Chinese clinical practice guidelines for the prevention and treatment of radiation-induced dermatitis
Abstract
Radiation-induced dermatitis is one of the most prevalent complications in patients undergoing cancer radiotherapy and poses a significant challenge to cancer therapy. The symptoms include erythema, dry desquamation, and moist desquamation, which are frequently observed in patients with breast, head and neck, anal, and vulvar cancers. Early skin reactions typically manifest within 2–4 weeks following the initiation of radiotherapy. In severe cases, acute dermatitis can cause radiotherapy interruptions, prolong treatment time, and ultimately affect patient outcomes and quality of life. Currently, there are numerous guidelines on radiation dermatitis, including the Multinational Association of Supportive Care in Cancer (MASCC), British Columbia Cancer Agency (BCCA), Oncology Nursing Society (ONS), and UK Society of Radiographers (SCoR) guidelines. In China, dermatology experts have drafted a consensus. However, due to the differing backgrounds of experts, recommendations among guidelines vary. These guidelines were first developed by Chinese radiation oncologists. The evidence-based guideline in this paper fully considers and adopts China's national conditions; hence, it can be easily applied in daily practice.
1 OVERVIEW
Radiation-induced dermatitis is a condition of inflammatory damage to the skin and mucous membranes caused by various forms of ionizing radiation, including X-rays, proton rays, beta rays, gamma rays, and other high-energy particle rays. This disease is mainly observed in patients who receive radiotherapy and in those exposed to radiation without proper protection. According to statistics, 90%-95% of patients who undergo radiation therapy experience radiation-induced dermatitis, manifesting as hair loss, dermatitis, pigment deposition, irreversible skin atrophy, and organic damage to sebaceous glands and sweat glands, eventually leading to radiation necrosis and ulcer formation.1, 2
Radiation-induced dermatitis affects the patient's appearance and quality of life; in severe cases, it can also cause radiotherapy interruptions, prolong treatment time, and ultimately affect the patient's treatment outcome and overall survival.3 Currently, there are numerous international guidelines on radiation-induced dermatitis, including those from the Multinational Association of Supportive Care in Cancer (MASCC), the British Columbia Cancer Agency (BCCA), the Oncology Nursing Forum (ONS), and the Society and College of Radiographers (SCoR).4, 5
In China, dermatology experts have drafted a consensus,6 but these guidelines differ significantly. To develop guidelines that aligned with China's national conditions, we convened domestic radiation oncologists. We developed evidence-based guidelines for the prevention and treatment of RDs by reviewing the literature. This guide combines clinical evidence with Chinese national conditions and can be easily applied in daily practice.
2 CLASSIFICATION
Based on the time of occurrence, radiation-induced dermatitis can be divided into two categories: acute and chronic. Acute radiation-induced dermatitis can occur within days or months after irradiation, whereas chronic radiation-induced dermatitis refers to skin damage that appears months to years after radiation exposure. In addition, chemotherapy or targeted therapy can cause localized acute skin inflammatory reactions, known as radiation recall reactions, in areas previously treated with radiotherapy, with an incidence rate of 6%-9%. These reactions can occur weeks, months, or even years after radiotherapy is completed.7, 8 In recent years, in the context of large-scale global vaccination against coronavirus disease 2019 (COVID-19), some patients who received COVID-19 vaccines experienced radiation recall reactions.9
3 PATHOGENESIS
The origin of radiation-induced dermatitis is multifaceted and involves factors such as the expression of genes related to apoptosis and alterations in growth factors (Figure 1). Various apoptotic genes can be induced by radiation, and research has indicated a strong correlation with genes, such as p53, Bax, and B-cell lymphoma-2 (Bcl-2).1, 3 Radiation can activate the p53, Bax, and Bcl-2 genes, inducing apoptosis in cells,1 while various cytokines play an essential role in wound healing. Research has indicated that radiation can lead to changes in the expression of growth factors, and the low expression of growth factors can affect wound repair and healing.3 In addition, changes in epithelial cells and subcutaneous blood vessels are important in radiation-induced skin injury.
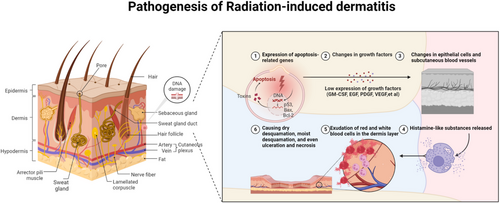
Cells in the basal germinative layer are sensitive to radiation, and the free radicals and reactive oxygen species generated by radiation can readily damage these basal cells, affecting cell division, proliferation, migration to the surface, and keratinization, thereby interfering with the normal metabolism of epidermal cells and causing radiation-induced dermatitis. In the early stages of radiotherapy, histamine-like substances are released at the irradiated site, increasing capillary permeability, and causing transient erythema and itching. In the later stages of radiotherapy, the exudation of red and white blood cells into the dermal layer leads to erythema. With an increased radiation dose, basal layer cells are damaged, causing dry desquamation, moist desquamation, ulceration and necrosis.3
4 CLINICAL MANIFESTATIONS
4.1 Acute radiation-induced dermatitis
Acute radiation-induced dermatitis typically manifests within 90 days of the first radiotherapy session, or after radiation exposure. Skin changes can appear within a few hours with mild symptoms including burning, itching, pain, pigmentation, dry or moist desquamation, and erythema. Severe cases may present with edema, ulceration, bleeding, necrosis, and local infections, leading to treatment interruption in extreme cases. Acute skin reactions generally heal within one month of stopping radiotherapy.
