Crystal structure of E. coli yddE protein reveals a striking homology with diaminopimelate epimerase
Awarded a GIP–Fond de Recherche Aventis postdoctoral fellowship.
Introduction.
As part of our structural genomics effort in solving crystal structures of Escherichia coli proteins of unknown function with widespread distribution among several Gram+ and Gram− bacteria,1-3 we selected the yddE open-reading frame (ORF) that encodes a hypothetical protein of 297 amino acid residues with a theoretical molecular weight of 32.3 kDa. In the Pfam database, YddE is annotated as a hypothetical protein probably involved in phenazine biosynthesis (family PhzF/PhzC; accession number: PF02567) and members of this family are also distantly related to diaminopimelate (DAP) epimerase.4 Phenazines are derived from the shikimic acid pathway, and one of the derived metabolites, phenazine-1-carboxylic acid (PCA), is a broad-spectrum antibiotic widely used by bacteria against fungal root pathogens and may also act as virulence factors.5 Here, we present the crystal structures of YddE from E. coli in the native and selenomethionine (Se-Met) forms arising from two different crystal forms and determined with use of the medium-scale throughput platform of the Marseilles Structural Genomics Program.
The structure of Se-Met YddE was solved at 2.5 Å resolution using the single-wavelength anomalous dispersion (SAD) method and refined to 2.05 Å resolution using the data set from the remote energy. The native protein was solved by the molecular replacement method using the Se-Met YddE coordinates as a template and refined to 2 Å-resolution.
Material and Methods.
Cloning, expression, and purification:
The yddE ORF was amplified from the genome of E. coli strain K-12 by polymerase chain reaction (PCR), and subcloned into the expression plasmid pDEST17 (Gateway, Invitrogen); the resulting construct encodes a protein extended at the N-terminus by 15 amino acids and 6 histidines.2, 6 Expression was carried out using E. coli Tuner (DE3)pLysS cells grown in Luria–Bertani (LB) medium at 37°C. Bacterial cells were lysed by treatment with lysozyme and consequent freeze-thawing. Nickel–ion affinity and subsequent preparative gel filtration chromatographies were performed using an Äkta FPLC (Amersham Biosciences). Se-Met YddE was produced using the same bacterial strain grown in minimal M9 medium and supplemented, before induction, with Se-Met and amino acids known to inhibit methionine biosynthesis.7 The purified proteins were further characterized by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), matrix-assisted laser desorption/ionization (MALDI-TOF) mass spectroscopy, circular dichroism (CD), and dynamic light scattering.
Crystallization and data collection:
Sitting drop vapor diffusion crystallization screens were set-up with a TECAN Genesis robot on deep-well microtiter plates containing 1.5 μL of protein solution (14 mg/mL) and equal amounts of 240 crystallization conditions provided by commercial kits: Stura Footprint and Structure Screens (Molecular Dimensions Ltd., Cambridge, UK), Wizard I and II (Emerald BioStructures, Baimbridge Island, WA). Several hits appeared with various conditions, most of them containing low-molecular-weight polyethylene glycol (PEG) as precipitant in the pH range 6–8. Refinement of these conditions by the hanging drop vapor diffusion method lead to well-diffracting crystals, grown from 20% PEG 600, 0.1 M imidazole-malate pH 6.5. Crystals for the Se-Met protein (6 mg/mL) were grown from 12% polyethylene glycol monomethyl ether (MPEG) 5 K, 0.2 M Na-acetate pH 5.0. Crystals of the native and the Se-Met protein belong to space group P21 with 2 and 4 molecules in the asymmetric unit, respectively. Cryosolution consisted of the mother liquor for native crystals and supplemented with 5–10% glycerol for the Se-Met YddE crystals. All data sets were collected at 100 K on flash-frozen crystals. A 3-wavelength MAD data set for Se-Met YddE was collected on beam line ID14-EH4, and a data set for the native protein was collected on beam line ID14-EH1 (ESRF, Grenoble). Data were indexed and integrated with DENZO,8 and were scaled and merged with SCALA.9
Structure solution, refinement, and validation:
The structure of YddE was solved by SAD on the Se-edge using SOLVE,10 and 16 out of 20 Se atoms present in the asymmetric unit of the Se-Met YddeE crystals were identified. The initial SAD phases had a mean figure of merit of 0.38 for data to 2.5-Å resolution, and of 0.60 after density modification using RESOLVE.11 The experimental electron density map was of good quality, and an initial model (883 out of 1188 residues) was automatically traced with RESOLVE.11 Automatic model building, including side-chain docking and placement of solvent molecules, was continued with ARP/wARP.12 Further manual rebuilding was done with the graphics program TURBO-FRODO,13 and refinement was carried out with REFMAC.14 One molecule was then used as a template for a molecular replacement search against the native data using MOLREP.15 The two molecules in the native crystal structure were subjected to rigid-body refinement with REFMAC.14 Further refinement and visual inspection of electron density maps was carried out as for the Se-Met YddE structure. The final native model comprises residues 1–297 for each of the two molecules, with two disordered regions from residues 72–73 and 100–101 in one molecule. Nine residues from the tag added for purification were at the N-terminus of one molecule. The final Se-Met YddE model encompasses residues 3–297 for each of the four molecules present in the asymmetric unit. The stereochemistry of the final models were verified with PROCHECK.16 Data collection, refinement and structure quality statistics are shown in Table I. Coordinates of native and Se-Met YddE have been deposited in the Protein Data Bank (PDB), with accession numbers 1QYA and 1QY9, respectively.
