Robot-Assisted Laparoscopic Nephrectomy for Wilms' Tumour in Children
Funding: This work was supported by the general program of National Natural Science Foundation of China (No. 32270853), the ‘Pioneer’ and ‘Leading Goose’ R&D Program of Zhejiang Province (No. 2024C03181), and the Medical Science and Technology Project of Zhejiang Province (No. 2022KY860).
Previous communication: The paper was not based on a previous communication to a society or meeting.
ABSTRACT
Background
The purpose of this study was to share our experiences with robot-assisted laparoscopic nephrectomy (RALN) for WT in children and to discuss the technical points and indications.
Methods
Patients with WT undergoing RALN between May 2020 and December 2022 were retrospectively analysed. Patient demographics, operative details, postoperative outcomes and follow-up were recorded.
Results
A total of 10 patients with WT who underwent RALN were enrolled in this study. The tumour diameter at operation was 70.2 ± 26.1 mm. The median tumour-abdominal volume ratio (TAVR) was 6.8% (range, 1.9%–14.8%). RALN was successfully performed in all the 10 patients without conversion, tumour rupture or operative complications. No local recurrence or death occurred during the follow-up period.
Conclusion
RALN for the treatment of WT in children appears to be safe and feasible in selected patients. Robotic surgery offers an effective alternative to laparoscopic surgery, appropriately expanding indications when performed by experienced surgeons.
1 Introduction
Wilms tumour (WT) is the most common renal tumour in children, accounting for more than 80% of cases [1]. Nephrectomy with adequate lymph node sampling is the mainstay of treatment for WT [2]. Minimally invasive surgery (MIS) has been widely used in the treatment of adult renal cell carcinoma [3], however it has played a relatively peripheral role in the resection of WT due to the frequently large tumour volume, concern for rupture, and small abdominal space of the toddler aged children who most at risk for developing WT. Nonetheless, a number of reports over the last 2 decades have illustrated that with optimised selection criteria, minimally invasive techniques can be used in the resection of WT. Duarte et al. first reported laparoscopic nephrectomy (LN) for WT in 2004 [4]; since then, this technology has been frequently reported and proved to be safe and feasible, and the criteria for LN are gradually being expanded [5, 6].
The Renal Tumour Study Group of the International Society of Paediatric Oncology (SIOP-RTSG) released the UMBRELLA protocol in 2016, which included surgical recommendations for treatment of WT [7]. LN was considered acceptable within these guidelines provided resection adhered to oncologic principles and included lymph node sampling (7 nodes). Tumours were required to be small and central with a surrounding rim of normal tissue, thus guaranteeing negative margins. Contraindications to laparoscopic nephrectomy included tumour infiltrating extra renal structures or extending beyond the border of the spinal column, venous thrombus, peripheral location, and tumour without response to chemotherapy. Robotic surgery was not specifically addressed within the 2015 UMBRELLA protocol, but the use of robotic surgery has gained increasing acceptance over the last decade and has been considered preferable to laparoscopic techniques by many paediatric surgeons [8-10]. Cost et al. reported the first applications of robot-assisted laparoscopic nephrectomy (RALN) for paediatric WT in 2015 [11]. Subsequent case reports published since this study have demonstrated encouraging outcomes [12, 13]. Here, we present a study of RALN for WT in children and discuss the technical points and indications.
2 Materials and Methods
2.1 Patients
We conducted a retrospective study of patients who underwent RALN for WT between May 2020 and December 2022. Chemotherapy response and selection criteria were pre-operatively evaluated by enhanced computed tomography scan. Patient demographics, operative details, postoperative outcomes and follow-up were recorded.
All patients underwent preoperative contrast-enhanced CT imaging either at admission or prior to surgery for tumour staging and vascular assessment. Tumour size, tumour-abdominal volume ratio (TAVR), tumour stage, lymph node metastases, venous thrombus, and tumour rupture were factors to take into account when choosing RALN. The inclusion criteria were unilateral tumours with stage I and II, diameter less than 10 cm or volume ratio less than 15%. Patients who had lymph node metastases, venous thrombosis, preoperative tumour rupture, bilateral WT, or extrarenal extension were deemed contraindications and excluded. The UMBRELLA protocol recommended that MIS could be acceptable in selected circumstances, including small central tumours with a rim of nonmalignant renal tissue, which still enable lymph node sampling. Tumours in this cohort were appropriately increased in diameter and included exogenous tumours. Considering the high cost and out-of-pocket of robotic surgery in China, the final decision to perform RALN was made by a solid tumour multidisciplinary team together with the Guardians. All procedures were performed by the same group of experienced surgeons.
