Paracrine regulation of granulosa cell development in the antral follicles in mammals
Abstract
Background
Development of ovarian follicles is regulated by a complex interaction of intra- and extra-follicular signals. Oocyte-derived paracrine factors (ODPFs) play a central role in this process in cooperation with other signals.
Methods
This review provides an overview of the recent advances in our understanding of the paracrine regulation of antral follicle development in mammals. It specifically focuses on the regulation of granulosa cell development by ODPFs, along with other intrafollicular signals.
Main Findings
Bi-directional communication between oocytes and surrounding cumulus cells is a fundamental mechanism that determines cumulus cell differentiation. Along with estrogen, ODPFs promote the expression of forkhead box L2, a critical transcription factor required for mural granulosa cells. Follicle-stimulating hormone (FSH) facilitates these processes by stimulating estrogen production in mural granulosa cells.
Conclusion
Cooperative interactions among ODPFs, FSH, and estrogen are critical in determining the fate of cumulus and mural granulosa cells, as well as the development of oocytes.
1 INTRODUCTION
Ovary plays a central role in the female reproductive system by producing functional oocytes capable of undergoing fertilization and embryogenesis and secreting several steroid hormones as part of its endocrine function. To achieve this, the normal development and function of ovarian follicles are crucial, with the oocyte playing a central role in this process. In fact, in mice, when follicles are reconstructed from oocytes and somatic cells collected separately from follicles at different developmental stages, the developmental stage of the follicle synchronizes with that of the oocyte rather than with that of the somatic cells.1 Therefore, the developmental stage of the follicle is determined by the oocyte, and this appears to be an important mechanism for establishing a suitable follicular environment for the developmental stage of the oocyte itself.
Many studies investigated the mechanism by which oocytes coordinate follicular development and function. These studies have identified critical growth factors produced by oocytes that are required for normal folliculogenesis. Oocyte-derived paracrine factors (ODPFs) regulate the way how follicular somatic cells, namely granulosa cells, respond to extra- and intrafollicular signals, profoundly by affecting the gene expression in these cells. In this review, we summarize the current understanding of paracrine regulation of antral follicle development, with an emphasis on the regulation of granulosa cell development by ODPFs and other intrafollicular signals in mammals.
2 FOLLICULOGENESIS
Follicular development begins with the formation of primordial follicles (Figure 1). Primordial follicles consist of a layer of squamous somatic cells, often called “pre-granulosa cells,” surrounding an immature and quiescent oocyte. The ovary contains thousands to millions of primordial follicles in a dormant state, which serve as a pool of oocytes produced by the animal throughout its lifetime. Some of the dormant primordial follicles initiate growth and progress to become primary follicles, where the oocyte starts to grow and the surrounding somatic cells transform into cuboidal morphology. The somatic cells surrounding the oocyte at this stage are now called “granulosa cells.” Formation and activation of primordial follicles are affected by oocytes, as evidenced by the ovarian phenotypes of mutant mouse models. For example, the ovaries of mice deficient in Figla (folliculogenesis specific basic helix–loop–helix) and Pten (phosphatase and tensin homolog) exhibit no primordial follicles and activation of entire pool of primordial follicles and consequent premature ovarian failure, respectively.2, 3
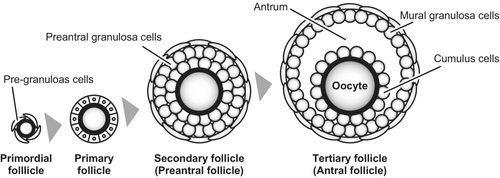
When the granulosa cells of the primary follicle continue to proliferate and form multiple cell layers, the follicle is called a secondary follicle, and the granulosa cells become round shape in mice. As a secondary follicle develops into a tertiary follicle, a fluid-filled cavity called the antrum is formed within the follicle. Hence, the tertiary follicle is also known as an “antral follicle,” while the secondary follicle is often referred to as a “preantral follicle.” Antrum formation appears to be dependent on oocytes, as shown in experiments in which oocytes or recombinant proteins of ODFPs promoted antrum formation in isolated granulosa cell complexes in vitro.4, 5
Formation of the antral cavity physically divides granulosa cells into two sub-populations in the antral follicles: cumulus cells and mural granulosa cells. Cumulus cells are located close proximate to oocytes and play a pivotal role in supporting oocyte development. In contrast, mural granulosa cells are located far from oocytes at the wall of the follicle and primarily perform endocrine functions such as estrogen production. In comparison to these granulosa cell populations in antral follicles, granulosa cells of preantral follicles (i.e., secondary follicles) are sometimes referred to as “preantral granulosa cells.” Normal development of these granulosa cell populations is crucial for ovarian function and, therefore, for normal female fertility.