| MAD | Native | ||
|---|---|---|---|
| Data collection | Peak (Se) | Remote (Se) | |
| Beamline | ESRF-EH4 | ESRF-EHI | |
| Wavelength (Å) | 0.9796 | 0.9540 | 0.934 |
| Space group | P21 | P21 | |
| Cell dimensions (Å)a | a = 80.49, b = 56.62, c = 148.59; β = 101.6° a (67.16), (79.63), (77.02), (115.2°) | ||
| Molecule/A.U. | 4 | 2 | |
| Resolution range (Å)b | 20–2.5 | 20–2.05 | 20–2.0 |
| Rmerge (%)c | 4.4 (17.6) | 6.6 (36.4) | 4.4 (43.1) |
| Ranom (%)d | 4.4 (11.4) | 4.3 (21.0) | — |
| No. of observations | 180,538 | 301,088 | 118,504 |
| No. of unique reflections | 41,929 | 80,627 | 39,830 |
| Completeness (%) | 98.5 (95.6) | 99.7 (100.0) | 94.6 (94.6) |
| Redundancy | 4.3 (4.3) | 3.9 (3.8) | 2.6 (2.7) |
| I/σI | 13.2 (4.0) | 7.3 (1.7) | 12.0 (1.7) |
| B from Wilson plot (Å) | 44.1 | 28.9 | 30.7 |
| Refinement | |||
| Resolution range (Å) | 20–2.05 | 20–2.00 | |
| Proteins atoms | 18,078 | 9,299 | |
| Solvent/ligand atoms | 622/18 | 270/— | |
| Rcryste/ Rfree (%) | 17.1/20.7 | 18.6/22.7 | |
| RMSD | |||
| Bond distances (Å) | 0.01 | 0.01 | |
| Bond angles (°) | 1.2 | 1.2 | |
| Chiral volume (Å3) | 0.07 | 0.07 | |
| B-factor (Å2) | |||
| Main/Side/Solvent | 34.0/37.2/43.2 | 40.9/44.4/45.1 | |
| RMSD on B (Å2) | |||
| Main/side-chain | 0.75/1.56 | 1.2/1.9 | |
| Ramachandran outliers | None | None | |
- a Values in parentheses are for the native data set.
- b Values in parentheses are for the highest resolution shell.
- c Rmerge = ∑hkl ∑i|Ihkli|−|〈Ihkli〉|/∑hkl ∑i 〈Ihkli〉
- d Ranom = ∑ | 〈I+〉|−|〈I〉| / ∑ (〈I+〉|+|〈I〉)
- e Rcryst = ∑||Fo|−|Fc||/∑|Fo|
Results.
YddE is dimeric in the crystalline state (Fig. 1) but elutes from the gel filtration column as a protein with an apparent molecular size of 105 kDa (data not shown). A YddE subunit consists of 18 β-strands (B1–B18) and 5 α-helices (A1–A5), and folds into two separate domains (domain 1: residues 1–119; domain 2: residues 122–297), sharing a similar topology and linked by a short 3-residue linker, and has an overall cylindrical shape with dimensions of 55 Å × 35 Å × 30 Å (Fig. 1). Each domain possesses a 3- and a 6-stranded β-sheet, almost perpendicular to each other, composed of B6–B8 (B15–B17) and B1–B5, B18 (B9–B14), respectively, with helix A1(A4) helix solvent-exposed, while the other A2(A5) is buried between the two β-sheets (Fig. 1). In addition to the domain interface, the two domains are further stabilized by the last B18 strand from domain 2 that wraps around domain 1 and participates in the 6-stranded β-sheet in domain 1. With the exception of a longer loop connecting B11–A4 and the additional α-helix A3 present in domain 2, the symmetry of the two domains is evident (Fig. 1). In fact, the two individual domains can be superimposed with a root-mean-square deviation (RMSD) of 1.65 Å for 81 Cα atoms. This suggests that the two domains of YddE arise from gene duplication.