4.2 Chronic radiation-induced dermatitis
Chronic radiation-induced dermatitis occurs from 90 days to several years after the first radiotherapy session or radiation exposure. Its main manifestations include skin atrophy, pigmentation, indurated edema, delayed ulceration, thickening, and fibrosis. Severe cases involve tissue contracture, limited mobility, and pain. In patients receiving epidermal growth factor receptor (EGFR) inhibitor therapy, the onset of radiation-induced dermatitis may occur earlier and be more severe, potentially inducing a pustular rash.10 In the absence of infection, the epidermis begins to regenerate 3–5 weeks after radiotherapy completion, with skin lesions gradually healing, and complete healing occurring within 1–3 months. If the patient develops epidermal or dermal necrosis (grade 4 radiation-induced dermatitis), the healing time is extended; in severe instances, skin fibrosis may occur (Table 1).
| Assessment Criteria | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| NCI CTCAE V5.0 (Dermatitis radiation) | No change | Faint erythema or dry desquamation | Moderate to brisk erythema; patchy moist desquamation, mostly confined to skin folds and creases; moderate edema | Moist desquamation in areas other than skin folds and creases; bleeding induced by minor trauma or abrasion | Life-threatening consequences; skin necrosis or ulceration of full thickness dermis; spontaneous bleeding from involved site; skin graft indicated | Death |
| RTOG Acute Radiation Injury Grading(skin) | No change over baseline | Follicular, faint, or dull erythema/epilation/dry desquamation/decreased sweating | Tender or bright erythema,patchy moist desquamation/moderate edema | Confluent, moist desquamation other than skin folds, pitting edema | Ulceration, hemorrhage, necrosis | |
| RTOG Chronic Radiation Injury Grading(skin) | None | Mild skin atrophy; pigment changes; hair loss | Patchy atrophy; moderate telangiectasia; complete hair loss | Significant skin atrophy; coarse telangiectasia | Ulceration | |
| LENT-SOMA Breast radiation skin lesions evaluation criteria | ||||||
| Subjective | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Pain | Occasional and minimal Hypersensation, pruritus | Intermittent and tolerable | Persistent and intense | Refractory and excruciating | |
| Objective | |||||
| Telangiectasia | <1 cm2 | 1∼4 cm2 | >4 cm2 | ||
| Fibrosis | Barely palpable, increased density | Definite increased intensity and firmness | Very marked density, retraction, and fixation | ||
| Edema | Asymptomatic | Symptomatic | Secondary dysfunction | ||
| Retraction, atrophy | 10%∼25% | >25%∼40% | >40%∼75% | Whole breast | |
| Ulcer | Epidermal only,<1 cm2 | Dermal only, >1 cm2 | Subcutaneous | Bone exposed, necrosis | |
| Lymphedema, arm circumference | Increased arm circumference 2∼4 cm | Increased arm circumference >4∼6 cm | Increased arm circumference >6 cm | Useless arm | |
| Skin | |||||
| Pigmentation change | Transitory, slight | Permanent, marked |
- Abbreviations: NCI - National Cancer Institute (USA); CTCAE - Common Terminology Criteria for Adverse Events; LENT-SOMA - Late Effects in Normal Tissues - Subjective, Objective, Management, and Analytic Scoring System.
5 DIAGNOSIS, GRADING, AND EVALUATION CRITERIA
The currently used assessment criteria for radiation-induced dermatitis include the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), Radiation Therapy Oncology Group (RTOG) toxicity grading system, and Late Effects Normal Tissue Task Force-Subjective, Objective, Management, and Analytic (LENT-SOMA) scoring system. In China, radiation oncologists generally adopt the RTOG and CTCAE criteria to diagnose, grade, and evaluate radiation-induced dermatitis.
6 RISK FACTOR ASSESSMENT
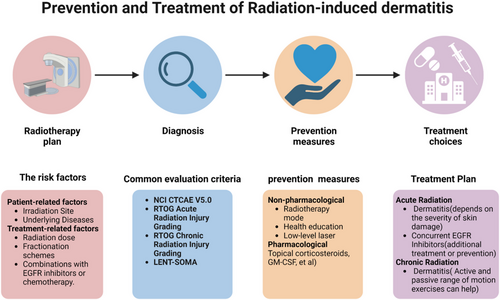
The assessment of the risk factors for radiation-induced dermatitis considers both patient- and treatment-related factors. The latter includes considerations, such as radiation dosage, fractionation strategies, and combinations with EGFR inhibitors or chemotherapy (Figure 2).
Prevention and Treatment of Radiation-induced Dermatitis
Abbreviations: EGFR, epidermal growth factor receptor; NCI - National Cancer Institute (USA); CTCAE, Common Terminology Criteria for Adverse Events; RTOG, Radiation Therapy Oncology Group; LENT-SOMA, The Late Effects Normal Tissue Task Force-Subjective, Objective, Management and Analytic; GM-CSF, granulocyte-macrophage colony-stimulating factor.
