Chinese clinical practice guidelines for the prevention and treatment of radiation-induced rectal injury
Abstract
Although radiotherapy plays an important role in the treatment of cancer, it may have negative effects in some individuals. Rectal injury is a common adverse effect of abdominal and pelvic radiotherapy. This injury is caused by administering radiation to the abdomen. Appropriate treatment techniques can be determined if doctors have a better understanding of the incidence, risk factors, and clinical symptoms of radiation-induced rectal injuries. Studies on the underlying pathophysiology of radiation-induced rectal injury may aid in the development of effective treatment and prevention strategies. The implementation of efficient preventive measures can improve the quality of life of patients with cancer and make it easier for them to complete their treatment. Therefore, comprehensive and accurate assessments are crucial for developing holistic and individualized treatment plans for patients who have already developed symptoms, with early intervention being a priority.
1 EPIDEMIOLOGY
Radiotherapy is an important modality for treating malignant tumors. Nearly 70% of patients with malignancies require either radical or palliative radiotherapy as part of their treatment regimens. The introduction of immunotherapy has revolutionized cancer therapy, with radiotherapy synergizing with immunotherapy to offer a new ray of hope for many patients. Although radiotherapy effectively eradicates cancer cells, it harms normal tissues within the radiation field. Although the incidence and severity of radiation damage have been significantly reduced by advances in radiotherapy technology, some patients are affected to varying degrees. A very common side effect of abdominopelvic irradiation is radiation-induced bowel injury, which can affect both the small bowel and colorectal tract. Rectal injuries are more common than other types of bowel injuries due to the fixed position of the rectum in the abdomen. Abdominopelvic radiotherapy is widely used to treat multiple malignancies, including rectal, anal canal, prostate, cervical, endometrial, and recurrent metastatic lesions, within the pelvic region.
Accurately calculating the incidence of radiation-induced intestinal injury poses challenges, owing to variations in tumor location and radiation treatment modalities utilized. According to the literature, approximately 70–80% of patients experience varying degrees of acute radiation-induced rectal injury, whereas late-stage injuries range between approximately 2–20%.1-3 The incidence of radiation-induced intestinal injury varies significantly, mainly due to differences in radiotherapy techniques, dosages, and concurrent medication use. In the early days of radiotherapy, patients received two-dimensional radiation treatment, which did not allow for a clear distinction of normal intestinal tissue volume, resulting in a higher rate of intestinal injury compared to modern, precise radiotherapy techniques. Patients with prostate and cervical cancers experience a higher rate of radiation-induced rectal injury because of the higher radiation dosages involved in their treatment. Several studies have reported that in patients receiving a combination of external beam radiotherapy and high-dose brachytherapy, the incidence of late-stage rectal injury surpasses 20%, exceeding 40% in some cases.4 In a retrospective survey of patients with cervical cancer treated with external irradiation combined with intracavitary brachytherapy, 541 patients with a median follow-up of 9.8 years indicated a five-year cumulative incidence of rectal injury of 49.7% and 32.7% in groups receiving 7.2 Gy and 4.8 Gy brachytherapy doses, respectively. At 10 years, incidence rates were 50.5% and 32.7%, with severe injury rates of 8% and 7%, respectively.5 In a phase III study of prostate cancer conducted by the Radiation Therapy Oncology Group (RTOG) 0126, 763 participants received 79.2 Gy of external beam irradiation using either three-dimensional conformal radiation therapy (3D-CRT) or intensity-modulated radiation therapy (IMRT). The rates of grade 2 or higher acute intestinal injury for 3D-CRT and IMRT are 15.2% and 9.7%, respectively, whereas the rates of grade 2 or higher late intestinal injury are 22% and 15.1%, respectively.6 Rectal cancer radiotherapy doses generally range between 45–50.4 Gy, or use large-fraction short-course radiotherapy, with doses typically lower than those for cervical and prostate cancers, thereby reducing the probability of radiation injury. Randomized controlled studies have shown that long-course radiotherapy combined with 5-FU chemotherapy, compared with short-course 25 Gy/5Fx radiotherapy for rectal cancer, has no significant difference in late toxicity, with grade 3 and 4 toxicities of 8.2% and 5.8%, respectively.7 Similarly, another Polish phase III randomized controlled study demonstrated that short- and long-course radiotherapies had comparable late toxicities, with grade 3 and 4 severe toxicities of 10.1% and 7.1%, respectively, and gastrointestinal injury-related adverse reactions of 5.1% and 1.4%, respectively.8 Only a small number of patients underwent radiotherapy alone, while most patients received combined chemotherapy, targeted therapy, surgical treatment, and immunotherapy. Consequently, the incidence of radiation-induced injury reported in the literature is not solely due to radiotherapy, but rather the result of combined treatment damage.
2 MECHANISM OF PATHOGENESIS
The pathophysiology of radiation-induced bowel injury is complex, and the mechanisms of acute and chronic radiation-induced bowel injuries are distinct from one another. Damage to cells caused by radiation is focused on DNA, which limits transcription and blocks cell replication, all of which can directly or indirectly lead to tissue damage. Radiation can directly damage DNA or cell membranes via various methods. These direct mechanisms can cause DNA double-strand breaks and decrease the stiffness of phospholipid bilayers and electric potential differences in cell membranes.9 Generation of free radicals by water molecules is one example of an indirect mechanism that contributes to oxidative stress-induced damage. Thus, DNA repair is triggered when it is damaged by radiation. Therefore, DNA damage can be efficiently repaired at radiation doses that are lower than optimal. However, when exposed to high levels of radiation, the damage is greater than what can be repaired, thereby causing apoptosis or inhibiting mitosis. Stem and cancer cells, both of which have high mitotic rates, are highly susceptible to radiation damage.10
The mucosa of the gastrointestinal tract is a rapidly reproducing tissue, and the stem cells that reside in the crypts of the intestinal tract are extremely vulnerable to radiation damage. Damage to the mucosa is the initial effect of radiation exposure, followed by late inactive connective tissue development and remodeling, and finally, a tissue response to prolonged ischemia.11 Radiation-induced destruction of crypt stem cells results in crypt depletion, mucosal destruction, and exposure of the underlying lamina propria to intestinal bacteria. This exposes the lamina propria to bacteria, which in turn, causes acute inflammatory reactions that often involve T lymphocytes, macrophages, and neutrophils. Secondary damage can occur in the submucosa, mucosa, and extracellular matrix due to enzymes and reactive oxygen species.12 Direct radiation or secondary mucosal damage in the early stages of injury releases inflammatory mediators, leading to infiltration and activation of inflammatory cells, increased capillary permeability, and increased severity of intestinal damage. Acute injury is primarily caused by inflammation, resulting in local edema and mucosal inflammation. Early damage includes mucosal edema, congestion, and ulceration.13 The most notable histological feature of acute phase is epithelial giant cell proliferation with a lack of mitotic activity and proliferation of lamina propria fibroblasts.14, 15 A recent study showed that mice with acute radiation-induced proctitis exhibited significant changes in the PI3K/AKT/NF-κB/vascular endothelial growth factor (VEGF) pathway compared to normal mice. The PI3K/AKT pathway activates NF-κB that promotes the transcription of VEGF and Bcl-2, which in turn induces angiogenesis and apoptosis.16 Histological changes were observed within hours after radiotherapy. Leukocyte infiltration and crypt abscesses appeared in the following 2–4 weeks, followed by occlusive vasculitis with intimal foam cell infiltration and hyaline thickening of the small arterial mesothelium, leading to occlusive arterial endarteritis and thickening of the entire intestinal wall due to ischemia.
