Indefinite noncooperative self-association of chicken deoxy hemoglobin D†
Mitra S. Rana
Section of Neurobiology, School of Biological Sciences, University of Texas, Austin, Texas 78712-0252
Institute for Cellular and Molecular Biology, University of Texas, Austin, Texas 78712-0252
Search for more papers by this authorCorresponding Author
Austen F. Riggs
Section of Neurobiology, School of Biological Sciences, University of Texas, Austin, Texas 78712-0252
Institute for Cellular and Molecular Biology, University of Texas, Austin, Texas 78712-0252
Section of Neurobiology, School of Biological Sciences, University of Texas, Austin, Texas 78712-0252===Search for more papers by this authorMitra S. Rana
Section of Neurobiology, School of Biological Sciences, University of Texas, Austin, Texas 78712-0252
Institute for Cellular and Molecular Biology, University of Texas, Austin, Texas 78712-0252
Search for more papers by this authorCorresponding Author
Austen F. Riggs
Section of Neurobiology, School of Biological Sciences, University of Texas, Austin, Texas 78712-0252
Institute for Cellular and Molecular Biology, University of Texas, Austin, Texas 78712-0252
Section of Neurobiology, School of Biological Sciences, University of Texas, Austin, Texas 78712-0252===Search for more papers by this authorPortions of this paper are based on the PhD dissertation of M.S. Rana at the University of Texas, Austin in 2010. (All work performed at The University of Texas at Austin)
Abstract
The minor tetrameric hemoglobin (Hb), Hb D, of chicken red blood cells self-associates upon deoxygenation. This self-association enhances the cooperativity of oxygen binding. The maximal Hill coefficient is greater than 4 at high Hb concentrations. Previous measurements at low Hb concentrations were consistent with a monomer-to-dimer equilibrium and an association constant of ∼1.3–1.6 × 104
M−1. Here, the Hb tetramer is considered as the monomer. However, new results indicate that the association extends beyond the dimer. We show by combination of Hb oligomer modeling and sedimentation velocity analyses that the data can be well described by an indefinite noncooperative or isodesmic association model. In this model, the deoxy Hb D associates noncooperatively to give a linear oligomeric chain with an equilibrium association constant of 1.42 × 104
M−1 at 20°C for each step. The data are also well described by a monomer–dimer–tetramer equilibrium model with monomer-to-dimer and dimer-to-tetramer association constants of 1.87 and 1.03 × 104
M−1 at 20°C, respectively. A hybrid recombinant Hb D was prepared with recombinant αD-globin and native β-globin to give a Hb D tetramer (α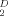 β2). This rHb D undergoes decreased deoxygenation-dependent self-association compared with the native Hb D. Residue glutamate 138 has previously been proposed to influence intertetramer interactions. Our results with recombinant Hb D show that Glu138 plays no role in deoxy Hb D intertetramer interactions. Proteins 2011. © 2011 Wiley-Liss, Inc.
β2). This rHb D undergoes decreased deoxygenation-dependent self-association compared with the native Hb D. Residue glutamate 138 has previously been proposed to influence intertetramer interactions. Our results with recombinant Hb D show that Glu138 plays no role in deoxy Hb D intertetramer interactions. Proteins 2011. © 2011 Wiley-Liss, Inc.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
| Filename | Description |
|---|---|
| PROT_22978_sm_suppinfo.pdf909.7 KB | Supporting Information. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1 Riggs AF. Self-association, cooperativity and supercooperativity of oxygen binding by hemoglobins. J Exp Biol 1998; 201: 1073–1084.
- 2 Rana MS,Knapp JE,Holland RA,Riggs AF. Component D of chicken hemoglobin and the hemoglobin of the embryonic Tammar wallaby (Macropus eugenii) self-associate upon deoxygenation: effect on oxygen binding. Proteins 2008; 70: 553–561.
- 3 Calvert SJ,Holland RA,Hinds LA. Blood O2 transport and Hb types in the embryonic Tammar Wallaby (Marsupialia, Macropus eugenii). Respir Physiol 1993; 91: 99–109.
- 4 Holland RA,Rimes AF,Comis A,Tyndale-Biscoe CH. Oxygen carriage and carbonic anhydrase activity in the blood of a marsupial, the Tammar wallaby (Macropus eugenii), during early development. Respir Physiol 1988; 73: 69–86.