6.1 Patient-related risk factors
6.1.1 Irradiation site
The skin of the anterior neck, limbs, chest, abdomen, face, and scalp hair follicles are more sensitive to radiation, and the probability of developing radiation-induced dermatitis is higher. Breast cancer patients with larger breasts who undergo breast reconstruction and implantation have a higher risk of severe radiation-induced dermatitis11-13 (Level II evidence).
6.1.2 Underlying diseases
Factors such as obesity, malnutrition (often characterized by weight loss), prolonged exposure to the sun, and smoking may increase the risk of radiation-induced dermatitis (Levels III-IV evidence). Additionally, individuals with genetic disorders associated with compromised DNA repair ability, including ataxia-telangiectasia, Bloom syndrome, Fanconi anemia, Gorlin syndrome, or xeroderma pigmentosum, are susceptible to severe forms of radiation-induced dermatitis1, 3 (Level III-IV evidence).
6.2 Treatment-related factors
6.2.1 Radiation therapy-related factors
The overall radiation dose, fractionation dose, irradiated volume, and surface area are factors that influence the risk of radiation-induced dermatitis.14, 15 In a multicenter randomized trial that included 331 patients with breast cancer who underwent postoperative adjuvant radiation therapy with either conventional wedge-tissue compensation methods or intensity-modulated radiation therapy (IMRT), the findings demonstrated a decreased occurrence of moist desquamation in the IMRT group compared with the conventional radiotherapy group (31% vs. 48%). Subsequent multivariate analysis showed that breast IMRT contributed to a nearly 60% reduction in the incidence of moist desquamation.16 Another prospective study comparing hypofractionated radiotherapy with standard fractional radiotherapy demonstrated that the incidence of dermatitis, itching, excessive pigmentation, and pain was lower in the hypofractionated radiotherapy group.17
Several studies have demonstrated that accelerated partial breast irradiation (APBI) may serve as a substitute for whole-breast irradiation after breast-conserving surgery in low-risk breast cancer patients. Compared with whole-breast radiotherapy, APBI exhibits a reduced frequency of acute toxicity without an increased risk of recurrence.18-20 In a study focusing on ultra-large fractionation for breast cancer, an evaluation of three regimens—a 3-week schedule of 40 Gy in 15 fractions, a 1-week schedule of 27 Gy in five fractions, and a 1-week schedule of 26 Gy in five fractions—revealed that the 1-week ultra-large fractionation regimen has the potential to reduce both the occurrence and severity of acute radiation dermatitis.21 IMRT-based precision radiotherapy can reduce acute skin toxicity compared with two-dimensional radiotherapy (level I evidence). Hypofractionated radiotherapy has a lower probability of acute skin toxicity than conventional fractionation (Level I evidence). In certain clinical situations (such as skin cancer or scar recurrence), the bolus delivery of sufficient skin doses significantly increases the incidence of radiation dermatitis (level III-IV evidence).
6.2.2 Combined therapy
A comprehensive meta-analysis of 15 randomized clinical trials, comparing the effects of concurrent chemoradiotherapy with radiotherapy alone on nasopharyngeal cancer, revealed an 80% increase in the risk of grade 3–4 radiation dermatitis with chemoradiotherapy (relative risk [RR] = 1.8, 95% confidence interval [CI]: 1.13-2.88).22 In addition, a systematic review of 48 studies encompassing 2,152 patients with locally advanced head and neck squamous cell carcinoma revealed that the incidence of grade 3–4 radiation dermatitis reached 32.5% (95% CI: 28.5%-36.5%) in those treated with a combination of radiotherapy and cetuximab.23 A smaller study that included 51 patients undergoing radiotherapy and cetuximab treatment observed a 43% incidence of grade 3–4 radiation dermatitis.24 Furthermore, a meta-analysis of 23 clinical trials exploring the combination of immune checkpoint inhibitors (ICI) and radiotherapy for different types of cancers revealed that the incidences of any-grade and severe dermatitis were 22% and 3%, respectively. These rates are analogous to the skin toxicity reported when using ICI alone.25
Additionally, research has shown that radiation dermatitis significantly increases in specific brain malignancies, such as glioblastoma, when treated with radiotherapy combined with electric-field therapy. Using scalp-sparing radiotherapy techniques can reduce the severity of radiation dermatitis26 (Level II-III evidence). Current clinical evidence suggests that combining radiotherapy with chemotherapy (e.g., paclitaxel, docetaxel, anthracyclines, methotrexate, and 5-fluorouracil), EGFR inhibitors, or electric field therapy increases the risk of severe radiation dermatitis (Level I evidence), whereas combining radiotherapy with ICI treatment does not increase the risk of radiation dermatitis (Level I evidence).
[Expert Recommendation 1] Before radiotherapy, patients’ risk of radiation dermatitis should be assessed from both their personal risk factors and treatment-related factors. Personal risk factors mainly include the irradiation site (Level II evidence, grade A recommendation), the presence of obesity, malnutrition, long-term sun exposure, smoking, or having a DNA repair-related genetic disease (Level III-IV evidence, grade C recommendation). Treatment-related risk factors include radiotherapy techniques, fractionation patterns, dose, and the use of combined chemotherapy/targeted therapy/electric field therapy (Level I evidence, grade A recommendation).
