Electronegativity equalization and the electronic structure of polyhedral clusters of main-group atoms
Abstract
The concept of the equalization of atomic electronegativities accompanving molecule formation is applied to a study of the electronic structure of polyhedral clusters of main-group atoms such as Ge, Sn, Pb, Tl, and Bi. Emphasis is placed upon charged clusters such as Sn9−x Pb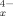 (x = 0 → 9), Sn9-xGe
(x = 0 → 9), Sn9-xGe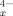 , Sn8−xPbx Tl5−, Sn2Bi
, Sn8−xPbx Tl5−, Sn2Bi , SnTe
, SnTe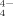 , etc. The role of the relativistic spin-orbit splitting of an np shell into np1/2 and np3/2 subshells in modifying atomic and hence molecular electronegativities is discussed. Correlations are made between calculated charge distributions and observed199 Sn NMR chemical shifts for clusters of a given size and charge. It is concluded that a useful picture of charge distributions in these clusters may be obtained from electronegativity equalization considerations.
, etc. The role of the relativistic spin-orbit splitting of an np shell into np1/2 and np3/2 subshells in modifying atomic and hence molecular electronegativities is discussed. Correlations are made between calculated charge distributions and observed199 Sn NMR chemical shifts for clusters of a given size and charge. It is concluded that a useful picture of charge distributions in these clusters may be obtained from electronegativity equalization considerations.