- 5 Holland RA,Calvert SJ,Hope RM,Chesson CM. Blood O2 transport in newborn and adult of a very small marsupial (Sminthopsis crassicaudata). Respir Physiol 1994; 98: 69–81.
- 6 Henty K,Wells RM,Brittain T. Characterization of the hemoglobins of the neonatal brushtailed possum Trichosurus vulpecula (Kerr): evidence for a highly cooperative, aggregated isoform of hemoglobin. Comp Biochem Physiol A Mol Integr Physiol 2008; 150: 52–57.
- 7 Saha A,Ghosh J. Comparative studies on avian hemoglobins. Comp Biochem Physiol 1965; 15: 217–235.
- 8 Bruns GA,Ingram VM. The erythroid cells and haemoglobins of the chick embryo. Philos Trans R Soc Lond B Biol Sci 1973; 266: 225–305.
- 9 Brown JL,Ingram VM. Structural studies on chick embryonic hemoglobins. J Biol Chem 1974; 249: 3960–3972.
- 10 Keane RW,Abbott UK,Brown JL,Ingram VM. Ontogeny of hemoglobins: evidence for hemoglobin M. Dev Biol 1974; 38: 229–236.
- 11 Chapman BS,Hood LE,Tobin AJ. Minor early embryonic chick hemoglobin M. Amino acid sequences of the ε and αD chains. J Biol Chem 1982; 257: 651–658.
- 12 Rücknagel KP,Reischl E,Braunitzer G. Hemoglobins of reptiles. Expression of alpha-D-genes in the turtles, Chrysemys picta bellii and Phrynops hilarii (Testudines). Hoppe Seylers Z Physiol Chem 1984; 365: 1163–1171.
- 13 Czelusniak J,Goodman M,Hewett-Emmett D,Weiss ML,Venta PJ,Tashian RE. Phylogenetic origins and adaptive evolution of avian and mammalian haemoglobin genes. Nature 1982; 298: 297–300.
- 14 Cooper SJ,Wheeler D,De Leo A,Cheng JF,Holland RA,Marshall Graves JA,Hope RM. The mammalian αD-globin gene lineage and a new model for the molecular evolution of α-globin gene clusters at the stem of the mammalian radiation. Mol Phylogenet Evol 2006; 38: 439–448.
- 15 Goh SH,Lee YT,Bhanu NV,Cam MC,Desper R,Martin BM,Moharram R,Gherman RB,Miller JL. A newly discovered human α-globin gene. Blood 2005; 106: 1466–1472.
- 16 Hoffmann FG,Storz JF. The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol Biol Evol 2007; 24: 1982–1990.
- 17 Cirotto C,Geraci G. Embryonic chicken hemoglobins. Studies on the oxygen equilibrium of two pure components. Comp Biochem Physiol A Comp Physiol 1975; 51: 159–163.
- 18 Baumann R,Goldbach E,Haller EA,Wright PG. Organic phosphates increase the solubility of avian haemoglobin D and embryonic chicken haemoglobin. Biochem J 1984; 217: 767–771.
- 19 Lapennas GN,Reeves RB. Oxygen affinity and equilibrium curve shape in blood of chicken embryos. Respir Physiol 1983; 52: 13–26.
- 20 Baumann R,Padeken S,Haller EA. Functional properties of embryonic chicken hemoglobins. J Appl Physiol 1982; 53: 1439–1448.
- 21 Baumann R,Meuer HJ. Blood oxygen transport in the early avian embryo. Physiol Rev 1992; 72: 941–965.
- 22 Holland RA,Calvert SJ. Oxygen transport by rabbit embryonic blood: high cooperativity of hemoglobin-oxygen binding. Respir Physiol 1995; 99: 157–164.
- 23 Cobb JA,Manning D,Kolatkar PR,Cox DJ,Riggs AF. Deoxygenation-linked association of a tetrameric component of chicken hemoglobin. J Biol Chem 1992; 267: 1183–1189.
- 24 Wagenbach M,O'Rourke K,Vitez L,Wieczorek A,Hoffman S,Durfee S,Tedesco J,Stetler G. Synthesis of wild type and mutant human hemoglobins in Saccharomyces cerevisiae. Biotechnology (N Y) 1991; 9: 57–61.