7 PREVENTION
7.1 Non-pharmacological prevention measures
7.1.1 Choice of radiotherapy mode
IMRT and volumetric-modulated arc therapy (VMAT) can reduce the incidence of skin reactions compared with two-dimensional radiotherapy (Level I evidence). Related studies on breast cancer found that the probability of developing acute radiation dermatitis is lower with hypofractionated radiotherapy than with conventional fractionated radiotherapy (Level I evidence). Accelerated partial breast irradiation can reduce the incidence and severity of acute radiation dermatitis compared to whole-breast radiotherapy (Level I evidence).
7.1.2 Health education
It is recommended to enhance health education, including psychological counseling, skincare, and dietary guidance. During radiotherapy and for a certain period after radiotherapy, it is necessary to minimize skin irritation, friction, and excessive sun exposure in the irradiated area (Level III-IV evidence); avoid patients wearing high-collared, tight clothing; recommend low-collared tops; and keep the irradiated skin area exposed, clean, and dry as much as possible. Several randomized controlled studies and a meta-analysis study on breast cancer and head and neck tumors have shown that washing with warm water or fragrance-free, non-irritating soap with a pH close to that of human skin (pH 4–6) can significantly reduce itching, redness, and scaling but does not reduce the overall incidence of radiation dermatitis (Level II evidence).
Avoid using alcohol, perfume, or baby talcum powder on the skin in the radiotherapy area (Level III-IV evidence). A study evaluating the effects of topical medications on radiation measurements for radiation dermatitis found that when the thickness of the topical medication was ≥3 mm, the skin surface radiation dose increased (Level II evidence); therefore, it is not recommended for patients to use topical moisturizers, gels, lotions, or dressings before radiotherapy. A randomized controlled study of 333 breast cancer cases showed that the sweating rate with the use of antiperspirants/deodorants during postoperative adjuvant conventional radiotherapy was markedly lower than that in the control group, and that antiperspirants/deodorants can be used for those who need it27 (Level I evidence).
7.1.3 Low-level laser
Low-level laser therapy, a type of phototherapy known to stimulate wound healing,28 has shown potential in warding off radiation-related side effects such as dermatitis, dry mouth, and oral mucositis. While the outcomes of these small-scale studies appear promising, indicating a need for a more comprehensive investigation into their effectiveness in both preventing and treating acute radiation dermatitis,29 current clinical evidence supporting their use in preventing radiation dermatitis is scarce, and they are not endorsed as a standard preventative measure (Level III-IV evidence).
7.2 Pharmacological prevention measures
7.2.1 Topical corticosteroids
Numerous randomized studies and meta-analyses have provided substantial evidence (Level I) that the consistent application of topical corticosteroids during the course of radiotherapy and for a period extending several weeks afterward can mitigate the occurrence of severe dermatitis, avert the development of grave radiation dermatitis, and alleviate associated discomfort and itching.4, 30, 31 Specifically, a meta-analysis encompassing 10 randomized trials involving 919 breast cancer patients determined that the daily application of topical corticosteroids to the breast or chest wall 1–2 times from the initiation of radiotherapy until up to 3 weeks following treatment yielded a significant reduction in the risk of moist desquamation compared to the use of a placebo cream.32
In a phase III randomized controlled trial involving 211 patients with head and neck squamous cell carcinoma, all of whom received bilateral neck radiotherapy and concurrent cisplatin chemotherapy, a significant finding was observed. The use of topical corticosteroids at a frequency of ≥1 time per day until 2 weeks post-radiotherapy led to a reduction in the occurrence of radiation dermatitis of ≥ grade 3, with an incidence of 13.9% compared to 25.5% in the placebo group.31 Based on these results, it is advisable to initiate the application of low-to-medium-potency topical corticosteroids (such as 0.1% mometasone furoate or 0.1% hydrocortisone butyrate cream) 1–2 times daily to the irradiated area. This regimen should begin on the first day of radiotherapy and persist throughout the treatment cycle to achieve optimal preventive effects against radiation dermatitis (Level I evidence).
7.2.2 Recombinant human granulocyte-macrophage colony-stimulating factor
Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) can stimulate macrophages to produce wound healing-related factors, induce the migration and proliferation of mucosal epithelial cells, activate keratinocytes in the regenerative state, and activate fibroblasts to promote granulation tissue formation, thereby promoting mucosal healing.33
A study involving 61 patients with vulvar cancer undergoing radiotherapy showed that GM-CSF solution-soaked gauze at 40 μg/cm2, applied 2 times a day reduced patients' skin reaction scores (p = 0.008) and the time interval for radiotherapy interruption (p = 0.037).34 After using the GM-CSF-soaked gauze, pain duration was shortened (p = 0.0017) (Level III evidence, p = 0.0017).