Endogenous tumours refer to those that are located inside the kidney with nonmalignant renal tissue on the surface of the tumour. Exogenous tumours refer to those that are located at the edge of the kidney and lack renal parenchyma in certain areas of their surface.
TAVR is the ratio between tumour volume and abdominal volume. Abdominal volume is defined as below the diaphragm and above the pelvic inlet plane. The volume of tumour and abdominal cavity was measured by 3D CT images (Figure 1).
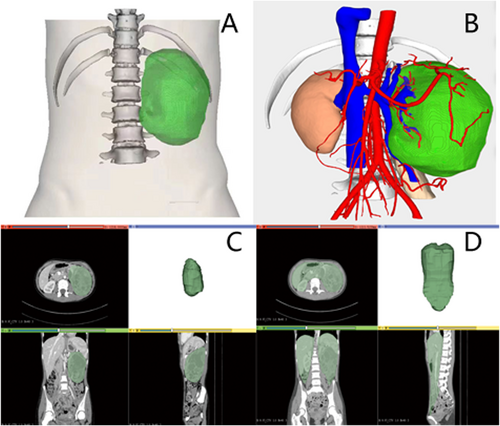
Measurement of tumour-abdominal volume ratio (TAVR) with 3D images. (A–B) 3D imaging of the tumour. (C) Tumour volume. (D) Abdominal volume.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Children's Hospital, Zhejiang University School of Medicine (2023-IRB-0178). Informed consent was obtained from all the study participants and their parents involved in the study.
2.2 RALN Procedures (Surgical Technique)
The temperature in the operating room was maintained between 23°C and 25°C, with relative humidity controlled at 40%–60%. The surgical equipment configuration is illustrated in Figure 2.
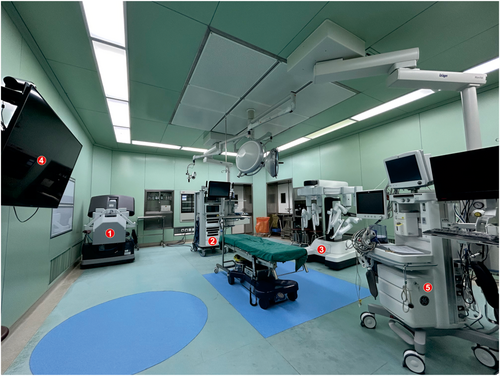
Operative room setting: 1. Surgeon console, 2. Vision cart, 3. Patient cart, 4. High-definition medical display screen, 5. Anaesthesia workstation.
We used the Da Vinci Xi Surgical System to perform the surgery with four ports: three 8-mm robotic arm ports and one 5-mm auxiliary port (Figure 3). The patient was placed in a 60° contralateral decubitus position.
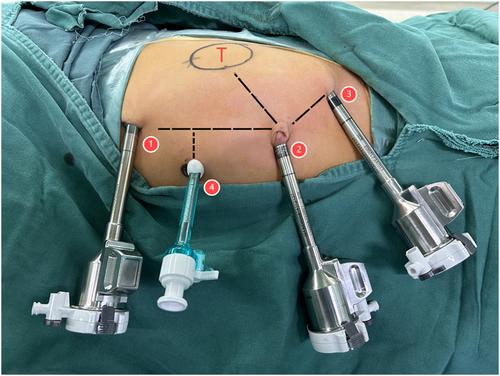
Port placement for right robotic nephrectomy: 1. Da Vinci port, 2. Camera port, 3. Da Vinci port, 4. Assistant port.
An 8-mm incision was made through the umbilicus, and pneumoperitoneum was established by a needle inserted to inject CO2 gas, followed by inserting an 8-mm Da Vinci trocar for the 30° 3D video laparoscope. Another two 8-mm robotic cannulae were placed at the ipsilateral lower abdomen and midline below the xiphoid, respectively, for fenestrated bipolar forceps and permanent cautery hook. Additionally, a 5-mm auxiliary port was placed on the contralateral upper abdomen. The robot was docked over the patient's back perpendicular to the operating room table, and instruments were inserted.