The acute inflammatory process resolves when radiation is stopped, and crypt cell regeneration occurs during this period. Radiation can also cause direct damage to blood vessels and endothelial cells, resulting in ischemia throughout the intestinal wall. Chronic injury is mainly caused by ischemia of small blood vessels and fibrosis of the intestinal wall. Late-stage injuries involve severe vascular changes, with submucosal fibrosis leading to small-artery stenosis, capillary and postcapillary venule dilation, endothelial degeneration, and platelet thrombosis. These changes are associated with severe lamina propria fibrosis and crypt distortion.14, 15 As the injury progresses to the final phase, the likelihood of inflammatory cell infiltration decreases. Cytokines play a significant role in radiation-induced bowel injury, as evidenced by higher levels of transforming growth factor-beta, tumor necrosis factor-alpha, interleukin-6, and other factors in late-stage injury.17 In the early stages of injury, there are mainly cellular and molecular changes in the DNA damage response, p53 signaling, and metabolic pathways. In the subacute and chronic phases, there are mainly changes in tissue remodeling.18 Matrix metalloproteinase 8 and urokinase-type plasminogen activator are two enzymes that work together to promote angiogenesis by degrading the extracellular matrix and basement membrane. Endothelial cell proliferation is promoted by VEGF and fibroblast growth factor 1, which induce blood vessel formation and angiogenesis.19 Huang et al. analyzed protein expression in a rat model of radiation-induced intestinal fibrosis; they found that changes in extracellular proteins were more significant. An imbalance among extracellular matrix synthesis, degradation, and remodeling plays a vital role in fibrosis.20 Studies conducted in animal models has demonstrated that damage to fat deposits in the pelvic region is associated with the degree of radiation-induced fibrosis. Adipocytes are responsible for the secretion of adipokines, such as adiponectin, which protect fibroblasts against cell death, myofibroblast development, and senescence caused by radiation.21
3 CLINICAL MANIFESTATION
Injuries to the rectum caused by radiation can be classified as either acute or late, depending on when the symptoms first appear. According to the RTOG, acute injury refers to an injury that arises within the first 90 days of initiating radiotherapy.22 In contrast, symptoms that manifest >90 days after treatment are classified as late injuries. The symptoms include diarrhea, urgency, pain, tenesmus, stool mucus, and mild bleeding.3, 13 These symptoms are mostly self-limiting and gradually recover after radiotherapy. However, in some patients, the symptoms are severe, leading to interruption or an inability to complete radiotherapy. Severe diarrhea and fecal incontinence during radiotherapy are more common in patients after rectal surgery, especially in those who underwent low anterior resection with coloanal anastomosis and poor postoperative anal function. Pre-existing symptoms and comorbidities can worsen with treatment. For example, patients with hemorrhoids before treatment may experience worsening of anal pain symptoms after radiotherapy. Prevention of radiation-induced bowel injury is critical, and modern precision radiotherapy techniques have decreased the incidence of acute reactions.23, 24 Chronic radiation-induced bowel injury is characterized by symptoms that arise three months after the initial radiotherapy, either as a continuation of acute reactions or as new symptoms that may manifest months to years after the completion of radiotherapy.3, 25 It includes all the clinical symptoms of acute injury. However, rectal bleeding is more common than that in the acute phase, and it includes manifestations that are not present in the acute phase, such as stenosis, obstruction, and fistula formation. Typically, approximately one year before the first chronic symptom emerges following radiotherapy. The risk of developing chronic radiation enteropathy is increased in patients with conditions that affect microvascular circulation, such as diabetes and peripheral artery disease. Symptoms that appear several years or decades after radiotherapy must be distinguished from infectious diseases and tumor recurrence.
4 RISK FACTORS
The risk factors for radiation-induced rectal injury include radiation- and patient-related factors and the treatment regimen used (Figure 1).
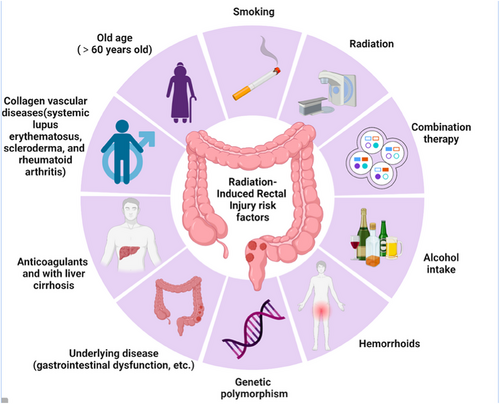
Radiation-induced rectal injury risk factors. The risk factors for radiation-induced rectal injury include radiation-related factors, combination therapies, and patient-related factors.
4.1 Radiation-related factors
Rectal irradiation dose and volume are primary factors that contribute to radiation-induced rectal injury26; the greater the high-dose irradiated rectal wall volume, the more severe the injury, and the earlier the symptoms occur. Long-term damage rarely occurs when the rectal irradiation dose is <45 Gy, whereas significant injury is common at doses >70 Gy.27 The recommended rectal dose limits for reducing the incidence of grade 2 or higher rectal toxicity are V50<50%, V60<35%, V65<25%, V70<20%, and V75<15%.28 Some scholars believe that evaluating the absolute rectal volume is more reliable, with rectal D5cc≥60 Gy significantly correlating with late grade 1 rectal bleeding.29 Therefore, in daily radiotherapy, careful assessment of the rectal irradiation dose and volume is essential to avoid excessively high dose volumes. In addition to external irradiation, supplemental brachytherapy for late-stage cervical cancer can increase the radiation dose to the adjacent rectum, thereby increasing the risk of rectal injury. Irradiation techniques and dose fractionation patterns can also cause radiation-induced rectal damage.
4.2 Patient-related factors
Patient-specific factors included general health, comorbidities, lifestyle, and genetic differences. Patients aged >60 years with gastrointestinal dysfunction, hemorrhoids, or smokers are at a higher risk of radiation-induced intestinal injury.26 Rectal bleeding is more likely to occur in patients taking anticoagulants and those with liver cirrhosis.30 Patients with collagen vascular illnesses, such as systemic lupus erythematosus, scleroderma, and rheumatoid arthritis, have an increased risk of developing radiation-induced proctitis.31 Genetic polymorphisms are intrinsic factors involved in the development of toxic reactions.32-34
4.3 Combination treatment regimens
Radiotherapy alone is rare, as most patients undergo combined treatments, including surgery, chemotherapy, targeted therapy, and immunotherapy.35, 36 Adverse effects of postoperative radiotherapy are more significant than those of preoperative radiotherapy, particularly in patients undergoing low anterior resection for rectal cancer. Concurrent chemotherapy, as well as pre- and post-radiotherapy chemotherapy, significantly increase the occurrence of radiotherapy reactions. Additionally, targeted drugs, such as cetuximab, increase the incidence of mucositis. For most tumors, radiotherapy is part of a combined treatment approach, and is seldom administered as a standalone therapy. Therefore, adverse reactions following radiotherapy should be recognized as a comprehensive response to overall treatment.
Expert Recommendation 1: Before initiating radiotherapy, assess the patient's risk of radiation-induced intestinal injury based on individual and treatment-related factors. Patient-specific risk factors include general health, smoking, age, genetic polymorphisms, and concurrent gastrointestinal dysfunction (Level II-III recommendation). Treatment-related factors mainly encompass radiotherapy techniques, irradiation field, fractionation patterns, concomitant medications, and surgery (Level I recommendation).
5 CLASSIFICATION CRITERIA
Several grading standards have been developed to improve comprehension, evaluate the severity of radiation-induced rectal injuries, and facilitate straightforward comparisons22 (Table 1). The RTOG and European Organization for Research and Treatment of Cancer are two organizations that commonly use evaluation criteria for adverse effects of radiotherapy. It is also common practice to employ the Late Effects of Normal Tissue/Subjective Objective Management Analytic scale (LENT/SOMA) when performing late toxicity assessments (Table 2).37 The National Cancer Institute of the United States National Institutes of Health has established definitions of adverse events. These definitions are based on a grading system known as the Common Terminology Criteria for Adverse Events grading system, which indicates the degree of toxicity caused by cancer treatment38 (Table 3). The severity of endoscopic lesions is an essential reference for evaluating disease severity, and the Vienna Rectoscopy Scoring System is the most widely used endoscopic scoring system (Tables 4 and 5).