7.2.3 Superoxide dismutase
Superoxide dismutase, which is known for its ability to effectively eliminate oxygen free radicals, has been examined for its protective effects against radiation damage. This well-designed randomized controlled study included 70 patients who underwent radiotherapy for head and neck tumors. In this study, patients in the experimental group were treated with a medical radiation protection spray containing superoxide dismutase as the primary active component, whereas those in the control group received no intervention. The findings were statistically significant (p < 0.05) and revealed that the tolerance dose for radiation-induced damage to the skin and mucosa was elevated in the experimental group compared to that in the control group. Furthermore, the likelihood of severe radiation-induced injury to the skin and mucosal tissues was significantly reduced in the group treated with the medical radiation protection spray (p < 0.05). These results strongly support the conclusion that a specialized spray provides targeted protection against acute radiation-induced injuries to the skin and mucosal surfaces35 (Level II evidence).
7.2.4 Silicone film-forming gel dressings
In a methodologically rigorous, randomized, single-blind clinical trial involving 197 patients diagnosed with head and neck tumors, the participants were randomly assigned to receive treatment with a silicone film-forming gel dressing or a conventional moisturizer. This treatment was administered 2 times per day until any skin reaction subsided, extending up to four weeks after the completion of radiotherapy. The results were marked and statistically significant, showing a 40% and 50% reduction in the risk of developing grade 2 and grade 3 radiation dermatitis, respectively, in the silicone film-forming gel dressing group compared to the control group (hazard ratio [HR] for grade 2 = 0.59, 95% CI: 0.44–0.80; HR for grade 3 = 0.51, 95% CI:0.32–0.80). These findings not only provide substantial evidence, but also emphasize that silicone film-forming gel dressings offer a specific protective effect against acute radiation-induced injuries to both skin and mucosal tissues. This approach represents a promising therapeutic intervention for enhancing the quality of care for patients undergoing radiotherapy36 (Level II evidence).
7.2.5 Silver ion dressings/ointments
Silver ions exhibit anti-inflammatory and barrier-enhancing effects.37 Studies have shown that they can reduce radiation-induced skin toxicity (Level II evidence).
A randomized controlled study involving 42 patients with anal or rectal cancer undergoing radiotherapy showed that compared to standard skin care, silver ion dressings alleviated the severity of radiation-induced dermatitis.38 In an alternative randomized trial that included 196 patients diagnosed with breast cancer, the application of silver ion dressings did not decrease either the occurrence or harshness of radiation dermatitis compared to standard skincare methods. Nevertheless, these dressings are effective in diminishing the sensations of itching, pain, and burning in the skin.39 In a randomized trial involving 102 patients with breast cancer, the effectiveness of 1% silver sulfadiazine cream (three times daily, for a period of three days each week over the course of five weeks, and then continued for an additional week following treatment) was investigated and compared with general skin care for preventing radiation dermatitis. The findings indicate that the group treated with silver sulfadiazine experienced a reduction in the severity of acute radiation dermatitis (average dermatitis score 5.49 vs. 7.21).40
7.2.6 Aloe vera
In a randomized trial that included 225 patients with breast cancer, the group treated with aloe vera gel had a higher number of patients experiencing dry desquamation, grade 2 or higher dermatitis, and intense pain than the group treated with aqueous cream.41 Additionally, a separate randomized controlled study determined that the use of aloe vera cream or placebo cream during radiotherapy resulted in an increased occurrence and severity of radiation dermatitis compared to a regimen involving non-metallic baby powder or cornstarch42, 43. Retrospective studies in China have suggested that aloe vera may alleviate the severity of radiation-induced dermatitis and delay its onset.44 However, there is controversy over whether aloe vera should be used for prevention, and it is not recommended to prevent radiation dermatitis (Level I evidence).
7.2.7 Triethanolamine
In a randomized controlled study, patients with head and neck squamous cell carcinoma undergoing radiotherapy were assigned to preventive triethanolamine (n = 166), interventional triethanolamine (n = 175), or institution-selected product (n = 165) groups.45 The findings demonstrated that the incidences of grade 2 or higher dermatitis in the three examined groups were 79%, 77%, and 79%, respectively, and the difference between them was not statistically significant. In a separate study that included 254 patients with breast cancer, triethanolamine cream or calendula cream was administered after each radiotherapy session. This study revealed that grade 2 or higher acute radiation dermatitis was less frequent in the calendula cream group than in the triethanolamine cream group (41% vs. 63%).46 Additional research has determined that the incidence of grade 2 or higher dermatitis in the group using calendula cream was comparable to that in the group using water-based moisturizer cream.47
However, these studies were conducted decades ago in the era of two-dimensional radiotherapy techniques. With the widespread use of IMRT technology, a series of studies have been conducted in China involving hundreds of patients undergoing radiotherapy in the head and neck, thoracic, and pelvic regions. The preventive use of triethanolamine cream has shown promising effects against radiation dermatitis.48-50 Based on China's experience and evidence, the use of triethanolamine cream for drug prophylaxis is recommended (level II–III evidence).
7.2.8 Sucralfate
In a randomized controlled study involving 357 patients undergoing head and neck, breast, or anorectal radiotherapy, the patients were divided into sucralfate cream, aqueous cream, and observation groups. The results showed that after 5 weeks of radiotherapy, self-reported erythema, desquamation, itching, pain, and discomfort levels were similar among the three groups.51 Therefore, sucralfate cream is not recommended for preventing radiation dermatitis (Level II evidence).