The colon was mobilised and pulled inside. After opening the Gerota's fascia following dissection of the retroperitoneal adipose tissue, the assistant provided additional medial retraction of the ascending colon and duodenum (right) or the descending colon, pancreas and spleen (left) through the auxiliary port, and the renal vein and renal artery were clearly visible. Once the renal hilar anatomy was defined and circumferentially mobilised, multiple hemoclips were used to seal the renal artery prior to its division, followed by the renal vein. The tail of Gerota's fascia was retracted laterally with the ureter and gonadal vein. Once identified, the ureter was ligated with a hemoclip and severed at the distal end to the iliac artery. If there were haematuria or imaging indications of pelvic and calyceal tumour invasion, ureter ligation was performed at the beginning of the operation. The nephrectomy process is shown in Figure 4.
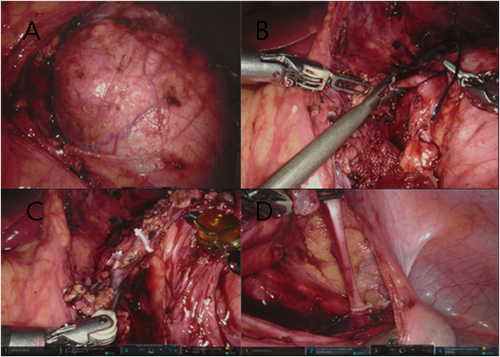
Robot-assisted laparoscopic nephrectomy procedures. (A) Three-dimensional visualisation of WT with robotic image magnification. (B–D) Ligation of renal artery, renal vein and ureter with hemoclips.
After releasing superior and lateral attachments of the kidney and tumour, the kidney was fully freed. After nephroureterectomy, lymph nodes around the renal hilum, inferior vena cava and aorta were sampled. Aortocaval nodes were not regularly sampled unless there were noticeable lymph nodes. A drainage tube was placed at the renal fossa under endoscopic monitoring, and the tumour along with the kidney was placed in a plastic bag and removed through the enlarged umbilicus incision.
Patients were transferred to the ICU for postoperative monitoring, and back to the general ward after successful weaning from mechanical ventilation and stabilisation of vital signs, typically within a 24-h ICU observation period. Intravenous patient-controlled analgesia (PCA) pumps were given for 2–3 days after operation, and for patients younger than 3 years, oral ibuprofen was used. Local infiltration anaesthesia was administered at the surgical incision site for supplementary pain control. Prophylactic antibiotics were administered for no more than 48 h following surgery, unless infection necessitated therapeutic antibiotic therapy. Drain removal occurred when output was < 30 mL/day for two consecutive days with normal characteristics. Discharge criteria required (1) tolerating oral intake, (2) drain removal, (3) normal renal function parameters, and (4) absence of major complications.
2.3 Complications and Outcome
Major intraoperative complications, including haemorrhage and tumour rupture, constitute the primary indications for conversion to open surgery. Postoperative morbidity predominantly includes lymphatic leakage, ureteral fistula, surgical site infection, and acute kidney injury. These complications significantly prolong hospital stay and necessitate protocol adjustments for adjuvant chemotherapy administration.
2.4 Statistical Analysis
For statistical analysis, the IBM SPSS software (Version 26.0.0; Armonk, NY, IBM Corp.) was used. The frequency of continuous variables was reported as median and range, and the categorical variables were expressed as counts and percentages.
3 Results
A total of 57 patients with WT underwent nephrectomy during the study period, of which 10 patients underwent RALN were enrolled in this study. The median age was 50.5 months (range, 9–156), and the median weight was 15.5 kg (range, 8.4–32.3). The tumours were located in the left kidney in 4 cases and the right kidney in 6 cases. The median maximum tumour diameter at operation was 70.2 ± 26.1 mm (range, 45–132). Median tumour volume was 147.1 cc (range, 20.9–671.1), accounting for 6.8% of the abdominal volume on average (TAVR, range, 1.9%–14.8%). Eight cases were exogenous and 2 cases were endogenous. Five patients (50%) received neoadjuvant chemotherapy, achieving a median tumour volume reduction of 46% (range: 39%–60%) prior to initial size. Patient demographics, preoperative data and clinical data are summarised in Table 1.