- 25 Martin de Llano JJ,Jones W,Schneider K,Chait BT,Manning JM,Rodgers G,Benjamin LJ,Weksler B. Biochemical and functional properties of recombinant human sickle hemoglobin expressed in yeast. J Biol Chem 1993; 268: 27004–27011.
- 26 Mould RM,Hofmann OM,Brittain T. Production of human embryonic haemoglobin (Gower II) in a yeast expression system. Biochem J 1994; 298: 619–622.
- 27 Hofmann O,Mould R,Brittain T. Allosteric modulation of oxygen binding to the three human embryonic haemoglobins. Biochem J 1995; 306: 367–370.
- 28 Nagai K,Thøgersen HC. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. Nature 309: 810–812.
- 29 Hoffman SJ,Looker DL,Roehrich JM,Cozart PE,Durfee SL,Tedesco JL,Stetler GL. Expression of fully functional tetrameric human hemoglobin in Escherichia coli. Proc Natl Acad Sci USA 1990; 87: 8521–8525.
- 30 Hernan RA,Hui HL,Andracki ME,Noble RW,Sligar SG,Walder JA,Walder RY. Human hemoglobin expression in Escherichia coli: importance of optimal codon usage. Biochemistry 1992; 31: 8619–8628.
- 31 Shen TJ,Ho NT,Simplaceanu V,Zou M,Green BN,Tam MF,Ho C. Production of unmodified human adult hemoglobin in Escherichia coli. Proc Natl Acad Sci USA 1993; 90: 8108–8112.
- 32 Villarreal DM,Phillips CL,Kelley AM,Villarreal S,Villaloboz A,Hernandez P,Olson JS,Henderson DP. Enhancement of recombinant hemoglobin production in Escherichia coli BL21(DE3) containing the Plesiomonas shigelloides heme transport system. Appl Environ Microbiol 2008; 74: 5854–5856.
- 33
Schnek AG,Paul C,Leonis J.
Evolution and adaptation of avian and crocodilian hemoglobins. In:
J Lamy, JP Truchot, R Gilles, editors.
Respiratory pigments in animals.
Berin:
Springer Verlag;
1985. pp
141–158.
10.1007/978-3-642-70616-5_10 Google Scholar
- 34 van Assendelft OW. Spectrophotometry of haemoglobin derivatives. Assen, The Netherlands: Royal VanGorcum; 1970. p 55, 72.
- 35 Good NE,Winget GD,Winter W,Connolly TN,Izawa S,Singh RM. Hydrogen ion buffers for biological research. Biochemistry 1966; 5: 467–477.
- 36 Laue TM,Shah BD,Ridgeway TM,Pelletier SL. Computer-Aided Interpretation of Analytical sedimentation data for proteins. In: SE Harding, AJ Rowe, JC Horton, editors. Analytical ultracentrifugation in biochemistry and polymer science. Cambridge: RCS; 1992. pp 90–125.
- 37 DeMoll E,Cox DJ,Daniel E,Riggs AF. Apparent specific volume of human hemoglobin: effect of ligand state and contribution of heme. Anal Biochem 2007; 363: 196–203.
- 38 Carrasco B,de la Torre JG. Hydrodynamic properties of rigid particles: comparison of different modeling and computational procedures. Biophys J 1999; 75: 3044–3057.
- 39 de La Torre JG,Huertas ML,Carrasco B. Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys J 2000; 78: 719–730.
- 40 Sontag CA,Stafford WF,Correia JJ. A comparison of weight average and direct boundary fitting of sedimentation velocity data for indefinite polymerizing systems. Biophys Chem 2004; 108: 215–230.
- 41 Schuck P. Sedimentation analysis of noninteracting and self-associating solutes using numerical solutions to the Lamm equation. Biophys J 1998; 75: 1503–1512.
- 42 Schuck P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal Biochem 2003; 320: 104–124.
- 43 Stafford WF,Sherwood PJ. Analysis of heterologous interacting systems by sedimentation velocity: curve fitting algorithms for estimation of sedimentation coefficients, equilibrium and kinetic constants. Biophys Chem 2004; 108: 231–243.
- 44 Demeler B. Ultrascan – a comprehensive data analysis software package for analytical ultracentrifugation experiments. In: DJ Scott, SE Harding, AJ Rowe, editors. Analytical ultracentrifugation. Cambridge: RCS; 2005. pp 210–229.
- 45 Creeth JM,Knight CG. On the estimation of the shape of macromolecules from sedimentation and viscosity measurements. Biochim Biophys Acta 1965; 102: 549–558.