7.2.9 Hyaluronic acid
A meta-analysis encompassing eight randomized trials, which included over 500 patients with breast cancer, discovered that hyaluronic acid led to a reduction in the grade of radiation dermatitis compared to treatments with plant sterols, vitamin E, omega-3, omega-6, and omega-9 fatty acids. However, no significant differences were observed between the effects of hyaluronic acid and those of moisturizers, vitamin C, and alginates.52 Based on this evidence, classified as Level II, routine application of hyaluronic acid for the prevention of radiation dermatitis is not advised.
7.2.10 Calendula cream
Calendula extracts have antioxidant and anti-inflammatory properties.53 Although laboratory data confirm the potential effectiveness of calendula extracts in mitigating radiation-induced skin toxicity, clinical studies examining the efficacy of calendula cream in preventing radiation dermatitis have produced varied outcomes.53 Some studies have demonstrated that calendula cream can reduce the occurrence of acute radiation dermatitis.46 However, follow-up research ascertained that the incidence of grade 2 or higher radiation dermatitis in the group treated with calendula cream was analogous to that in the group administered a water-based moisturizer cream.47 Therefore, the routine use of calendula cream to prevent radiation dermatitis is not recommended (Level II evidence).
7.2.11 Oral medications
Several small size randomized trials have evaluated the effects of various oral medications including proteolytic enzymes (papain, trypsin, and chymotrypsin mixtures),54 pentoxifylline,55 zinc supplements,56 and curcumin.57 However, there is a lack of high-level evidence on the efficacy and safety of these systemic treatments. Although pentoxifylline has been shown to be effective in minimizing delayed skin alterations, fibrosis, and skin necrosis, a compact randomized study involving 78 patients who underwent surgery for head and neck squamous cell carcinoma found otherwise in acute conditions. Specifically, the administration of pentoxifylline at 400 mg per dose, administered orally three times daily, was not found to be superior to a placebo in the prevention of acute radiation dermatitis.54 Another multicenter randomized trial involving 686 patients with breast cancer patients used 1.5 g/day of curcumin throughout radiotherapy and one week after its completion. The results showed that curcumin was not better than placebo in alleviating radiation dermatitis.58 Zinc, known for its antioxidant properties, has been examined in studies focusing on protecting normal tissues from radiation. While there are some reports with small sample sizes suggesting zinc's potential role in preventing radiation dermatitis, these findings require further comprehensive evaluation.56 Therefore, oral systemic medications are not recommended to alleviate radiation dermatitis (Level II evidence).
7.2.12 Amifostine
Amifostine acts as a prodrug, transforming into an active free thiol metabolite once its phosphate is removed by an alkaline phosphatase within tissues. This metabolite selectively protects normal cells from radiation-induced damage by neutralizing free oxygen radicals. Investigations have revealed that patients administered intravenous amifostine experience a notable decline in the severity of radiation dermatitis.59 However, the body of evidence remains incomplete and additional research is required to definitively ascertain the effectiveness and safety of amifostine as a cytoprotective agent against acute radiation dermatitis. Since there are insufficient data available to completely dismiss the possibility that amifostine might have a protective effect on tumors, its use for the prevention of radiation dermatitis is not advised based on Level III–IV evidence.
7.2.13 Epidermal growth factor
In a prospective, self-controlled study involving 193 patients with rectal/anal cancer receiving pelvic radiotherapy, the left and right pelvic skin regions were randomly assigned to either a recombinant human epidermal growth factor (rhEGF) group or a control group. This study conducted an analysis examined the relationship between radiation dermatitis and various factors, including rhEGF, dose of radiotherapy, and distance from the tumor to the anal margin. The findings revealed that while rhEGF did not exert a significant influence on the grading of radiation dermatitis, it notably improved the area where radiation dermatitis manifested, as evidenced by a statistically significant value (p = 0.0007).60 Therefore, using the epidermal growth factor has a preventive effect by reducing the severity of radiation dermatitis (Level II evidence).
[Expert Recommendation 2] Adopt individualized prevention strategies based on patient-related factors and treatment factors. For patients with risk factors, preventive measures for radiation dermatitis can be used at the beginning of radiotherapy. It is recommended to combine multiple prevention modes and methods. (1) Non-pharmacological prevention: Choose appropriate radiotherapy techniques and segmentation modes based on the patient's condition and stage (Level I evidence, Grade A recommendation). Strengthen health education, dietary and psychological guidance, and avoid skin irritation, friction, and excessive sun exposure in the radiation area. Keep the skin clean and dry, and use warm water or neutral soap for local cleaning (Level II evidence, Grade A recommendation). Antiperspirants/deodorants can be used for those in need (Level I evidence, Grade A recommendation). Routine use of topical moisturizers, gels, lotions, or dressings before radiotherapy is not recommended for patients without risk factors (Level II evidence, Grade A recommendation). (2) Pharmacological prevention: It is recommended to use low to medium potency topical corticosteroids on the irradiation field for patients with high-risk factors, 1–2 times/d (e.g., 0.1% mometasone furoate or 0.1% hydrocortisone butyrate ointment) (Level I evidence, Grade A recommendation). GM-CSF, superoxide dismutase, silicone film-forming gel dressings, silver ion dressings/ointments, triethanolamine, and epidermal growth factor can also be used to prevent radiation dermatitis (Level II-III evidence, Grade B recommendation).