| Patient no. | Sex | Age | Weight (kg) | Side | Clinical presentation | Exophytic/Endophytic | NLNA | Cross the midline | MTD (cm) | Tumour volume (cm3) | TAVR (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | F | 3Y7M | 13.5 | R | Abd. pain | Ex | 6 | No | 6.0 | 68.3 | 5.4 |
| #2 | M | 1Y1M | 9 | R | None | En | 6 | No | 5.8 | 32.1 | 3.5 |
| #3 | M | 3Y5M | 13.9 | R | Abd. pain | Ex | 5 | No | 4.7 | 68.1 | 4.9 |
| #4 | M | 5Y3M | 19.2 | L | Abd. pain | Ex | 4 | No | 6.0 | 42.2 | 2.1 |
| #5 | M | 2Y2M | 13.8 | R | Abd. pain | Ex | 12 | No | 8.8 | 150.9 | 9.1 |
| #6 | F | 11M | 8.4 | L | Hematemesis | Ex | 12 | Yes | 8.7 | 194.6 | 13.0 |
| #7 | M | 9M | 8.5 | L | None | En | 8 | No | 4.5 | 20.9 | 1.9 |
| #8 | F | 5Y1M | 22.1 | R | Hematemesis | Ex | 8 | No | 6.7 | 124.9 | 6.6 |
| #9 | M | 6Y | 14.2 | R | Abd. pain | Ex | 6 | No | 5.8 | 96.7 | 6.4 |
| #10 | F | 13Y | 32.3 | L | Abd. pain | Ex | 23 | Yes | 13.2 | 671.1 | 14.8 |
- Abbreviations: Abd. pain, abdominal pain; En, endogenous; Ex, exogenous; MTD, maximum tumour diameter; NLNA, number of lymph nodes sampling; TAVR, tumour-abdominal volume ratio.
All 10 patients underwent RALN successfully, without conversion, tumour rupture or operative complications. Tumours were completely resected with clear margins. The number of lymph nodes sampling was 9 ± 5.3 (range, 4–23), and none of the patients had metastases. The median operation time was 3.9 ± 1.1 h (range, 2.5–6), the intraoperative haemorrhage was 24 ± 15.1 mL (range, 5–50), and the median postoperative hospital stay was 4.6 ± 1.8 days (range 2–7). Histological patterns were mixed type, blastemal type, epithelial type and stromal type in 3 cases, 3 cases, 2 cases, and 2 cases, respectively. There was no local recurrence over the median follow-up time of 35.7 ± 12.9 months (range, 18–50). All patients were disease-free before the deadline of follow-up (April 30, 2023). Surgical and postoperative data of patients are summarised in Table 2.
| Patient no. | Operation time (hour) | IBL (mL) | PHS (day) | Histological patternsa | Local recurrence | Follow-up time (Mon) |
|---|---|---|---|---|---|---|
| #1 | 2.5 | 30 | 6 | S | No | 50 |
| #2 | 3 | 10 | 3 | M | No | 49 |
| #3 | 6 | 50 | 5 | B | No | 49 |
| #4 | 5 | 20 | 3 | S | No | 47 |
| #5 | 3 | 20 | 7 | M | No | 36 |
| #6 | 3.5 | 10 | 6 | E | No | 35 |
| #7 | 2.9 | 5 | 3 | E | No | 32 |
| #8 | 3.5 | 15 | 2 | B | No | 23 |
| #9 | 4.6 | 50 | 6 | B | No | 18 |
| #10 | 5 | 30 | 6 | M | No | 18 |
- Abbreviations: IBL, intraoperative blood loss; PHS, postoperative hospital stay.
- a Histological patterns include blastemal (B), epithelial (E), stromal (S) and mixed (M) types.
4 Discussion
In this study, we summarise the experience of 10 patients undergoing RALN for Wilms tumour. The results demonstrate that Robotic surgery appears to be safe and feasible in selected patients, offering an effective alternative to laparoscopic surgery, appropriately expanding indications when performed by experienced surgeons.
Significant tumour shrinkage and flexible encapsulation caused by preoperative neoadjuvant chemotherapy make MIS possible in patients with WT. Of course, MIS for WT should strictly adhere to the principles of oncologic removal, including complete tumour resection, avoidance of tumour rupture, and a negative surgical margin [14]. Due to these high standards, the wide application of MIS in WT is limited.
With improvements in instrumentation and precision, as well as optimization of ergonomics, MIS has been successfully applied to even the smallest paediatric patients [15]. Since the first report of robotic-assisted surgery in children was published with the successful completion of a robotic Nissen fundoplication in a 10-year-old girl [16], an increasing number of reports have been published about robotic surgery in paediatric surgery [15, 17]. It allows for significant benefits over laparoscopy, including three-dimensional magnification, tremor filtering, precision and flexibility, all of which play significant roles in the success of MIS for retroperitoneal tumours [18]. Paediatric oncology surgeons are hopeful about MIS for WT once again and exploring its indications further.
