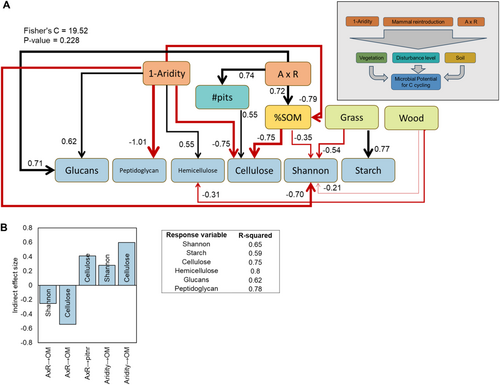
4 Discussion
In line with our initial expectations, we found that both the diversity and composition of the microbial CAZyme genetic pool were affected by aridity. Aridity is an important driver of microbial community structure and function (Egidi et al. 2023; Maestre et al. 2015; Chen et al. 2020), especially in Australian soils (Egidi et al. 2019; Maisnam et al. 2023). In arid environments, limited water and nutrient availability constrains microbial activity, leading to lower overall microbial diversity and shifts in community composition towards more drought-tolerant taxa (Coleine et al. 2024). These conditions can favour microbial communities that are adapted to stress resistance, and thus result in a reduced diversity of functional genes related to nutrient cycling (Li et al. 2022; Piton et al. 2023), including those encoding CAZymes (Zhang et al. 2024). Further, we found that the structure of CAZyme genes associated with the SOM content and soil properties such as nitrogen availability and total carbon content. These properties determine nutrient cycling, water retention, and soil structure, influencing various microbial processes. Our findings align with current evidence showing that aridity-driven changes in resource availability and microbial community structure directly influence the functional potential of microbial communities in soil ecosystems (Hu et al. 2021). Interestingly, we also found a significant correlation between grazing history and the CAZyme composition. This association highlights the significant impact of grazing, and its legacy effects, on microbial dynamics, in line with previous studies demonstrating that grazing pressure can substantially alter plant community composition, soil properties, and overall ecosystem function (He et al. 2022).
Contrary to our first hypothesis, however, we did not detect significant changes in the genetic structure or diversity of CAZymes between reintroduction and control areas across sites. This result contrasts with previous studies reporting an increase in decomposition rates (i.e., cellobiosidase enzymatic activity) and abundance of decomposer microbes mediated by digging mammal activity in the same study areas (Decker et al. 2023; Decker, Leonard, and Gibb 2019). This inconsistency suggests that bioturbation might enhance the activity levels of existing enzymes and alter the taxonomic composition of the resident community, possibly by inducing the proliferation of ‘dormant’ microbes (Binet et al. 1998), without leading to significant changes in the microbial potential for decomposition (Louca, Parfrey, and Doebeli 2016). A possible explanation can be attributable to the relatively high degree of functional redundancy of soil microbes (Allison and Martiny 2008), that could be particularly exacerbated when considering ‘general’ ecosystem processes carried out by a wide range of microbes, such as decomposition (Banerjee, Schlaeppi, and van der Heijden 2018; Rousk, Brookes, and Bååth 2009).
However, when examining the changes in the abundance of CAZy genes involved in the degradation of plant and microbial biomass, we found evidence of both direct and indirect responses to digging mammals and aridity. For instance, digging activity increased the abundance of glucanase-encoding genes in arid sites. Glucans are the most abundant polysaccharides in fungal cell walls (Ruiz-Herrera and Ortiz-Castellanos 2019). This result aligns with our previous study linking digging mammal reintroductions to enhanced incidence of fungi in arid environments (Decker et al. 2023). Such increases in genes encoding for glucanases in arid sites following mammal reintroduction can thus be explained by a greater activity, and thus faster turnover, of the fungal community in reintroduction areas (Ren et al. 2021). Notably, fungi store carbon in their mycelia (Hawkins et al. 2023), and a potentially higher biomass turnover rate could lead to quicker microbial carbon decomposition and potentially greater soil nutrient availability (López-Mondéjar et al. 2020), which could lead to greater carbon capture via plants. However, it also implies that carbon stored in fungal biomass may be released more rapidly (Emilia Hannula and Morriën 2022), potentially reducing the long-term carbon storage capacity of the soil.