| Grade | Definition (Lower GI including the pelvis) |
|---|---|
| Grade 1 | Increased frequency or change in the quality of bowel habits not requiring medication; rectal discomfort not requiring analgesics. |
| Grade 2 | Diarrhea requiring parasympatholytic drugs (e.g., Lomotil); mucous discharge not necessitating sanitary pads; rectal or abdominal pain requiring analgesics. |
| Grade 3 | Diarrhea requiring parenteral support; severe mucous or blood discharge necessitating sanitary pads; abdominal distention (flat plate radiograph demonstrates distended bowel loops). |
| Grade 4 | Acute or subacute obstruction, fistula or perforation; GI bleeding requiring transfusion; abdominal pain or tenesmus requiring tube decompression or bowel diversion. |
- Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; GI, gastrointestinal; RTOG, Radiation Therapy Oncology Group.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Subjective | ||||
| Tenesmus | Occasional urgency | Intermittent urgency | Persistent urgency | Refractory |
| Mucosal loss | Occasional | Intermittent | Persistent | Refractory |
| Sphincter control | Occasional | Intermittent | Persistent | Refractory |
| Stool frequency | 2–4 per day | 4–8 per day | >8 per day | Uncontrolled diarrhea |
| Pain | Occasional& Minimal | Intermittent& Tolerable | Persistent& Intense | Refractory& Excruciating |
| Objective | ||||
| Bleeding | Occult | Occasionally >2/week | Persistent/daily | Gross hemorrhage |
| ulceration | Superficial ≤1 cm2 | Superficial >1 cm2 | Deep ulcer | Perforation, Fistulae |
| Stricture | >2/3 normal diameter with dilatation | 1/3~2/3 normal diameter with dilatation | <1/3 normal diameter | Complete obstruction |
| Management | ||||
| Tenesmus & stool frequency | Occasional, ≤2 antidiarrheals/week | Regular, >2 antidiarrheals/week | Multiple, >2 antidiarrheals/week | Surgical intervention/ Permanent colostomy |
| Pain | Occasional non-narcotic | Regular non-narcotic | Regular narcotic | Surgical intervention |
| Bleeding | Stool softener, iron therapy | Occasional transfusion | Frequent transfusions | Surgical intervention/ Permanent colostomy |
| Ulceration | Diet modification, stool softener | Occasional steroids | Steroids per enema, hyperbaric oxygen | Surgical intervention/ Permanent colostomy |
| Stricture | Diet modification | Occasional dilatation | Regular dilatation | Surgical intervention/ Permanent colostomy |
| Sphincter control | Occasional use of incontinence pads | intermittent use of incontinence pads | Persistent use of incontinence pads | Surgical intervention/ Permanent colostomy |
| Analytic | ||||
| Barium enema | Assessment of lumen and peristalsis | |||
| Proctoscopy | Assessment of lumen and mucosal surface | |||
| CT | Assessment of wall thickness, sinus, and fistula formation | |||
| MRI | Assessment of wall thickness, sinus, and fistula formation | |||
| Anal manometry | Assessment of rectal compliance | |||
| Ultrasound | Assessment of wall thickness, sinus, and fistula formation | |||
- Abbreviations: CT, computed tomography; LENT/SOMA, late effects of normal tissues/subjective-objective-management-analytical scale; MRI, magnetic resonance imaging.
| Grade | Definition (Rectal Mucositis) |
|---|---|
| Grade 1 | Asymptomatic or mild symptoms; intervention not indicated |
| Grade 2 | Symptomatic: medical intervention indicated; limiting instrumental ADL |
| Grade 3 | Severe symptoms; limiting self-care ADL |
| Grade 4 | Life-threatening consequences; urgent operative intervention indicated |
| Grade 5 | Death |
- Abbreviations: ADL, activities of daily living; CTCAE, Common Terminology Criteria for Adverse Events.
| VRS | Congested mucosa | Telangiectasia | Ulceration | Stricture | Necrosis |
|---|---|---|---|---|---|
| Score 0 | Grade 1 | None | None | None | None |
| Score 1 | Grade 2 | Grade 1 | None | None | None |
| Score 2 | Grade 3 | Grade 2 | None | None | None |
| Score 3 | Any | Grade 3 | Grade 1 | None | None |
| Score 4 | Any | Any | Grade 2 | Grade 1 | None |
| Score 5 | Any | Any | Grade ≥3 | Grade ≥2 | Any |
- Abbreviation: VRS, Vienna Rectoscopy Score.
| Endoscopic Findings | Classification |
|---|---|
| Capillary Dilation |
|
| Mucosal Congestion |
|
| Ulceration |
|
| Stricture |
|
| Necrosis |
|
6 DIAGNOSIS
A diagnosis is crucial before devising an appropriate treatment plan. Objective diagnosis and grading of observed symptoms are prerequisites for clinicians to make correct treatment decisions. When patients present with suspected radiation-induced intestinal injury-related symptoms, it is important to consider their radiotherapy history, including treatment timing, dose, irradiation field, intestinal irradiation volume and dose, concurrent comprehensive treatment plans, and other related risk factors. It is also necessary to rule out other possible causes of enteritis, including recent antibiotic use that causes enteritis symptoms, an overdose of nonsteroidal anti-inflammatory drugs (NSAIDs), PD-1 inhibitor immunotherapy, and bacterial or viral infections. In particular, in patients with late radiation-induced injuries, it is essential to carefully exclude enteritis caused by other factors. Enteritis-related symptoms in patients with a history of intestinal radiotherapy does not automatically lead to a diagnosis of late radiation-induced intestinal injury.
For patients with a high suspicion of radiation-induced intestinal injury, the assessment should be combined with appropriate examination methods, including a thorough physical examination, digital rectal examination to determine intestinal stenosis and fistulas, and hematological tests for anemia. The most direct examination method is endoscopy, which can reveal acute-phase mucosal congestion, edema, and later-stage manifestations, such as pale, friable mucosa, vascular dilation, and intestinal stenosis. An endoscopic biopsy of the affected area is not required, but can be helpful in determining the nature of the lesion and ruling out conditions, such as inflammatory bowel disease or cancer. Exercise caution when performing biopsies in endoscopically suspected intestinal injuries may lead to complications, such as perforation and fistula formation. Endoscopy can also be used to grade the severity of injury. The two key markers of intestinal inflammation generated by neutrophils migrating through the colorectum are fecal calprotectin and lactoferrin. Studies39, 40 have demonstrated that pelvic radiotherapy causes an increase in the levels of fecal calprotectin and lactoferrin, and that this increase is related to radiation-induced intestinal injury in patients with cancers other than colon cancer. Fecal calprotectin and lactoferrin levels are difficult to use as diagnostic indicators of radiation-induced intestinal injury in patients with colorectal cancer because they tend to be elevated. Another sign is the concentration of DNA in the feces, which originates from damaged and shed epithelial cells. These characteristics are extremely helpful in monitoring acute mucosal injuries and improving treatment strategies. Radiation-induced intestinal injury can be classified as either acute or chronic, based on the period during which symptoms first appear. A three-month mark following the beginning of radiotherapy is typically used as a dividing line between the two types of injuries. These two types of injuries have different symptoms, incidence rates, and histopathological manifestations; consequently, their treatment approaches differ. Severe acute injury can persist for an extended period and progress to late-stage injury. Sometimes, late-stage injuries develop directly without a prior acute injury, with a possible asymptomatic latency period, lasting for several months or even years.
7 PREVENTION
Once a radiation-induced injury occurs, it is difficult to treat and can significantly affect a patient's quality of life and treatment tolerance. Therefore, prevention is vital for reducing the incidence and severity of injuries. Preventive measures mainly focus on patient education, optimization of radiotherapy techniques, physical protection measures, and pharmacological interventions (Figure 2).
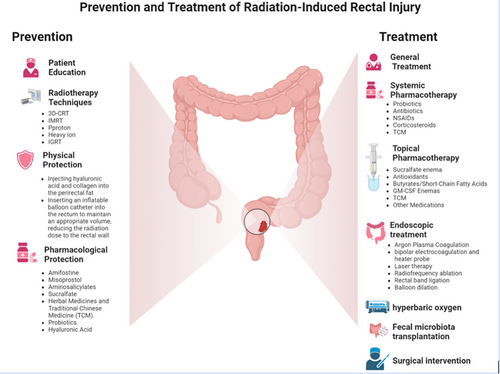
Prevention and treatment of radiation-induced rectal injury. Preventive measures mainly focus on patient education, optimization of radiotherapy techniques, physical protection measures, and pharmacological interventions. Treatment options include observation, medication, hyperbaric oxygen therapy, endoscopic treatment, and surgery.
7.1 Patient education
Patients should be informed that when undergoing abdominopelvic radiotherapy, a certain degree of radiation-induced intestinal injury is inevitable during tumor treatment. Symptoms and their periods of onset can vary, ranging from asymptomatic to life-threatening, and they can appear a few days to several decades after radiotherapy. Therefore, patients should be fully informed, and consent to treatment should be obtained, which can help reduce anxiety and tension when symptoms appear.
If symptoms arise, patients should promptly consult a physician for advice and treatment. During radiotherapy, patients should relax, maintain a reasonable schedule, and follow a balanced diet. Eating high-protein, nutritious meals, avoiding spicy and irritating foods, abstaining from smoking and alcohol consumption, and reducing exposure to risk factors can help minimize complications.