In the present study, the results show that robotic surgery is safe and feasible for tumours less than 10 cm in diameter or less than 15% TAVR, whether endogenous or exogenous tumours without extrarenal extension. But currently there is no standardisation of the technical approach, patient selection, size and extension of tumours, which have been identified as critical parameters for performing MIS safely [19]. TAVR reduces the impact of patient weight and age and substitutes tumour size as a reference index to some extent. The patients in our cohort had TAVR of less than 15%, which was proved to be a secure level for robot surgery. Large sample statistics in the future are needed to estimate the upper limit value of TAVR.
Tumours beyond the midline do not get in the way of MIS [5]. The key technical challenge is the separation and ligation of the renal pedicle. The lateral side of the kidney can be separated initially if the renal pedicle cannot be made visible. When the range of motion of the kidney grows, ligation of the renal pedicle can be safely completed by a robotic system with a more flexible joint and more stable control.
Although the UMBRELLA protocol did not specify the indications for MIS, central tumours with a rim of nonmalignant renal tissue were recommended [7]. Different from renal carcinoma, WT typically has a large tumour at the time of diagnosis and endogenous tumours are relatively uncommon. Preoperative chemotherapy reduces tumour size but cannot transform exogenous tumour into endogenous tumour. In this cohort, exogenous WT within a reasonable size could be safely removed by the robotic surgical system with greater magnification and a precision multi-angle rotating manipulator, which enabled finer separation of tumour capsule and surrounding tissue. It doesn't seem like exogenous or endogenous tumour is one of the main determinants of whether MIS is feasible. The indications could be improved, for example, by recommending small, unruptured tumours, regardless of endogenous or exogenous tumours. Of course, it also needs to be supported by a sizeable sample of data in the future. However, MIS is not advised when the tumour penetrates the renal envelope and invades nearby, which could raise the risk of tumour rupture.
Lymph node sampling is an essential step in WT surgery and is usually performed around the inferior vena cava and aorta [20]. The highest proportion of positive nodes is discovered when at least 7 lymph nodes are sampled [21]. For favourable histology WT, it appears that the desired lymph node yield to reduce the risk of false-negative lymph node sampling to ≤ 10% is between 6 and 10 [22]. Otherwise, it may downstage the tumour and lead to inadequate adjuvant therapy and increase the risk of local recurrence [21]. Patients with lymph node metastases around the aorta and vena cava were not advised to undergo MIS. We found that while the robot surgical system can successfully perform lymph node dissection on the side of the tumour, it proved to be challenging beyond the spine, particularly in the interaortocaval area, which may raise the risk of vascular injury and lymph node rupture. Naturally, there are sufficient lymph nodes in the area adjacent to the renal hilum and ipsilateral area.
Many malignant diseases have seen changes in treatment paradigms as a consequence of improvements to adjuvant therapy, and meanwhile fuelled the growth of organ-sparing surgery in an effort to preserve native form and function [23]. Robot-assisted laparoscopic partial nephrectomy has been successful in managing renal cell carcinoma in adult urology [24], and will continue to be applied to WT in children. In the near future, perhaps this rapidly evolving technology could be generally applied to aid in nephrectomy for WT and even larger tumours in younger patients. Looking even further ahead, it's possible that this technology will be used to revolutionise nephron spring surgery for WT.
5 Limits
The limitations of the present study are its retrospective nature, small sample size and short follow-up period; in order to validate the advantages of this technique, a prospective larger multi-centre clinical trial and long-term follow-up is necessary.
6 Conclusion
In conclusion, RALN for the treatment of WT in children is feasible in selected patients. Robotic surgery offers a safe and effective alternative to laparoscopic surgery, appropriately expanding indications when performed by surgeons who have had special training and are experienced in the procedures. Further studies are needed to evaluate the long-term outcomes of RALN for the treatment of WT and to create an effective scoring system to assess the grounds for MIS.
Author Contributions
Article conception and design: Min He, Jinhu Wang and Xiang Yan. Operation implementation: Min He, Jiabin Cai and Jinhu Wang. Data collection and analysis: Xiaohui Ma, Xuan Wu, Junqin Mao, Linjie Li, Lifeng Zhang, Meidan Ying, Ziqi He and Ting Tao. Manuscript writing: Min He, Shuangai Liu and Xiang Yan.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Children's Hospital, Zhejiang University School of Medicine (2023-IRB-0178).
Consent
Informed consent was obtained from all the study participants and their parents involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Permission to Reproduce Material From Other Sources
The authors agree to the permission to reproduce material from other sources.
Open Research
Data Availability Statement
The datasets generated during the current study are available from the corresponding author upon reasonable request.