3 BI-DIRECTIONAL COMMUNICATIONS BETWEEN OOCYTES AND CUMULUS CELLS
Cumulus cells contact oocytes through narrow cytoplasmic extensions known as transzonal projections (TZPs) that penetrate the zona pellucida.6, 7 At the end of TZPs, cumulus cells form heterologous gap junctions with oocytes and support oocyte development by supplying small-molecule substances, such as metabolic products.8 For example, oocytes do not express enzymes in the glycolytic pathway and therefore cannot efficiently utilize glucose as an energy substrate.9 In contrast, cumulus cells have high glycolytic activity, actively metabolize glucose, and supply metabolic products (such as pyruvate) to the oocytes.10, 11 Through this process, cumulus cells support the energy metabolism of oocytes. Similarly, cumulus cells supply substances that oocytes are not capable of producing or taking up, such as cholesterol and certain amino acids, to support oocyte development.12-14 Moreover, oocytes depend on cumulus cells for transcriptional regulation15 and meiotic controls.16
In contrast, oocytes are not merely the recipients of support from cumulus cells; rather, they actively participate in regulating the development and function of cumulus cells by secreting various growth factors. For example, oocytes promote the proliferation of cumulus cells,17 prevent apoptosis,18 and, at least in mice, enable cumulus expansion, a prerequisite process for normal ovulation.19, 20 Moreover, the aforementioned metabolic activities in cumulus cells, that is, glycolysis, amino acid uptake, and cholesterol biosynthesis, are also regulated by oocytes.12, 21, 22 In addition, while meiotic arrest of oocytes requires cyclic guanosine monophosphate (cGMP) supplied from cumulus cells through gap junctions, cGMP production by cumulus cells is regulated by oocytes.23
Therefore, while oocytes regulate the development and function of cumulus cells, cumulus cells support the normal development of oocytes. This bi-directional communication between oocytes and cumulus cells is an essential mechanism for the normal development of both cell types.
4 OOCYTE-DERIVED PARACRINE FACTORS (ODPFS)
4.1 Members of transforming growth factor beta (TGF-β) superfamily
The critical requirement for ODPFs in normal follicular development was first reported in a study using a mutant mouse model deficient in growth differentiation factor 9 (GDF9), an oocyte-secreted member of the TGF-β superfamily.24 Female mice deficient in Gdf9 gene exhibit infertility due to the arrest of follicular development at the primary follicle stage.24, 25 Similarly, in vivo administration of GDF9 or treatment with GDF9 in ovarian organ cultures has been shown to stimulate primary follicle progression in rats.26, 27 Moreover, in human ovarian organ cultures, treatment with GDF9 promotes survival and progression of follicular development to the secondary stage.28 Therefore, GDF9 promotes follicular development during the early stages.
The arrest of follicular development in Gdf9-deficient mice appears to be attributable to increased production of inhibin, which suppresses the proliferation of granulosa cells.29 In fact, in the ovaries of mice deficient in both Gdf9 and inhibin α genes, the arrest of follicular development was not observed, and follicles develop beyond the secondary stage. However, these mice eventually develop granulosa cell tumors and become infertile.29
Because the ovaries of Gdf9-deficient mice exhibit folliculogenesis arrest at the primary follicle stage, the role of GDF9 in the later stages of follicular development has mainly been studied using recombinant proteins. These studies have shown in mice that GDF9 promotes the proliferation of granulosa cells,30, 31 suppresses the expression of luteinizing hormone receptor (LHCGR) in cumulus cells,32 and promotes cumulus expansion,32 thus, highlighting its crucial role in the regulation of granulosa cell differentiation and function in the later stages of follicular development. Moreover, recombinant GDF9 and/or oocyte coculture can enhance the developmental competence of oocytes in cattle,33 mice,34 goats,35 and pigs.36
Oocytes also produce bone morphogenetic proteins (BMPs), such as BMP15 and BMP6, which are the other members of the TGF-β superfamily. The profound requirement of BMP15 for female fertility was first reported in sheep. Inverdale and Hanna sheep carrying a naturally occurring mutation in BMP15 gene exhibited increased ovulation rates in heterozygotes, whereas homozygotes exhibit primary ovarian failure due to impaired follicular development beyond the primary stage.37 Similarly, sheep carrying mutations in genes encoding BMP receptors exhibited increased ovulation rate.38-40
In contrast to sheep, mice deficient in Bmp15 gene show no significant abnormalities in the developmental dynamics of follicles; however, they are subfertile due to decreased ovulation and fertilization rates.41 Similarly, female mice deficient in Bmp6 or both Bmp15 and Bmp6 genes exhibit reduced ovulation and fertilization rates and are subfertile.42 On the other hand, the ovaries of mice deficient in the genes encoding SMAD1/5/8, which are intracellular mediators of BMP signaling, develop granulosa cell tumors, ultimately resulting in infertility.43, 44 Moreover, deficiency in the BMP receptor gene Bmpr1b results in female infertility and functional ovarian defects including lower aromatase production in granulosa cells.45 These findings suggest that an intrafollicular BMP signal consisting of BMP15 and BMP6 produced by oocytes, together with BMPs derived from other cells within the follicle, is essential for normal female fertility in mice.