8 Treatment
8.1 Acute radiation dermatitis
Patients undergoing radiotherapy should be evaluated at a minimum frequency of once a week, with careful observation of the skin response to treatment during each visit. Management of radiation dermatitis is based on the degree of skin damage. Accordingly, the specific treatment administered is determined by the severity of the skin damage.
8.1.1 Grade 1 radiation dermatitis
Grade 1 radiation dermatitis, as evaluated by both the Radiation Therapy Oncology Group (RTOG) and the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), manifests as mild redness (erythema) and dry peeling (desquamation). Standard care measures for this grade of dermatitis include gentle cleaning of the affected area and the application of water-in-oil-type hydrophilic moisturizers to maintain skin hydration. In addition to these measures, patients are advised to use topical corticosteroids (1–2 times/d) and continue using them for 2 weeks after radiotherapy (Level I evidence). A 4-week randomized trial involving 278 breast cancer patients with Grade 1 radiation dermatitis found that after four weeks of treatment with hydrocolloid dressings or placebo (water-based spray), the local treatment failure rates in both the treatment and placebo groups were comparable, with 49% in the treatment group and 51% in the placebo group, as were the erythema, pain levels, and quality of life scores.61 Therefore, treating patients with Grade 1 radiation dermatitis with special dressings is not recommended (Level II evidence).
8.1.2 Grade 2 and 3 radiation dermatitis
Grade 2 radiation dermatitis manifests as moist desquamation involving skin folds, whereas Grade 3 radiation dermatitis affects other skin areas with similar symptoms. Treatment includes preventing skin infections and dressing skin desquamation sites.4, 62 Topical and/or systemic antibiotics should be used when bacterial infections are present (Level IV evidence). A moist environment can accelerate reepithelialization and wound healing; therefore, dressings are used to treat moist desquamation.63 Studies have shown that combining silver sulfadiazine dressings40 with epidermal growth factor dressings64 can significantly alleviate Grade 2 and higher radiation dermatitis (Level II-III evidence). Dressings must be changed daily, or even more frequently, with the frequency determined by the severity of exudation, as supported by Level III-IV evidence. Although various approaches have been made in recent years to treat radiation dermatitis, evidence from randomized controlled trials is still lacking.
8.1.3 Grade 4 radiation dermatitis
Grade 4 radiation dermatitis is an uncommon condition characterized by full-thickness skin necrosis and ulcers. Management of this condition should be individualized, possibly necessitating the discontinuation of radiotherapy. Comprehensive treatment should be administered by a multidisciplinary team tailored to each patient's specific needs, including trauma surgeons, radiation oncologists, dermatologists, and nursing staff.62 The treatment options for affected patients include surgical debridement, full-thickness skin grafts, myocutaneous flaps, or pedicled skin flaps. In cases of infected wounds or those at risk of infection, careful consideration should be given to the use of systemic or topical antimicrobial agents (Levels IV-V evidence).
8.2 Patients treated with concurrent EGFR inhibitors
For patients treated with concurrent EGFR inhibitors, if Grade 1, 2, or 3 radiation dermatitis occurs, it is generally not necessary to reduce the dose of EGFR inhibitors62, 65-68 (Level III-IV evidence). Based on Level V evidence, some experts suggest reducing the cetuximab dosage for patients with severe Grade 3 dermatitis.68 In the event of Grade 4 radiation dermatitis, it is recommended that both radiotherapy and cetuximab treatment be discontinued. Cetuximab should be resumed only once the skin reaction has been downgraded to at least Grade 265, 66 (Level III evidence). When using cetuximab, additional prevention of papulopustular rashes related to EGFR inhibitors is required (Levels III-IV evidence).
8.3 Chronic radiation dermatitis
8.3.1 Pharmacological treatment
There are a few reports on drug treatments for chronic radiation dermatitis. Some small randomized trials have shown that combining pentoxifylline and vitamin E for more than 3 years may help treat subcutaneous radiation fibrosis.69-71 However, the optimal dose, duration, and role of vitamin E remain unclear (Levels II-III evidence).
8.3.2 Non-pharmacological treatment
Non-drug treatments for radiation-induced conditions may involve active and passive range of motion exercises, which have been shown to improve mobility and reduce contractures, as supported by Level IV evidence. While hyperbaric oxygen has been assessed as a potential treatment for radiation-induced fibrosis, existing evidence is insufficient to conclusively demonstrate its effectiveness72 (Level III evidence). A few studies have found that laser treatment can heal radiation-induced telangiectasia and excessive pigmentation73, 74 (Level III-IV evidence).
[Expert Recommendation 3] The treatment of acute radiation dermatitis should be carried out according to the severity: (1) For Grade 1 radiation dermatitis, general skin care measures should be applied, and topical corticosteroids can be used 1–2 times/d (Level I evidence). Special dressings are not recommended for patients with Grade 1 radiation dermatitis (Level II evidence). (2) Treatment for Grade 2 and 3 radiation dermatitis includes preventing skin infections and using dressings on the desquamated skin area. Topical dressings such as silver sulfadiazine can be used (Level II evidence), and in case of infection, topical and/or systemic antibiotics should be applied (Level IV evidence). (3) For Grade 4 radiation dermatitis, radiotherapy should be discontinued, and a multidisciplinary team should provide individualized treatment. (4) For patients treated with concurrent EGFR inhibitors, if Grade 1, 2, or 3 radiation dermatitis occurs, it is generally not necessary to reduce the drug dose (Level III-IV evidence); if Grade 4 radiation dermatitis occurs, it is recommended to discontinue both radiotherapy and EGFR monoclonal antibody treatment (Level III evidence).