7.2 Optimization of radiotherapy techniques
With the advancement of modern radiotherapy technologies, such as 3D-CRT, IMRT, proton and heavy ion therapy, image-guided radiotherapy, and magnetic resonance imaging (MRI)-guided radiotherapy, the incidence of adverse reactions has been significantly reduced compared to early two-dimensional radiotherapy methods.41-43 Compared with 3D-CRT, IMRT lowers the radiation dose delivered to normal pelvic organs, such as the small intestine, bladder, and urethra. Consequently, the frequency and severity of radiation-induced intestinal injuries are reduced to some degree. In patients with locally advanced cervical cancer, the incidence of late radiation-induced rectal injury was reduced from 48% to 19%, when comparing IMRT combined with brachytherapy to 3D-CRT combined with brachytherapy.44 This was the result of the comparison between the two treatment modalities. In prostate cancer, IMRT and MRI accelerator radiotherapy reduces rectal irradiation volume, thereby decreasing the incidence and severity of radiation-induced intestinal injury.45 In addition to its physical advantages and dose distribution in tumor tissues, proton therapy offers physical benefits, allowing for better tumor control while reducing the response of normal tissues.46 However, the rectum is mostly within the irradiation field used for the radiotherapy in rectal cancer. Intensity-modulated radiotherapy cannot reduce rectal volume within the radiation field; therefore, it does not alleviate rectal injuries. However, this may reduce the irradiation volume to the small intestine within the pelvis. Radiation dose and fractionation patterns are essential factors affecting radiation-induced injuries. When developing a radiotherapy plan, it is necessary to consider the controlling effect on the tumor and potential adverse effects on normal organs at risk. A reasonable radiotherapy plan should be developed based on normal intestinal volume within the irradiation field. Small details of the radiotherapy process may also affect the irradiated volume of normal tissues. In some patients, the prone position can reduce the volume of irradiation to the small intestine. The degree of bladder filling significantly affects rectal irradiation volume in prostate cancer and gynecological tumor radiotherapy. Consumption of gas-producing foods and bowel movements before and after treatment can also affect rectal filling status.
7.3 Physical protection
The main emphasis of physical protection for alleviating radiation-induced rectal injury is the creation of barriers around the intestine. This can be achieved by injecting hyaluronic acid and collagen into the perirectal fat, and using an inflatable balloon catheter inserted into the rectum to maintain an appropriate volume. These measures effectively reduce the radiation dose to the rectal wall and alleviate radiation-induced rectal injury.47
7.4 Pharmacological protection
Studies on the prevention of radiation-induced intestinal injuries focus on pharmacological protection. The use of drugs with protective effects on normal tissues before and after radiotherapy can alleviate radiation-induced intestinal damage. Common medications include amifostine, misoprostol, aminosalicylates, hormones, mucosal protectants, and probiotics.
7.4.1 Amifostine
Amifostine is a potent superoxide radical scavenger. Preclinical studies have shown that amifostine protects intestinal cells by reducing free radicals, thereby alleviating radiation-induced rectal injury. Intravenous administration of amifostine 30 min before radiotherapy can help treat radiation-induced intestinal injuries. Rectal and subcutaneous administration of amifostine prevents radiation-induced intestinal injury.48 However, the inconvenience and inherent toxicity of amifostine limit its clinical application. Additionally, amifostine can cause severe hypotension.49
7.4.2 Misoprostol
Misoprostol, a prostaglandin E1 analog, is another radioprotective agent used to treat acute radiation-induced rectal injury. However, related research results have been mixed. Studies have demonstrated that providing misoprostol suppositories to patients with prostate cancer 1 h before irradiation can considerably improve the symptoms of acute and chronic radiation-induced rectal injuries.50 This is true for both acute and chronic forms of the condition. However, as only 16 patients were included in this study, the sample size was limited. Further studies are required to confirm these findings.
7.4.3 Aminosalicylates
Aminosalicylate compounds, such as sulfasalazine, balsalazide, and olsalazine, as well as the active ingredient mesalazine, can reduce inflammation. After oral administration of balsalazide, the inactive form is converted to its active form by the action of intestinal flora, with only modest absorption into the systemic circulation. Compared to other oral drugs, such as mesalazine, balsalazide has a higher concentration of active components in the distal colon, which enables it to treat disorders more effectively in this region. The prophylactic use of balsalazide at a dosage of 2,250 mg twice daily, beginning on the fifth day of irradiation and continuing for two weeks after the conclusion of radiotherapy, has been shown to minimize the symptoms of radiation-induced rectal damage in patients with prostate cancer.51
7.4.4 Sucralfate
Sucralfate is a non-absorbable aluminum salt of sucrose sulfate with cellular protective, angiogenic, epithelial proliferative, mucosal protective, and bile acid-binding properties. Randomized controlled trials have shown that sucralfate prevents radiation-induced rectal injury.52 However, other studies have been unable to prove whether oral or rectal administration of sucralfate can alleviate acute radiation-induced rectal injury53-55, which may be related to insufficient dosing frequency.
7.4.5 Herbal Medicines and Traditional Chinese Medicine (TCM)
Similar studies have been conducted on the use of herbal medicines and TCM. Some studies have shown that topical application of aloe vera gel can improve diarrhea symptoms in patients receiving pelvic radiotherapy. Gel was applied twice daily at a dose of 1 g for six weeks,56 compared with a control group.
7.4.6 Probiotics
Probiotics are living bacteria that are beneficial to human health, and several studies have demonstrated that they prevent radiation-induced intestinal damage. Probiotics can be found in foods, such as yogurt and fermented foods. Probiotics boost anti-inflammatory activity through a process known as the synthesis of superoxide dismutase, glutathione, and extracellular polysaccharides. The mechanism underlying this effect is as follows: to prevent acute radiation-induced proctitis, the daily consumption of probiotics has been the subject of randomized, double-blind, placebo-controlled clinical studies.57 Patients with prostate cancer undergoing radiotherapy were offered either probiotics or placebo as a preventive intervention. A probiotic group showed fewer instances of proctitis symptoms and lower fecal calprotectin expression levels than a control group. However, this study had a sample size of only approximately 20 patients; therefore, additional studies are required to validate these findings.
7.4.7 Hyaluronic acid
Hyaluronic acid is a polysaccharide that reduces epithelial cell death and provides protection against radiation-induced intestinal injury.58
Based on the current findings, the use of preventive treatments, such as sucralfate, misoprostol, hyaluronic acid, balsalazide, and probiotics is not recommended in clinical settings.
Expert Recommendation 2: For patients with high-risk factors for radiation-induced intestinal injury, it is advised to implement preventive measures at the beginning or early stage of radiotherapy, strengthen patient education (Level I recommendation), and select appropriate radiation techniques and dose fractionation patterns (Level I recommendation). In some high-risk patients, physical protection measures can be used (Level II-III recommendation). Although the evidence level for pharmacological protection is lower, it can be used in high-risk patients (Level III recommendation).
8 TREATMENT
Treating radiation-induced intestinal injury requires collaboration among a multidisciplinary team, comprising nutritionists, nurses, endoscopists, urologists, gynecologists, radiotherapists, and surgery specialists, for assessment and treatment. Treatment options include observation, medication, hyperbaric oxygen therapy (HBOT), endoscopic treatment, and surgical treatment (Figure 2).12, 59-63 Due to the lack of randomized controlled studies comparing various therapies, there is no definitive optimal treatment method; many treatments are based on small-sample studies, and there is still a lack of strong evidence support.64 Although acute radiation-induced intestinal injury has a high incidence, most cases are mild and self-limiting, and they gradually recover after the end of radiotherapy. Treatment may not be necessary, or symptomatic treatment may be administered to relieve symptoms. In severe cases that do not respond to treatment, radiotherapy may need to be temporarily halted or terminated. In some patients, the injury may not heal after radiotherapy, which may progress to chronic intestinal injury.
The incidence of chronic radiation-induced intestinal injury is low; however, once it occurs, treatment is challenging, and recovery is slow. Medication is a commonly used treatment method combined with a long-term management with diet, exercise, lifestyle habits, and psychological counseling. Medications can alleviate clinical symptoms to some extent and reduce pain in patients with severe symptoms. However, there is currently no highly effective standard medication, and most treatments are based on the experience of individual centers. Clinical practice guidelines include American Society of Colon and Rectal Surgeons Clinical Practice Guideline for Chronic Radiation Proctitis,65 Chinese Consensus on the Diagnosis and Treatment of Radiation Proctitis (2018 version),66 and Chinese Multidisciplinary Consensus on the Diagnosis and Treatment of Radiation-induced Rectal Injury (2021 version).67 However, most evidence supporting these guidelines or the consensus comes from small, single-center retrospective studies and a few small prospective studies. Several treatment methods are supported by high-quality study evidence.