BMP15 and GDF9 have also been implicated in fertility of woman. For example, mature GDF9 levels in follicular fluid significantly correlated with oocyte nuclear maturation and embryo quality in patients who underwent in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI).46 Mutations in the GDF9 genes are significantly correlated with dizygotic twinning.47, 48 Moreover, the association of mutations in the GDF949-52 and BMP1550, 53-57 genes with primary ovarian insufficiency (POI) has been reported. In addition, dysregulation of BMPR1B in granulosa cells is associated with reduced ovarian reserves and age-related decline in human fertility.58
Synergistic interaction between BMP15 and GDF9 has been reported in the regulation of granulosa cell development. For instance, as mentioned earlier, while Bmp15-deficient mice exhibit relatively mild ovarian defects, mice with an additional heterozygous deletion of Gdf9 (i.e., Bmp15−/−/Gdf9+/−) show more severe ovarian abnormalities and become infertile.41 Although the precise mechanisms underlying the synergistic function of BMP15 and GDF9 require further investigation, heterodimerization of BMP15 and GDF9 is likely to be involved. This BMP15/GDF9 heterodimer, known as cumulin, exhibits higher activity than the homodimers of BMP15 or GDF9.59 More recently, highly potent GDF9 variant, designated as “super-GDF9,” has been developed.60 Both cumulin and Super-GDF9 have been reported to improve the developmental potential of mouse oocytes in culture, and their application is anticipated to enhance oocyte development in livestock and infertility treatment in humans.61, 62
4.2 Fibroblast growth factors (FGFs)
Another growth factor family produced by mammalian oocytes is FGFs. Expression of FGF8 by oocytes has been reported in several mammalian species, including mice63 and cows.64 Although FGF8 transcript was not detected in normal human ovaries, it was detected in ovarian tumors and cancer cell lines.65 These findings suggest that FGF8 plays an important role in ovarian tumorigenesis in human. Furthermore, FGF17, one of the FGF8 subfamily ligands, is expressed by oocytes in mice,66 and by oocytes, theca cells, and granulosa cells in cows.67 Other FGF8 subfamily ligand, FGF18, is expressed by mouse oocytes, but not by bovine oocytes; instead, FGF18 was detected in bovine theca, granulosa, and luteal cells.68 Moreover, the expression of FGF receptors has been reported in granulosa cells of various mammalian species, including mice, rats, cows, and humans.64, 69-73
As mentioned above, mouse oocytes promote glycolysis in the surrounding cumulus cells and utilize the metabolic products provided by the cumulus cells as energy substrates.22 One of the ODPFs that control glycolysis in cumulus cells is FGF8.74 FGF8 cooperates with BMP15 to promote the expression of glycolytic enzymes, such as PFKP and LDHA, by cumulus cells, thereby enhancing glycolytic activity in these cells. SPRY2, an antagonist of FGF signaling, has been implicated in the coordinated action of FGF8 and BMP15.75 A cooperative interaction between FGF8 and BMP signaling has also been reported in rats. FGF8 promotes the suppressive effects of BMPs on follicle-stimulating hormone (FSH)-induced cyclic adenosine monophosphate (cAMP) production and BMP-stimulated SMAD1/5/8 phosphorylation in rat preantral granulosa cells.76
Ovarian follicles are enriched in FGF signals, and many studies have emphasized the importance of FGFs in the regulation of ovarian function. In fact, mice carrying mutations in FGF receptor genes have been reported to exhibit female infertility.77-79 However, compared to the TGF-β superfamily, there is relatively limited in vivo evidence regarding the requirement of FGF signaling in normal follicle development, such as the phenotypes observed in knockout mouse models. Future studies involving the conditional deletion of genes encoding FGF ligands and receptors are crucial to gain a deeper understanding of FGF function in the ovaries and its impact on female fertility.