- 46 Rollema HS,Bauer C. The interaction of inositol pentaphosphate with the hemoglobins of highland and lowland geese. J Biol Chem 1979; 254: 12038–12043.
- 47 Shine J,Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature 1975; 254: 34–38.
- 48 Qiu Y,Maillett DH,Knapp J,Olson JS,Riggs AF. Lamprey hemoglobin. Structural basis of the bohr effect. J Biol Chem 2000; 275: 13517–13528.
- 49 Nagai M,Nagai Y,Aki Y,Imai K,Wada Y,Nagatomo S,Yamamoto Y. Effect of reversed heme orientation on circular dichroism and cooperative oxygen binding of human adult hemoglobin. Biochemistry 2008; 47: 517–525.
- 50 Lebowitz J,Lewis MS,Schuck P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci 2002; 11: 2067–2079.
- 51 Frigon RP,Timasheff SN. Magnesium-induced self-association of calf brain tubulin. I. Stoichiometry. Biochemistry 1975; 14: 4559–4566.
- 52 Na GC,Timasheff SN. Stoichiometry of the vinblastine-induced self-association of calf brain tubulin. Biochemistry 1980; 19: 1347–1354.
- 53 Rowe AJ. The concentration dependence of transport processes: a general description applicable to the sedimentation, translational diffusion, and viscosity coefficients of macromolecular solutes. Biopolymers 1977; 16: 2595–2611.
- 54 Harding SE,Johnson P. The concentration-dependence of macromolecular parameters. Biochem J 1985; 231: 543–547.
- 55 de La Torre JG. Sedimentation coefficients of complex biological particles. In: SE Harding, AJ Rowe, JC Horton, editors. Analytical ultracentrifugation in biochemistry and polymer science. Cambridge: RCS; 1992. pp 333–345.
- 56 Na GC,Timasheff SN. Velocity sedimentation study of ligand-induced protein self-association. Methods Enzymol 1985; 117: 459–495.
- 57 Naghibi H,Tamura A,Sturtevant JM. Significant discrepancies between van't Hoff and calorimetric enthalpies. Proc Natl Acad Sci USA 1995; 92: 5597–5599.
- 58 Liu Y,Sturtevant JM. Significant discrepancies between van't Hoff and calorimetric enthalpies. II. Protein Sci 1995; 4: 2559–2561.
- 59 Zhukov A,Karlsson R. Statistical aspects of van't Hoff analysis: a simulation study. J Mol Recognit 2007; 20: 379–385.
- 60 Winzor DJ,Jackson CM. Interpretation of the temperature dependence of equilibrium and rate constants. J Mol Recognit 2006; 19: 389–407.
- 61 Prabhu NV,Sharp KA. Heat capacity in proteins. Annu Rev Phys Chem 2005; 56: 521–548.
- 62 Stites WE. Protein-protein interactions: interface structure, binding thermodynamics, and mutational analysis. Chem Rev 1997; 97: 1233–1250.
- 63 Ross PD,Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 1981; 20: 3096–3102.
- 64 Undritz E,Betke K,Lehmann H. Sickling Phenomenon in Deer. Nature 1960; 187: 333–334.
- 65 Evans ET. Sickling phenomenon in sheep. Nature 1968; 217: 74–75.
- 66 Simpson CF,Taylor WJ. Ultrastructure of sickled deer erythrocytes. I. The typical crescent and holly leaf forms. Blood 1974; 43: 899–906.
- 67 Simpson CF,Taylor WJ,Jacobson ER. Sickling hemoglobin polymerization in iguana erythrocytes. Comp Biochem Physiol A Comp Physiol 1982; 73: 703–708.
- 68 Ball S,Hawkey CM,Hime JM,Keymer IF,Brambell MR. Red cell sickling in genets. Comp Biochem Physiol A Comp Physiol 1976; 54: 49–54.
- 69 Hárosi FI,von Herbing IH,Van Keuren JR. Sickling of anoxic red blood cells in fish. Biol Bull 1998; 195: 5–11.
- 70 Koldkjaer P,Berenbrink M. In vivo red blood cell sickling and mechanism of recovery in whiting, Merlangius merlangus. J Exp Biol 2007; 210(Pt 19): 3451–3460.