Expert Recommendation 3: For patients with intestinal injury, acute and chronic injuries should be defined according to the time limit, and grading should be based on clinical manifestations and auxiliary examinations. For grade 1 acute injuries, general treatments, such as psychological and dietary guidance should be provided. For grades 2–3 injuries, systemic or local symptomatic medication should be given. For grade 4 injuries, patients need to pause radiotherapy and receive symptomatic medicines. If they cannot recover after an extended period, radiotherapy may need to be terminated (Level I recommendation). Early treatment is recommended for chronic injuries. Grades 1–2 injuries can be treated with local or systemic symptomatic medication or TCM. For grades 3–4 injuries, if medication is not effective, endoscopic treatment, formalin enema, or surgery may be considered (Level I recommendation).
8.1 General treatment
General treatment primarily involves nutritional and psychological guidance. Dietary adjustments can alleviate urgency and incontinence. Most cancer patients experience malnutrition, and those with gastrointestinal tumors are more prone to malnutrition. Patients with chronic radiation-induced intestinal injuries have a higher risk of malnutrition, owing to intestinal bleeding and narrowing, which causes nutrient loss and absorption problems.68 Some studies have reported that approximately 10–20% of patients with cancer die from malnutrition rather than from tumors.69 Therefore, a comprehensive nutritional assessment is crucial for cancer treatment and radiation-induced intestinal injury. Simultaneously, unhealthy habits, such as smoking and alcohol consumption70, may exacerbate radiation-induced intestinal injuries. Therefore, educating patients and encouraging healthy lifestyle habits are essential.
Similarly, patients with cancer are prone to psychological disorders, such as anxiety and depression, and their mental state can also trigger or exacerbate physical symptoms.71 The assessment of patients’ mental and psychological states is often overlooked in clinical practice or not properly assessed and intervened. However, this is crucial, especially for patients who have undergone multiple antitumor treatments and subsequently experience severe radiation-induced intestinal injury or late complications. These conditions can significantly affect physical and mental well-being.72
Active and repeated communication with the patients is essential. Before and during treatment, patients and their families should be informed of common adverse reactions that may occur during and after their treatment. This allows patients and their families to fully understand the situation, alleviate their tension and anxiety when radiation-induced intestinal injuries occur, cooperate with treatment, and avoid the emergence and worsening of adverse psychological states to complete the radiotherapy course smoothly.
In clinical practice, self-assessment scales, such as the SOMA and Generalized Anxiety Disorder 7-item scores, can be used to assess patients’ mental and psychological states and physical symptoms, to accurately and systematically evaluate patients’ psychological conditions, and to intervene promptly.73, 74
8.2 Pharmacotherapy
Drug therapy is a common approach for treating radiation-induced intestinal injury, and clinically used symptomatic treatment drugs include systemic medications and local enema treatments.75 Systemic medications include probiotics, antibiotics, NSAIDs, and corticosteroids. The routes of administration include oral and intravenous. Corticosteroids, antibiotics, and NSAIDs can also be used for enemas. Additionally, growth factors and glutamine enemas, which promote intestinal mucosal repair, can accelerate the healing of intestinal injuries.
Patients with radiation-induced intestinal injuries often present with multiple symptoms, and a single-drug treatment may not be sufficient to control all symptoms. The combined use of these enema agents may yield better results. Multiple mixed compound enema drugs are often used in clinical practice; however, their formulations vary.
8.2.1 Systemic Pharmacotherapy
8.2.1.1 Probiotics
The intestinal tract comprises a diverse range of microbial species that form the gut microenvironment. Studies in animals and humans have demonstrated that significant changes in the gut microbiota occur after radiotherapy, and that these changes are closely related to the development of radiation-induced intestinal injury. Probiotics can help maintain balance in the gut microbiota, restore normal intestinal pH levels, and alleviate symptoms, such as diarrhea during the acute phase of radiotherapy. Existing clinical studies have shown that probiotics can reduce diarrhea and abdominal pain during radiotherapy and improve the quality of life of patients. The probiotics commonly used in clinical practice include Lactobacillus, Bifidobacterium, Enterococcus, and Lactococcus.76, 77
8.2.1.2 Antibiotics
Radiation-induced damage to the intestinal mucosal barrier may lead to bacterial translocation and intestinal infections, thereby causing increased abdominal pain and bloating. The addition of antibiotics can alleviate these symptoms. Cavci et al.78 conducted a study in which 60 patients with radiation-induced proctitis were randomly assigned to receive either mesalazine (1 g, three times/day) or betamethasone enema (once per day for four weeks), with or without oral metronidazole (400 mg, three times/day). Mesalazine was administered in combination with a betamethasone enema. The incidences of rectal bleeding, mucosal ulceration, diarrhea, and mucosal edema were lower in the group that received metronidazole than those in the other groups. In another trial, 50 patients with hemorrhagic radiation-induced proctitis were administered either an enema plus oral metronidazole (500 mg, three times/day) and oral ciprofloxacin (500 mg, twice/day) for one week, or a 4% formalin solution applied during rectoscopy for 3 min. Both treatments were compared using 4% formalin solution. The group that received metronidazole and ciprofloxacin experienced a more substantial reduction in rectal bleeding, urinary urgency, and diarrhea than the other groups.79 Both groups experienced an improvement in stool frequency and the amount of blood in their stools. In clinical practice, corticosteroids and antibiotics, including those used for rectal and other radiation-induced injuries, are commonly used to treat radiation-induced injuries. In cases where the symptoms are severe and difficult to mitigate, local or systemic antibiotics and corticosteroids can be considered; however, they are not recommended for long-term use. However, the current evidence is based on small-sample studies and clinical experience, and large-scale studies are required to validate these findings.
8.2.1.3 NSAIDs
sulfasalazine, balsalazide, mesalazine, olsalazine, and 5-aminosalicylic acid (5-ASA) effectively inhibit the formation and release of inflammatory mediators and reduce the production of prostaglandins, thereby suppressing intestinal mucosal inflammation and alleviating intestinal injury. Additionally, 5-ASA is an intestine-specific aminosalicylate salt with few systemic side effects, and is commonly used to treat ulcerative colitis and Crohn's disease. Seo et al.80 reported a small non-randomized study of 23 patients who received oral mesalazine (3 g, once per day) and rectal mesalazine suppositories (1 g before bedtime) for four weeks. Although no significant changes were observed in stool frequency, pain, urgency, bleeding, capillary dilation, or mucosal fragility scores, no adverse reactions were observed. Kilic et al.81 conducted a prospective, randomized, double-blind study with 31 patients. They found that gastrointestinal toxicity was significantly reduced in patients with pelvic cancer taking oral sulfasalazine (500 mg, twice/day) during radiotherapy compared to placebo, with grade 2 or higher gastrointestinal toxicity rates of 20% and 63% (p = 0.017), respectively. In a study conducted by Jahraus et al.,51 which included 27 patients with prostate cancer undergoing pelvic radiotherapy, those taking balsalazide (2.25 g, twice/day) experienced significantly reduced intestinal adverse reactions (such as diarrhea, weight loss, fatigue, nausea, and vomiting) compared with those who received a placebo. This improvement was observed from five days before the initiation of radiotherapy until two weeks after treatment completion. The study was conducted over the course of two weeks. The incidence of injury to the rectal organs ranged between 35.3–74.1%. These studies have shown that NSAIDs significantly alleviate radiation-induced intestinal injury. However, these were early small-sample studies and further large-scale studies are required for confirmation.
8.2.1.4 Corticosteroids
Glucocorticoids are commonly used to treat radiation-induced injuries. They also inhibit the production and release of inflammatory mediators and cytokines. Animal studies and small-sample clinical trials have shown that glucocorticoids alleviate radiation-induced intestinal injuries. However, there is currently a lack of large-scale evidence confirming their role in the treatment of radiation-induced intestinal injury.82 Nevertheless, in clinical practice, intravenous and topical enema corticosteroids are still used to alleviate symptoms, particularly anal pain. Owing to the potential adverse reactions caused by the long-term use of corticosteroids, their extended use is generally not recommended.