5 DIFFERENTIATION BETWEEN CUMULUS AND MURAL GRANULOSA CELLS
5.1 Intrafollicular signal gradients of FSH and ODPFs
Both cumulus and mural granulosa cells differentiate from preantral granulosa cells during the transition from preantral to antral follicles. The currently accepted model for the regulation of the differentiation of these cell types is that preantral granulosa cells located near the oocytes differentiate into cumulus cells under the influence of ODPFs (Figure 2). In contrast, preantral granulosa cells located relatively far from the oocytes differentiate into mural granulosa cells. Differentiation into mural granulosa cells depends to a great extent on FSH from the pituitary gland. Therefore, the differentiation and function of cumulus and mural granulosa cells are determined by opposing signal gradients within a follicle, with FSH signaling from outside the follicle and ODPFs signaling from inside.80
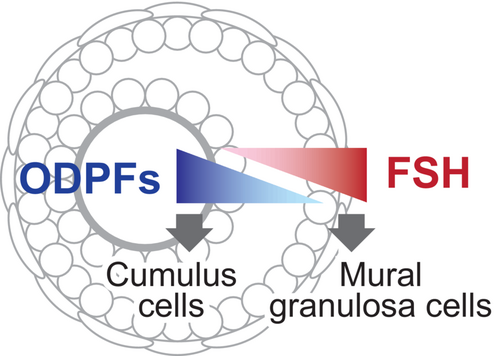
In addition to ODPFs and FSH, normal follicular development requires signals of estrogen. In fact, the disruption of estrogen signaling by deletion of the Esr2 gene encoding estrogen receptor 2 (also known as estrogen receptor-β), a predominant estrogen receptor expressed by granulosa cells, results in female subfertility,81 attenuated follicular development81-83; and reduced ovulation rates in mice.81, 84 Moreover, loss of both Esr1 (encoding estrogen receptor-α) and Esr2 results in female infertility associated with granulosa cell defects.85 Our study also revealed that the cooperative interaction between oocyte and estrogen signals is critical for the normal development and function of cumulus cells in mice.86-88 Therefore, estrogen is critical for the development and function of both cumulus cells and mural granulosa cells.
A comprehensive analysis of cellular transcripts is a valuable approach for understanding the processes and regulation of cell differentiation and function. We have previously attempted to elucidate the differentiation processes and regulatory mechanisms of cumulus and mural granulosa cells through transcriptomic analysis.12, 87, 89, 90 According to these studies, there are significant differences in the expression of over 3000 genes between cumulus and mural granulosa cells.89 Transcripts highly represented in cumulus cells are often associated with cell proliferation and metabolism-related processes such as glycolysis and cholesterol production, whereas those related to steroid production show higher expression in mural granulosa cells. These transcriptomic differences between these cell types are in agreement with in vitro experiments showing that oocytes promote the expression of transcripts encoding enzymes involved in glycolysis and cholesterol biosynthesis in cumulus cells,12, 22, 74 whereas FSH regulates the expression of enzymes involved in steroid synthesis in mural granulosa cells.91, 92 Furthermore, by comparing these data with the transcriptomic differences between cumulus cells of Bmp15−/−/Gdf9+/− mice and wild-type mice12 and the changes in gene expression upon stimulation of cumulus cells with ODPFs,87 it was suggested that approximately half of the significantly upregulated genes in cumulus cells compared to mural granulosa cells are directly regulated by oocytes.89 These findings strongly support the importance of oocyte-derived signals in cumulus cell differentiation.
Although the importance of ODPFs in the development of cumulus cells has been reported, oocytes are also required for the development and maintenance of mural granulosa cells. For example, in the reconstructed follicles where the somatic cells of newborn ovaries were combined with mid-growth stage oocytes, the development of not only cumulus cells but also that of mural granulosa cells was accelerated.1 Moreover, removing cumulus-oocyte complexes from antral follicles in rabbits results in precocious luteinization of mural granulosa cells,93 and oocytes suppress the luteinization of cultured mural granulosa cells in rats.94 These observations indicate that oocyte-derived signals are required not only for the development of mural granulosa cells but also for maintaining the characteristics of mural granulosa cells; however, the mechanism by which oocytes influence these processes is not well understood.