[Expert Recommendation 4] Recommendations for treating chronic radiation dermatitis: (1) Pharmacological treatment: Evidence is limited. Long-term combined pentoxifylline and vitamin E used to treat subcutaneous radiation fibrosis remains uncertain (Level II-III evidence). (2) Non-pharmacological treatment: hyperbaric oxygen therapy is not recommended for treating radiation-induced skin fibrosis (Level III evidence). After careful evaluation, laser treatment can be used for radiation-induced telangiectasia and excessive pigmentation in selected patients (Level III-IV evidence). Through active and passive range of motion exercises, it is possible to enhance mobility and minimize contractures (Level IV evidence).
9 CONCLUSION
Various methods can be used to prevent and treat radiation dermatitis. For the prevention of radiation dermatitis, personalized strategies based on patient-related and treatment factors should be adopted. Prevention measures include the rational selection of radiotherapy techniques and fractionation patterns, skin care education, and various medications; acute radiation dermatitis should be treated according to severity. Symptom management should be the focus in chronic radiation dermatitis. Due to the lack of large-scale, multicenter, randomized controlled studies, many prevention and treatment methods remain controversial. Subsequent high-quality studies are required to optimize the generation of and practical strategies for preventing and treating radiation dermatitis.
ACKNOWLEDGMENTS
Guiding experts: Jinming Yu (Shandong Cancer Hospital).
Writing experts:Ming Fan, Sichuan Cancer Hospital, Mei Feng, Sichuan Third People's Hospital
Editorial Board Members: (in alphabetical order by surname)
Weiqing Chen, Chongqing University Cancer Hospital
Xiaozhong Chen, Zhejiang Cancer Hospital
Yan Cheng, First Affiliated Hospital of Zhengzhou University
Mei Feng, Sichuan Third People's Hospital
Ming Fan,Sichuan Cancer Hospital
LiYing Gao, Gansu Provincial Cancer Hospital
Xianshu Gao, Peking University First Hospital
Yuanhong Gao, Sun Yat-sen University Cancer Center
Xia He, Jiangsu Cancer Hospital
Man Hu, Shandong Cancer Hospital
Xiaobo Huang, Sun Yat-Sen Memorial Hospital
Baosheng Li, Shandong Cancer Hospital
Guiling Li, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology
Jie Li, Shanxi Provincial Cancer Hospital
Jingao Li, Jiangxi Provincial Cancer Hospital
Qin Lin, The First Affiliated Hospital of Xiamen University
Fang Liu, The People's Liberation Army General Hospital
Qing Liu, The Third Affiliated Hospital of Air Force Medical University
Zi Liu, The First Affiliated Hospital of Xi'an Jiaotong University
Tenghui Ma, The Sixth Affiliated Hospital of Sun Yat-sen University
Shuhuai Niu, The Fourth Hospital of Hebei Medical University
Qiao Qiao, The First Affiliated Hospital of China Medical University
Jianguang Qiu, The Sixth Affiliated Hospital of Sun Yat-sen University
Mei Shi, Xijing Hospital of Air Force Military Medical University
Hui Wang, Hunan Cancer Hospital
Jun Wang, The Fourth Hospital of Hebei Medical University
Qifeng Wang, Sichuan Cancer Hospital
Rensheng Wang, The First Affiliated Hospital of Guangxi Medical University
Ruozheng Wang, Cancer Hospital Affiliated to Xinjiang Medical University
Sangang Wu, The First Affiliated Hospital of Xiamen University
Qin Xu, Fujian Provincial Cancer Hospital
Junlin Yi, The Cancer Hospital of Chinese Academy of Medical Sciences
Shuanghu Yuan, Shandong Cancer Hospital
Xianglin Yuan, Tongji Hospital of Tongji Medical College,Huazhong University of Science and Technology
Daxin Zhang, the First Affiliated Hospital of Harbin Medical University
Fuquan Zhang, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College
Liyuan Zhang, The Second Affiliated Hospital of Soochow University
Yunyan Zhang, Harbin Medical University Cancer Hospital
Zhen Zhang, Fudan University Shanghai Cancer Center
Anping Zheng, Anyang Tumor Hospital
Li Zhu, Tianjin Medical University Cancer Institute and Hospital
Hongqing Zhuang, Peking University Third Hospital
Dongling Zou, Chongqing University Cancer Hospital
CONFLICTS OF INTEREST STATEMENT
Jinming Yu is the Editor-in-Chief of the journal and co-author of this article. He was excluded from the peer review process and from all editorial decisions related to the acceptance and publication of this article. Peer reviews were handled independently by another editor to minimize bias.
ETHICS STATEMENT
The authors are accountable for all aspects of this work and ensure that questions related to the accuracy or integrity of any part are appropriately investigated and resolved.