Expert Recommendation 4: For patients with acute injury of grade 2 or higher and chronic injury of grades 1–3, systemic medication or a combination of systemic medication and topical enema treatment can be administered. Systemic medications can include probiotics (Level III recommendation), antibiotics (Level II-III recommendation), NSAIDs (Level III recommendation), and corticosteroids (Level III recommendation). However, it is essential to be cautious of adverse reactions caused by the long-term use of antibiotics and corticosteroids, and short-term use is generally recommended.
8.2.2 Topical Pharmacotherapy
Chronic radiation-induced intestinal injury requires a long treatment period, and the prolonged use of systemic medications can increase patient's burden and cause adverse reactions. Topical enema treatment is considered one of the most effective methods of treating radiation-induced intestinal injury, as it provides high local drug concentrations and mild systemic side effects, and can be easily performed at home. It is one of the most widely used treatments in clinical practice. The medications used for enemas are diverse, including hemostatic agents, corticosteroids, antibiotics, mucosal protectants, nonsteroidal anti-inflammatory drugs, and growth factors. Although formaldehyde is used for local treatment, it is difficult to administer at home, and it requires the involvement of a professional doctor.
8.2.2.1 Sucralfate enema
Sucralfate is a highly sulfated polyanionic disaccharide that accelerates mucosal injury healing by boosting angiogenesis.83 It protects the intestinal mucosa by establishing a protective layer on its surface, thereby preventing mucosal damage. As an intestinal mucosal protectant, sucralfate is widely used to treat radiation-induced rectal injuries. Domestic and international guidelines recommend the use of sucralfate for the treatment of radiation-induced intestinal injuries.65-67 It has minimal side effects, and has shown efficacy. Sucralfate can heal both acute and chronic intestinal injuries caused by radiation and prevent their future occurrence. Kochhar et al.84 reported the results of a randomized, double-blind, controlled study in which a control group was administered a treatment comprising oral mesalazine (3.0 g/day) and prednisolone enemas (20 mg twice daily) for a period of four weeks. The experimental group was administered an oral placebo in addition to sucralfate enemas at a dose of 2 g twice daily. Based on these findings, the therapeutic benefits and tolerability of the treatment significantly improved in a sucralfate group. Long-term follow-up data showed that the median time to maintain bleeding was 45.5 months.85 After four weeks of sucralfate use, 77% of the patients experienced an improvement in bleeding symptoms, and after 16 weeks, bleeding control reached 92%, with no treatment-related complications. Other randomized controlled studies have shown that oral sucralfate combined with endoscopic argon plasma coagulation (APC) treatment does not effectively control bleeding; therefore, a sucralfate rectal administration treatment plan is recommended.86 For patients in the active phase who find it challenging to retain enemas, a low-volume paste (2 g sucralfate + 15 mL water mixture) can be prepared to ensure better contact between the drug and damaged intestinal mucosa.87
8.2.2.2 Antioxidants
Radiation plays a major role in radiation-induced injury, and antioxidants are expected to treat radiation-induced damage. The oxidative damage caused by free radicals plays a substantial role in radiation-induced injury. The effects of antioxidants on radiation-induced intestinal injury have been investigated in two separate studies. In the first study, a randomized, double-blind group of 19 patients with radiation-induced rectal injury were administered either oral retinyl palmitate (1000 IU of vitamin A) or a placebo. The vitamin A group showed an efficacy rate of 70%, higher than 20% in the placebo group.88 The second study was a non-controlled trial,89 in which 20 patients with radiation-induced rectal injury received oral vitamin E (400 IU, three times/day) and vitamin C (500 mg, three times/day) treatment; among 10 patients who completed one year of treatment, bleeding, diarrhea, and urgency symptoms significantly improved. In contrast, rectal pain symptom did not show significant improvement. It is difficult to draw conclusions given the limited sample size and absence of a control group in the study.
8.2.2.3 Butyrates/Short-Chain Fatty Acids
Butyrates are unique short-chain fatty acids that serve as primary energy sources for colon cancer cells and promote epithelial cell proliferation and differentiation. A randomized, crossover, placebo-controlled study of 20 patients with acute radiation-induced rectal injury was reported in Lancet in 2000,90 which showed that butyrate enemas for three weeks had significant therapeutic effects. Another non-randomized prospective study in patients with prostate cancer undergoing radiotherapy demonstrated the effectiveness of butyrate enemas in treating acute radiation-induced rectal injuries.91 However, some studies have reported inconsistent results. A large-sample (166 patients) randomized controlled trial investigated the effect of daily butyrate enemas (1 g, 2 g, 4 g, or placebo) during radiotherapy and two weeks after radiotherapy in preventing acute radiation-induced rectal injury.92 Our results showed that this intervention did not reduce the incidence, severity, or duration of acute radiation-induced rectal injury. A study involving 19 patients showed that short-chain fatty acid enemas for five weeks significantly alleviated bleeding symptoms in chronic radiation-induced intestinal injuries. However, long-term follow-up did not show any advantage over placebo, suggesting that continuous long-term medication may be required.93 Another randomized placebo-controlled crossover trial did not demonstrate a therapeutic effect of butyrate on chronic radiation-induced rectal injury.94 The recommended dose of butyrate for the treatment of acute radiation-induced rectal injury is 80 mL/enema once daily or 40 mL/enema twice daily.
8.2.2.4 Granulocyte-macrophage colony-stimulating factor (GM-CSF) Enemas
Recombinant human GM-CSF has anti-infective and mucosal repair-promoting effects and has been included in the treatment guidelines for radiation-induced oral mucositis.95 Studies have shown that GM-CSF enemas combined with traditional medications are effective against radiation-induced proctitis and improve symptoms, such as hematochezia, diarrhea, tenesmus, and abdominal pain after enemas.96
8.2.2.5 Other medications
Other enema medications used in clinical practice include kangfuxin solution, berberine, lidocaine, montmorillonite powder, Yunnan Baiyao, gentamicin, and dexamethasone. Patients with radiation-induced intestinal injuries often exhibit various symptoms, and single-drug treatments may not control all the symptoms. Combining these medications may provide better results, and clinicians often use mixed-compound enema medications with different formulations. For example, kangfuxin combined with berberine enemas is more effective than berberine alone in treating radiation-induced rectal injury than berberine alone,97 and a combination of peifukang and berberine helps prevent acute radiation-induced rectal injury.98
8.2.2.6 Formaldehyde local treatment
Formaldehyde causes coagulative tissue necrosis when in contact with tissue, leading to sclerosis and closure of blood vessels, and is used to treat bleeding symptoms associated with radiation-induced rectal injury. Several studies have demonstrated significant effects in the treatment of bleeding, with response rates, ranging between 70–100%.99 The concentrations used were between 4–10%, and the application methods included direct instillation, endoscopic instillation, and direct application with gauze. During endoscopy, 50 mL of formaldehyde solution was applied to the damaged mucosa for 30 s, and followed by rinsing with saline. This process was repeated five or six times. Alternatively, a formaldehyde-soaked gauze was applied directly to the damaged mucosa. However, its use may also cause severe adverse reactions, such as severe pain, colitis, fistulas, anal/rectal stenosis, fecal incontinence, and intestinal necrosis, with serious complication rates, ranging between 1–7%, limiting its clinical application. Luna-Pérez et al.100 studied 20 patients with steroid- or mesalamine-resistant hemorrhagic radiation-induced rectal injuries treated rectally with 500 mL formaldehyde. The hemostasis success rate reached 90%, with 85% of bleeding stoppage after a single treatment. Five patients experienced moderate pelvic pain, one had bleeding colonic necrosis, two had rectovaginal fistulas, and one had a pelvic abscess. De Parades et al.101 evaluated the efficacy and safety of local formaldehyde treatment under anesthesia for refractory hemorrhagic rectal injuries. Among 33 patients, effectiveness rate was 70%, with six cases of anal/rectal stenosis, four cases of anal function incontinence, and five cases of worsened anal incontinence. Adverse reactions are more common in patients with anal cancer; therefore, careful patient selection is required.