Simultaneously, reintroduction had a negative effect on the abundance of cellulose-degrading enzymes mediated by increases in SOM content in arid sites. These patterns are apparently in contrast. However, it is possible that the increased availability of SOM reduces competition for cellulose, as higher SOM levels in these reintroduced sites may provide an abundance of easily decomposable organic matter across the landscape. This could result in a decreased relative abundance of genes encoding for cellulose-degrading enzymes, as microbial communities prioritize breaking down more readily available substrates. This observation is consistent with our previous studies from the same areas (Decker et al. 2023) showing that mammal reintroduction resulted in decreased abundance of bacterial phyla with well-known cellulolytic activities, such as Actinobacteriota (Lewin et al. 2016), and reduced abundance of fungal litter saprotrophs. Notably, our landscape-scale study targeted soil from patches outside foraging pits, and the abundance of cellulose-degrading enzymes was positively linked to the number of pits present at each site. Foraging pits act as ‘litter traps’ (James, Eldridge, and Moseby 2010) and, in arid systems, are considered hotspots for litter decomposition (Travers and Eldridge 2016). The density of pits, therefore, drives litter decomposer communities via niche partitioning (Smith et al. 2018), while undisturbed soil lacks litter heterogeneity (such as different species of plant litter, stages of decomposition, etc.).
Further, we report that the overall diversity of CAZy genes, and the abundance of genes encoding for hemicellulose and starch-degrading enzymes, were influenced by site-level differences in plant structure (i.e., abundance of woody or grassy plants), regardless of aridity or digging mammal presence. Previous reports from the same areas have shown a strong impact of the feeding behavior of the mammals (e.g., herbivory) on the vegetation structure (Michael et al. 2022) and food webs (Gibb et al. 2021). Our study suggests that the indirect influence of digging mammals, extending beyond ecosystem engineering, might also affect the microbial decomposition potential in soil. However, in this study, we did not consider the traits and composition of the reintroduced animal assemblages, and thus this potential linkage remains unquantified.
Taken together, our results imply potential trade-offs in microbial carbon cycling processes linked with mammal reintroduction. Specifically, while the higher incidence of genes linked to glucan degradation due to digging activity may enhance the decomposition of microbial biomass and accelerate nutrient cycling, the decreased potential for cellulose degradation could slow the breakdown of plant litter and other recalcitrant organic matter at the landscape scale. This trade-off could have important implications for long-term carbon storage and soil health, particularly in arid environments, where plant-derived (litter and root) input to soil is a large part of the carbon pool targeted by microbial decomposers (Egidi et al. 2024). Further, the multiple pathways by which reintroduced native fauna might modify the ecosystem (e.g., via shifts in plant attributes) could also have repercussions for the functional potential of the resident microbial community. Restoration of landscapes for fauna-driven functions will thus need to consider the implications of these potential shifts, especially in arid sites. This might be particularly important in light of the absence of baseline data from these ecosystems preceding the European settlement (Gibb et al. 2021; Michael et al. 2022). Future studies should focus on the long-term effects of mammal reintroduction on soil microbial communities and carbon cycling, as well as the potential interactions with other environmental factors such as climate change and land use practices. Understanding these dynamics is essential for informing land management practices and ensuring the sustainability of restoration efforts in dryland ecosystems.
Author Contributions
Eleonora Egidi: conceptualization, formal analysis, data curation, funding acquisition, writing–original draft. Orsi Decker: conceptualization, funding acquisition, data curation, writing–review and editing. Claudia Coleine: data curation, writing–review and editing, Davide Albanese: data curation. Heloise Gibb: conceptualization, funding acquisition, writing–review and editing.
Acknowledgments
This study was funded by the Australian Academy of Science, Thomas Davies fund for Marine, Plant and Soil Sciences. E.E. is supported by an Australian Research Council DECRA Fellowship (DE210101822); H.G. is supported by an Australian Research Council Future Fellowship (FT130100821); C.C. is supported by the European Commission, Marie Sklodowska-Curie Fellowship (Grant Agreement No. 702057; DRYLIFE). Open access publishing facilitated by Western Sydney University, as part of the Wiley - Western Sydney University agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




