A theoretical study of the structure and properties of uric acid: A potent antioxidant
Abstract
A detailed study of tautomeric properties of uric acid, its different anions, and radical species was performed at the DFT level, employing B3LYP functional and the 6-311++G(d,p) basis set. Single-point energy calculations were also performed at the MP2 level using B3LYP/6-311++G(d,p) optimized geometries and the cc-pVTZ and 6-311++G(d,p) basis sets. The effect of aqueous solvation on the relative stability of the neutral and anionic species was investigated using Tomasi's polarized continuum model. The keto form of the molecule was found to be the most stable in the gas phase and in aqueous medium. The proton transfer barrier height was also computed. The gas-phase barrier height is high; however, the inclusion of a water molecule in the proton transfer reaction path reduces the barrier significantly. Among monoanions of uric acid, the species obtained by the deprotonation of the N3 site is the most stable, whereas among dianions, the anion obtained by deprotonation of both N3 and N9 sites is the most stable both in the gas phase and in aqueous medium. It appears that both forms of radicals UAN and UAN
and UAN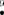 would exist. Among radical anions, the species obtained by the dehydrogenation of the N3 site of the N7 anion (the N7 anion was obtained by deprotonation of the N7 site of uric acid) is the most stable in the gas phase and in aqueous solution. The molecular electrostatic potential, ionization potential, and electron affinity are also reported. © 2004 Wiley Periodicals, Inc. Int J Quantum Chem, 2004
would exist. Among radical anions, the species obtained by the dehydrogenation of the N3 site of the N7 anion (the N7 anion was obtained by deprotonation of the N7 site of uric acid) is the most stable in the gas phase and in aqueous solution. The molecular electrostatic potential, ionization potential, and electron affinity are also reported. © 2004 Wiley Periodicals, Inc. Int J Quantum Chem, 2004