Expert Recommendation 5: Local rectal enema administration is an effective treatment for radiation-induced rectal injury, with high local drug concentrations, mild systemic side effects, and the ability for long-term home use. Sucralfate is a mucosal protectant recommended by domestic and international guidelines (Level I-II evidence).65-67 Antioxidants, such as vitamin A and E, currently have less evidence (Level III recommendation). Butyrate/short-chain fatty acids show some effect in acute intestinal injury but not in chronic injury (Level III recommendation). Granulocyte-macrophage colony-stimulating factor is included in the guidelines for radiation-induced mucositis in head and neck tumors.95 Additionally, it shows some efficacy in radiation-induced intestinal injury (Level III recommendation). Formaldehyde local treatment is a more specialized local treatment, mainly used for treating bleeding symptoms caused by radiation-induced intestinal injury. The response rate for treating bleeding is high, but the incidence of adverse reactions is also high, especially with the potential for a certain proportion of serious adverse reactions. For patients who are unresponsive to conventional drug therapy, it can be used in experienced treatment centers while paying attention to contraindications and adverse reactions (Level I-II recommendation).
8.2.3 TCM treatment for radiation Proctitis
Traditional Chinese medicine has been used for thousands of years in China, and is extremely valuable in the Chinese traditional culture. Traditional Chinese medicine treatments for radiation-induced intestinal injury include oral Chinese medicine, Chinese medicine retention enemas, acupuncture, moxibustion, Chinese medicine suppositories, acupoint injections, acupoint massage, and topical applications. In TCM, one of the most successful methods for treating radiation-induced rectal injuries is retention enema. In a systematic evaluation, a comparison was made between TCM and Western medicine enemas, as well as between a combination of Chinese and Western medicine enemas compared to Western medicine enemas. It was found that TCM enemas and a combination of Chinese and Western medicine enemas had more advantages than Western medicine enemas.102
Chinese medicine enema liquids include the Kangfuxin liquid, Xuejie Enema liquid, Pingkui Powder, and Cenbai granules. Chinese herbal compound enema liquids mainly include Diyu, Huanglian, Baiji, Huangbai, Licorice, Baijiangcao, and Baitouweng, which are responsible for clearing heat, detoxification, astringent sore regeneration, and dispelling dampness and turbidity. Chinese medicine has a specific impact on the adjustment of the intestinal flora. For the common intestinal microecological imbalance in radiation-induced intestinal injury, formulas, such as Baitouweng decoction, Wumei pill, and Sijunzi decoction can improve the intestinal flora and inhibit the levels of inflammatory cytokines, thereby alleviating the symptoms of intestinal injury.103 Semi-finished products can be tailored to individuals and adjusted according to conditions. However, the preparation process is complicated, lacks procedural preparation technology and quality control, and it is difficult to store and promote on a large scale. Some Chinese medicines, such as Kangfuxin liquid, Kusen gel, and Yunnan Baiyao enema liquid, have been converted into finished products for clinical use.104 Recently, the use of acupuncture and massage therapies has increased. Acupuncture can unblock the meridians, promote local blood circulation, alter tissue nutritional status, and promote the absorption of inflammatory factors. Commonly used acupoints include Guanyuan, Tianshu, Zusanli, the spleen, and the stomach. Moxibustion has a warming effect and can relieve abdominal pain and diarrhea. Chinese and Western medicines have advantages, and their combined treatments can complement each other, relieving patient symptoms faster and more durable. In summary, TCM treatment for radiation-induced intestinal injury has specific efficacy and merits further investigation.
Expert Recommendation 6: TCM is a unique feature of China. Chinese medicine retention enema is one of the effective methods for treating radiation-induced intestinal injury (Level I-II recommendation). At the same time, acupuncture and massage can alleviate symptoms to a certain extent (Level III recommendation). However, it is challenging to standardize and unify the practices in TCM.
8.3 Endoscopic treatment
Endoscopy is an effective method for treating rectal bleeding and stenosis. Endoscopic treatment may be considered if no improvement is observed after medical treatment.99 These include APC, bipolar cautery/heating probes, endoscopic laser treatment, radiofrequency ablation (RFA), endoscopic rectal ligation, and other practical methods for treating chronic radiation-induced rectal injuries. Balloon dilatation has been used to treat rectal stenosis.
8.3.1 Argon plasma coagulation (APC)
Argon plasma coagulation is a noncontact thermal coagulation method for hemostasis that can control the penetration depth (<2–3 mm) and improve treatment safety. It ionizes argon gas into plasma using high-voltage sparks and deposits thermal energy in nearby tissues to achieve hemostasis. Sato et al.105 found that using a power of 40 W and single pulse of 2 s could achieve adequate treatment of submucosal capillary dilation without affecting the deep muscle layers. In a prospective study involving 65 patients, 98.5% of the patients were successfully treated. The reported incidence of complications ranges between 5–20%, primarily pain and ulcers, with a low risk of fistulas.106 The incidence of severe adverse reactions was low, and appropriate treatment parameters should be used to improve treatment safety. A randomized controlled study comparing the efficacy of APC treatment with that of local formalin treatment for rectal bleeding in 27 randomly selected patients found similar success rates (approximately 92%) and adverse reactions in both groups, with >20% of the patients experiencing mild adverse reactions.107 Another randomized study reached similar conclusions, with both APC and local formalin treatments being effective with similar rates of efficacy and adverse reactions.108
8.3.2 Bipolar electrocoagulation and heater probe
Bipolar electrocoagulation probes function by heating the tissue to cause coagulation, which serves as a hemostatic method. The depth of the heating probe is not controlled, allowing for deep coagulation; however, this also increases the risk of serious complications, such as perforation. Jensen et al.109 reported a randomized prospective trial that evaluated the efficacy of endoscopic bipolar or heater probe coagulation for the treatment of bleeding angiodysplasia. Bleeding stopped within four treatment sessions in 21 patients in whom drug therapy failed, with no serious complications observed. Another randomized study compared the treatment outcomes of APC and bipolar electrocoagulation for chronic radiation-induced intestinal injury bleeding.110 Thirty patients were randomly assigned to two groups, with similar treatment outcomes, treatment frequencies, recurrence rates, and mild/serious complications. However, the total incidence of complications was higher in a bipolar electrocoagulation group, suggesting that APC was safer than bipolar electrocoagulation. However, further studies with larger sample sizes are required.
8.3.3 Laser therapy
Endoscopic laser therapy has been reported to effectively treat rectal bleeding.111, 112 However, compared with other endoscopic treatments, the cost is relatively high, and inability to control the depth increases the risk of perforation, especially in patients with severe bleeding. Consequently, laser therapy is used less commonly in clinical practice.
8.3.4 RadioFrequencing Ablation (RFA)
Endoscopic RFA works by heating cells in the electrode area to cause necrosis through variable frequency currents. Rustagi et al.113 reported a retrospective study involving 39 patients with an average follow-up period of 28 months, in which bleeding completely stopped in all the patients. Common side effects include mild-to-moderate pain, temporary fecal incontinence, and perianal ulcers. No severe adverse reactions were reported. However, randomized controlled studies to verify these findings are lacking.
8.3.5 Rectal band ligation
Treatment of severe and difficult-to-treat bleeding can be challenging. A recent study showed that endoscopic rectal band ligation (RBL) using a multiband ligator to ligate an affected intestinal mucosa had therapeutic effects in patients with severe or recurrent hemorrhagic colitis. Adverse reactions were mild, including mild tenesmus and pelvic pain.114
8.3.6 Balloon dilation
Endoscopic mechanical dilation using a balloon is a simple and effective method for the treatment of rectal stenosis.115 Patients show significant symptom improvement, and treatment complications are generally minimal. However, long or angulated stenoses are associated with the risk of perforation.
Expert Recommendation 7: Endoscopic treatment is an invasive treatment option. Argon plasma coagulation therapy has an excellent hemostatic effect on bleeding caused by radiation-induced intestinal injury. Argon plasma coagulation therapy can be considered for patients who are unresponsive to drug therapy (Level II recommendation). By choosing appropriate parameters, adverse reactions can be reduced. Bipolar coagulation probes and laser therapy have similar effects on bleeding as APC therapy, but have a higher probability of adverse reactions (Level III recommendation). There is limited evidence for radiofrequency ablation and RBL (Level III recommendation). Balloon dilation is an effective method for relieving mild to moderate stenosis in patients with fewer complications. However, perforation is risky for patients with more prolonged or angulated lesions (Level III recommendation).