- 71 Agarwal G,Wang JC,Kwong S,Cohen SM,Ferrone FA,Josephs R,Briehl RW. Sickle hemoglobin fibers: mechanisms of depolymerization. J Mol Biol 2002; 322: 395–412.
- 72 Weickert MJ,Curry SR. Turnover of recombinant human hemoglobin in Escherichia coli occurs rapidly for insoluble and slowly for soluble globin. Arch Biochem Biophys 1997; 348: 337–346.
- 73 Vasseur-Godbillon C,Hamdane D,Marden MC,Baudin-Creuza V. High-yield expression in Escherichia coli of soluble human α-hemoglobin complexed with its molecular chaperone. Protein Eng Des Sel 2006; 19: 91–97.
- 74 Bucci E. Preparation of isolated chains of human hemoglobin. Methods Enzymol 1981; 76: 97–106.
- 75 Mishra R,Seckler R,Bhat R. Efficient refolding of aggregation-prone citrate synthase by polyol osmolytes: how well are protein folding and stability aspects coupled? J Biol Chem 2005; 280: 15553–15560.
- 76 Mishra R,Bhat R,Seckler R. Chemical chaperone-mediated protein folding: stabilization of P22 tailspike folding intermediates by glycerol. Biol Chem 2007; 388: 797–804.
- 77 Yu Z,Li B. The effect of polyols on the reactivation of guanidium chloride-denatured arginine kinase from shrimp feneropenaeus chinensis muscle. Protein Pept Lett 2003; 10: 199–211.
- 78 Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature 1957; 180: 326–328.
- 79 Jeffrey PD,Milthorpe BK,Nichol LW. Polymerization pattern of insulin at pH 7.0. Biochemistry 1976; 15: 4660–4665.
- 80 Attri AK,Fernández C,Minton AP. pH-dependent self-association of zinc-free insulin characterized by concentration-gradient static light scattering. Biophys Chem 2010; 148: 28–33.
- 81 Romberg L,Simon M,Erickson HP. Polymerization of Ftsz, a bacterial homolog of tubulin. is assembly cooperative? J Biol Chem 2001; 276: 11743–11753.
- 82 Rivas G,López A,Mingorance J,Ferrándiz MJ,Zorrilla S,Minton AP,Vicente M,Andreu JM. Magnesium-induced linear self-association of the FtsZ bacterial cell division protein monomer. The primary steps for FtsZ assembly. J Biol Chem 2000; 275: 11740–11749.
- 83 Cole JL,Gehman JD,Shafer JA,Kuo LC. Solution oligomerization of the rev protein of HIV-1: implications for function. Biochemistry 1993; 32: 11769–11775.
- 84 Henniker A,Ralston GB. Reinvestigation of the thermodynamics of spectrin self-association. Biophys Chem 1994; 52: 251–258.
- 85 Tojo H,Horiike K,Shiga K,Nishina Y,Watari H,Yamano T. Self-association mode of a flavoenzyme D-amino acid oxidase from hog kidney. I. Analysis of apparent weight-average molecular weight data for the apoenzyme in terms of models. J Biol Chem 1985; 260: 12607–12614.
- 86 Smith R. Self-association of myelin basic protein: enhancement by detergents and lipids. Biochemistry 1982; 21: 2697–2701.
- 87 Fernández C,Minton AP. Static light scattering from concentrated protein solutions II: experimental test of theory for protein mixtures and weakly self-associating proteins. Biophys J 2009; 96: 1992–1998.
- 88 Fernández C,Minton AP. Automated measurement of the static light scattering of macromolecular solutions over a broad range of concentrations. Anal Biochem 2008; 381: 254–257.
- 89 Weber RE. High-altitude adaptations in vertebrate hemoglobins. Respir Physiol Neurobiol 2007; 158: 132–142.
- 90 Gou X,Li N,Lian L,Yan D,Zhang H,Wei Z,Wu C. Hypoxic adaptations of hemoglobin in Tibetan chick embryo: high oxygen-affinity mutation and selective expression. Comp Biochem Physiol B Biochem Mol Biol 2007; 147: 147–155.
- 91 Liu C,Zhang LF,Song ML,Bao HG,Zhao CJ,Li N. Highly efficient dissociation of oxygen from hemoglobin in Tibetan chicken embryos compared with lowland chicken embryos incubated in hypoxia. Poult Sci 2009; 88: 2689–2694.