5.2 Forkhead box L2 (FOXL2): A transcriptional regulator of cumulus and mural granulosa cell differentiations
To explore the basic mechanisms that determine the transcriptomic differences between cumulus and mural granulosa cells, we attempted to identify the “transcriptional regulators” controlling the differences in gene expression between these two granulosa cell populations using mice as a model.90 The results showed that a transcription factor FOXL2 is a critical transcriptional regulator responsible for the differentiation of mural granulosa cells. In fact, the target transcripts of FOXL2 are enriched in those involved in steroid metabolism, which is one of the critical functions of mural granulosa cells, but not cumulus cells.95
FOXL2 is an essential transcription factor involved in normal ovarian development and function. In Foxl2-deficient ovaries, granulosa cells do not complete the squamous-to-cuboidal transition, which normally occurs during the transition from primordial to primary follicles, and result in infertility.96, 97 In contrast, when Foxl2 genes are deleted in the ovaries of adult mice, developing granulosa cells become express testis-specific genes such as SOX9 and sexually transdifferentiate into Sertoli cell-like cells.98 In humans, mutations in FOXL2 gene cause the blepharophimosis-ptosis-epicanthus inversus syndrome (BPES), which is often associated with POI.99 Additionally, approximately 97% of adult-type granulosa cell tumors harbor a somatic missense mutation in the FOXL2 gene.100, 101 Based on these findings, FOXL2 is considered an essential transcription factor for the development and maintenance of granulosa cells.
We previously reported in mice that FOXL2 expression increases during the development of mural granulosa cells, while it is maintained at a lower level in cumulus cells due to suppression by ODPFs.88 In addition, the high level of FOXL2 expression in mural granulosa cells requires the stimulation of both ODPFs and estrogen.102 Therefore, oocytes exert two antagonistic effects on FOXL2 expression during granulosa cell differentiation: they cooperate with estrogen signaling to promote mural granulosa cell differentiation by enhancing FOXL2 expression and facilitate cumulus cell differentiation by suppressing FOXL2 expression.
Ovarian follicles are composed of various cell types including oocytes, cumulus cells, and mural granulosa cells. The coordinated development and function of each cell type is crucial for normal ovarian function and female fertility (Figure 3). Bi-directional communication between oocytes and cumulus cells is essential for the normal development of both cell types.8, 103 Mural granulosa cells facilitate this bi-directional communication by producing estrogen since ODPFs and estrogen cooperatively regulate the development and function of cumulus cells.86, 87 At the sametime, along with estrogen, oocytes regulate the development and function of mural granulosa cells by promoting FOXL2 expression.88, 102 Importantly, estrogen production by mural granulosa cells is controlled by FSH.104-106 Therefore, FSH indirectly influences oocyte development by promoting cumulus cell development via estrogen, at least, in mice. These interactions are likely part of the complex regulatory network governing granulosa cell development and warrant further research. Furthermore, since these studies were conducted in mice, it is important to determine whether a similar mechanism exists in other mammals.
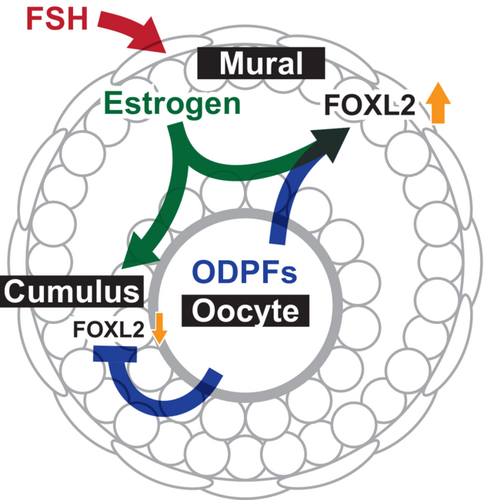
In addition to FOXL2 expression, differences are observed in the expression of epigenetic regulators between cumulus and mural granulosa cells.88 Therefore, epigenetic regulation may play a role in determining the fate of these cells. Further investigation of these factors will provide deeper insights into the cellular mechanisms regulating the differentiation of cumulus and mural granulosa cells.
6 CONCLUSION
The potential importance of nutrient support from the surrounding granulosa cells during oocyte development was first noted over 100 years ago.107 Subsequent studies have shed light on the regulation of granulosa cell differentiation and revealed many roles played by them. These studies have greatly improved our understanding of follicular development. However, the specific mechanism involved remains unclear. Recent advances in next-generation sequencing and genome-editing technologies have drastically accelerated the research progress. Further investigations using these advanced techniques will accelerate our understanding of follicular development and aid in the production of efficient assisted reproductive technologies.
ACKNOWLEDGEMENTS
The authors are grateful to the members of the Laboratory of Applied Genetics (The University of Tokyo) for their helpful input and critical reading of this manuscript. This work was supported by the Japan Society for the Promotion of Science (grant/award numbers: JP23H02359, JP21K19183, and JP20H03124).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