8.4 Hyberbaric oxygen therapy (HBOT)
Hyperbaric oxygen therapy (HBOT) is used to treat radiation-induced injury. Daily inhalation of 100% concentrated oxygen in a hyperbaric chamber for several weeks can enhance the intrinsic repair ability of the body for therapeutic purposes. Endothelial cell regeneration and increased antioxidant enzyme activity are induced by exposure to hyperbaric oxygen, which ultimately results in less damage caused by free radicals. Chronic radiation-induced rectal injury is mediated by damage to tiny arteries, ultimately resulting in submucosal fibrosis. It is anticipated that hyperbaric oxygen will heal damaged blood vessels. Clarke et al.116 reported 120 cases receiving 2.0 atmospheric pressure of hyperbaric oxygen and 1.1 atmospheric pressure of sham treatment, with symptom improvement and increased quality of life in the hyperbaric oxygen group. Glover et al.117 published a phase III randomized, double-blind HOT2 study in Lancet Oncology in 2016, with 84 patients randomly receiving 2.4 atmospheric pressure of 100% oxygen treatment at an 1.3 atmospheric pressure of 21%, 90 min/day, 5 times/week for a total of eight weeks. No improvement was observed in chronic radiation-induced gastrointestinal symptoms, including rectal bleeding. A meta-analysis of multiple studies has shown that patients with wound complications benefit more from HBOT.118 Adverse reactions of HBOT are generally mild and temporary and include anxiety, ear barotrauma, and transient myopia. However, its cost is relatively high, equipment accessibility is complicated, and it generally serves as an alternative when conventional treatments fail.
Expert Recommendation 8: Study results of HBOT are inconsistent, treatment duration is long, and cost is high. It can be considered an alternative option when conventional treatments fail (Level III recommendation).
8.5 Fecal Microbiota Transplantation (FMT)
Growing evidence suggests that radiotherapy is associated with changes in the types and quantities of gut bacteria.119-121 Fecal microbiota transplantation involves transferring a fecal microbial community from a specific population to a patient's body to regulate the gut microbiota for therapeutic purposes. Microbial transplantation has been used to treat various diseases, including gastrointestinal and non-gastrointestinal diseases, such as inflammatory bowel disease and autism. Ding et al.122 reported that after FMT treatment in five patients with radiation-induced bowel injury, symptoms significantly improved in three patients, with high treatment safety and no apparent adverse reactions. Ning et al.123 reported FMT treatment outcomes in 2010 patients with intestinal diseases, including 127 patients with radiation-induced bowel injury. After FMT at 3, 12, and 36 months, clinical improvement and cure rates were 80%, 70%, and 60%, respectively. Studies on FMT for treating radiation-induced rectal injury has shown promising results; however, it is still in its early stages, with few studies available. Further studies are required to select appropriate microbial donors, administration routes, adverse reactions, and treatment stability.
Expert Recommendation 9: As a new treatment method, fecal microbiota transplantation is still in a study process, and its safety cannot be guaranteed. It is not recommended for clinical use at this time. However, patients with severe diarrhea may consider participating in clinical studies on fecal microbiota transplantation.
8.6 Surgical intervention
Surgical intervention is an invasive treatment option for this condition. It is only considered for patients with persistent symptoms after medical treatment. Endoscopic treatment is ineffective for emergencies, such as acute massive bleeding, perforation, and obstructive strictures requiring immediate surgical intervention. Less than 10% of patients with radiation-induced bowel injuries require surgery.124 The main surgical options include diversion stoma, local excision/flap reconstruction, and bowel resection.125 In patients with radiation-induced rectal injury, fecal excretion aggravates pain, tenesmus, and infection. Clinical stoma creation can alleviate bowel injury symptoms by reducing fecal irritation and bleeding.126, 127 Small vessel lesions can cause chronic radiation-induced bowel injuries. In theory, the local excision of poorly perfused tissue and reconstruction with well-perfused flaps can achieve therapeutic goals. However, long-term complications and flap necrosis can occur.128 Resection is necessary for irreversible and progressive damage to bowel segments. However, pelvic adhesions and fibrosis are often present in patients with chronic radiation-induced bowel injury, leading to unclear structures and poor tissue healing ability, making resection difficult, and resulting in complications.
Expert Recommendation 10: Surgical treatment has the most significant trauma, but is also the most effective. Surgical treatment can be considered for patients who do not respond to medical treatment or have emergencies (Level I recommendation). Surgery itself may also have certain complications, especially for patients with chronic injury, pelvic fibrosis, and adhesions. Before surgical treatment, patients need to be fully informed, consent obtained, and surgical risks and procedures assessed.
9 CONCLUSION
It is helpful for physicians to understand the incidence, risk factors, and clinical symptoms of radiation-induced intestinal injuries. This allowed the selection of more appropriate treatment options. Studies on the etiology of radiation-induced intestinal injury will assist in the development of efficient techniques for the prevention and treatment of these conditions. Patients can complete their anticancer therapies more successfully if effective preventive measures are implemented. This improves the quality of life of the patients. To develop comprehensive and individualized treatment plans and initiate early interventions, a thorough and accurate assessment of patients who have already developed symptoms of radiation-induced bowel injury is essential. However, it is important to note that current evidence for treatment methods in this context is relatively limited. To strengthen the validity of certain treatment options and explore novel and effective strategies, well-designed studies with larger sample sizes are required.
ACKNOWLEDGMENTS
Guiding experts: Jinming Yu (Shandong Cancer Hospital).
Writing experts: Hui Zhang, Fudan University Shanghai Cancer Center, Zhen Zhang, Fudan University Shanghai Cancer Center.
Editorial Board (Alphabetize by Last Name)
Weiqing Chen, Chongqing University Cancer Hospital
Xiaozhong Chen, Zhejiang Cancer Hospital
Yan Cheng, First Affiliated Hospital of Zhengzhou University
Mei Feng, Sichuan Third People's Hospital
Liying Gao, Gansu Provincial Cancer Hospital
Xianshu Gao, Peking University First Hospital
Yuanhong Gao, Sun Yat-sen University Cancer Center
Xia He, Jiangsu Cancer Hospital
Man Hu, Shandong Cancer Hospital
Xiaobo Huang, Sun Yat-Sen Memorial Hospital
Baosheng Li, Shandong Cancer Hospital
Guiling Li, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology
Jie Li, Shanxi Provincial Cancer Hospital
Jingao Li, Jiangxi Provincial Cancer Hospital
Qin Lin, The First Affiliated Hospital of Xiamen University
Fang Liu, The People's Liberation Army General Hospital
Qing Liu, The Third Affiliated Hospital of Air Force Medical University
Zi Liu, The First Affiliated Hospital of Xi'an Jiaotong University
Tenghui Ma, The Sixth Affiliated Hospital of Sun Yat-sen University
Shuhuai Niu, The Fourth Hospital of Hebei Medical University
Qiao Qiao, The First Affiliated Hospital of China Medical University
Jianguang Qiu, The Sixth Affiliated Hospital of Sun Yat-sen University
Mei Shi, Xijing Hospital of Air Force Military Medical University
Hui Wang, Hunan Cancer Hospital
Jun Wang, The Fourth Hospital of Hebei Medical University
Qifeng Wang, Sichuan Cancer Hospital
Rensheng Wang, The First Affiliated Hospital of Guangxi Medical University
Ruozheng Wang, Cancer Hospital Affiliated to Xinjiang Medical University
Weiping Wang, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College
Sangang Wu, The First Affiliated Hospital of Xiamen University
Qin Xu, Fujian Provincial Cancer Hospital
Junlin Yi, The Cancer Hospital of Chinese Academy of Medical Sciences
Shuanghu Yuan, Shandong Cancer Hospital
Xianglin Yuan, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology
Daxin Zhang, the First Affiliated Hospital of Harbin Medical University
Fuquan Zhang, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College
Liyuan Zhang, The Second Affiliated Hospital of Soochow University
Yunyan Zhang, Harbin Medical University Cancer Hospital
Zhen Zhang, Fudan University Shanghai Cancer Center
Hui Zhang, Fudan University Shanghai Cancer Center
Anping Zheng, Anyang Tumor Hospital
Li Zhu, Tianjin Medical University Cancer Institute and Hospital
Hongqing Zhuang, Peking University Third Hospital
Dongling Zou, Chongqing University Cancer Hospital
CONFLICTS OF INTEREST STATEMENT
Jinming Yu is the Editor-in-Chief of the journal. He was excluded from the peer review process and all editorial decisions related to the acceptance and publication of this article. Peer reviews were handled independently by another editor to minimize bias.
ETHICS STATEMENT
The authors are accountable for all aspects of this study and ensure that questions related to the accuracy or integrity of any part are appropriately investigated and resolved.