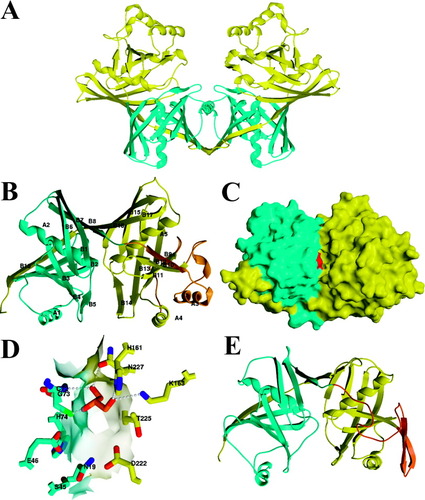
Structure of E. coli YddE. (A) View of the dimer with the two separate domains from each subunit shown in blue and yellow. (B) Ribbon diagram of the molecule colored as in (A) and viewed perpendicular to the internal approximate 2-fold axis. The secondary structure elements are indicated. (C) Molecular surface representation of the molecule oriented 90° from (B). The large cleft at the domain interface is visible (center) with Cys72 indicated in red. (D) Close view of the predicted catalytic site with side-chains shown through a transparent surface and colored blue for domain 1 and yellow for domain 2. The glycerol molecule is shown in orange. (E) Ribbon diagram of DAP epimerase (accession number 1BWZ) oriented and colored as in (B). The regions that significantly differ from YddE are between DAP epimerase and YddE are indicated in orange. Figure produced with SPOCK (http://quorum.tamu.edu/spock) and Raster3D.19
The molecular surface of YddE displays a 10-Å-deep and 20-Å-long cleft separating the two domains and lined by polar and charged residues (Fig. 1). It is likely that Cys72, along with Asn19, Ser45, Glu46, and His74, which are located midway along this cleft in domain 1, are key residues for the biological activity of YddE, with the cysteine probably functioning as a nucleophile, as often seen in enzyme-catalyzed reactions. His161, Lys163, Asp222, Thr225, and Asn227, which are positioned in domain 2 across the cleft, may complete the architecture of the active site. Poor electron density was observed for the Cys72-containing region in the native structure, suggesting that the active-site region is flexible. A glycerol molecule, arising from the cryoprotectant solution used for flash-cooling of the Se-Met YddE crystals, is covalently bound to Cys72, suggesting that it could mimic part of the natural substrate (Fig. 1). Sequence alignment of members of the Pfam PhzF/PhzC family indicates that Asn19, Cys72, His74, and Asp222 are mostly invariant, consistent with our hypothesis on the biological importance of these residues. However, a few members within this family lack the central Cys72 residue, suggesting that they do not share the same function. The localization of the putative active site at the interface of the two domains suggests that a more “open” conformation could exist in solution for the substrate to accommodate the catalytic site. This is consistent with a structural comparison between the native and Se-Met YddE structures that evidence a small rigid domain motion of one domain relative to the other.
A structural similarity search was performed by the program DALI using the coordinates of YddE as a template. The DAP epimerase from Haemophilus influenzae,17 a representative of the pyridoxal-5-phosphate-independent amino-acid racemases was identified as the single structural homolog (Z score = 21.4). The RMSD value is 2.9 Å over 243 residues, but the sequence identity is only 16%. DAP epimerase, which has no detectable sequence similarity with YddE using BLAST searches, shares the same two-domain organization as found for YddE, but has a higher internal symmetry (Fig. 1). Structural comparison between each of the two domains separately reveals that the two domains of DAP epimerase and YddE are highly homologous, with RMSD values of 1.6 Å and 1.7 Å over 82 and 107 residues for domains 1 and 2, respectively. DAP epimerase lacks the large surface loop region containing the additional helix A3 present in the domain 2 of YddE (Fig. 1). This structural comparison also exhibits a 15° motion between the positions of the two domains in a direction roughly perpendicular to the internal approximate 2-fold axis, resulting in a more “open” conformation in DAP epimerase compared to YddE.
This striking structural homology detected between DAP epimerase and YddE suggests that YddE may act as an epimerase for a yet unknown substrate. However, although they share a common location of the active site, the nature of the residues that form the catalytic site are drastically different between these two proteins, indicating that they have unrelated functions. Indeed, YddE lacks the catalytically important Cys73–Cys217 disulfide bridge17, 18 located at the domain interface in DAP epimerase, and only Cys72 in YddE is positioned close to Cys73 in DAP. With our structural information, it would be now interesting to assess the biological function of YddE and to identify the nature of its substrate within the phenazine-biosynthesis pathway, along with its catalytic mechanism. (A similar structure (PDB accession number: 1P9V) has been solved by the Northeast Structural Genomics Consortium.)
Acknowledgements
The ESRF staff is greatly acknowledged for beam time allocation. We thank the structural genomics team of the AFMB laboratory for technical assistance. This study is a collaboration with the IGS laboratory and the Aventis-Pharma pharmaceutical company.




