PD-L1 expression by different scoring methods and different cutoff values and correlation with clinicopathological characteristics in gastric cancer: A retrospective study
Lixiang Si and XiaoHua Pan contributed equally to this work.
Abstract
We retrospectively enrolled 325 gastric cancer (GC) patients to investigate the associations of programmed death ligand-1 (PD-L1) expression with clinicopathological characteristics by different scoring methods and different cutoff values. PD-L1 expression was evaluated by the tumor proportion score (TPS) and the combined positive score (CPS). The positive rate of PD-L1 TPS ≥1%, CPS ≥1, CPS ≥5 and CPS ≥10 in our study were 12.0%, 87.4%, 69.8% and 42.2%, respectively. Multivariate analysis showed that PD-L1 CPS ≥5 was related to high expression of Ki67 (OR = 2.658, 95% CI: 1.401–5.045, p = .003) and pTNM staging (p = .033). PD-L1 CPS ≥10 was correlated with larger tumor size (OR = 2.322, 95% CI: 1.052–5.127, p = .037) and lymph node metastasis (OR = 2.495, 95% CI: 1.293–4.814, p = .006). It is expected that these results can provide a reference for screening GC patients with high PD-L1 expression level.
1 INTRODUCTION
Gastric cancer (GC) ranks 5th and 4th in incidence and mortality worldwide, respectively.1 Recently, immune checkpoint therapy (ICT) is transforming the treatment of advanced cancer which brings more therapeutic options for some patients. ICT has been implemented for therapy of metastatic disease and the most widely predictive biomarker is programmed death ligand-1 (PD-L1).2, 3 In GC, two scoring methods are used to assess the expression of PD-L1: the tumor proportion score (TPS) and the combined positive score (CPS). These two methods were widely used for clinical trials but the connection between PD-L1 expression and clinicopathological characteristics and prognosis of GC remains controversial. These may be related to the selection of different scoring methods and cutoff values in different studies.
Therefore, we retrospectively enrolled 325 GC patients who received surgical treatment and investigated the rate of PD-L1 expression by different scoring methods and different cutoff values. We also examined the associations of PD-L1 expression with clinicopathological features in GC patients.
2 METHODS
2.1 Patients
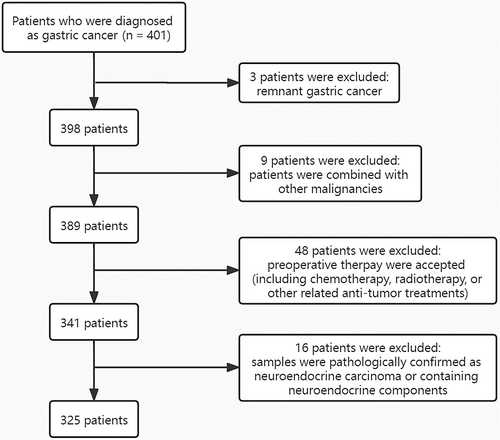
We retrospectively evaluated 401 GC patients in Jiangsu Cancer Hospital from August 2020 to May 2021. The inclusion criteria include: (1) tumors were histologically verified for GC; (2) postoperative samples received immunohistochemistry (IHC). The exclusion criteria include: (1) patients had remnant gastric cancer (RGC) or GC combined with other malignancies; (2) preoperative chemotherapy, radiotherapy, or other related anti-tumor treatments were accepted; (3) patients were confirmed to have neuroendocrine carcinoma (NEC) or containing neuroendocrine components based on pathology. A flow diagram depicting the screening process is shown in Figure 1. As a result, 325 patients were enrolled in our study based on inclusion/exclusion criteria.
2.2 Immunohistochemistry
We used 10% formaldehyde to fix the tumor samples, embedded them in paraffin, and then cut into four-micrometer-thick sections. After dewaxing and hydrating, citrate buffer was used for thermal remediation, and 3% hydrogen peroxide was used to inactivate the endogenous peroxidase. After that, we incubated the slides with primary antibody including anti-PD-L1 (clone MXR003), anti-Ki67 (clone MIB-1), anti-human epidermal growth factor receptor 2 (HER2) (clone MXR001), and p53 (clone Do-7) at 4°C in the refrigerator overnight. After washing with PBS for three times, we added the secondary antibody on the slides and used diaminobenzidine (DAB) for visualization. Finally, hematoxylin was applied for counterstaining.
2.3 PD-L1 expression and evaluation of molecular markers
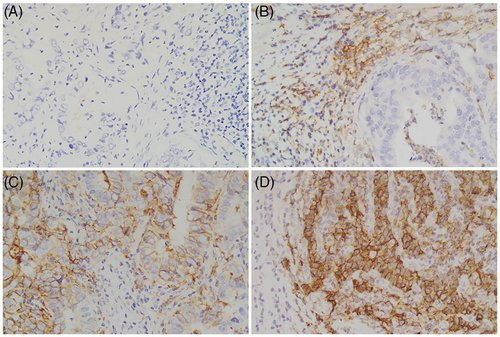
TPS was calculated as PD-L1 positive tumor cells/viable tumor cells × 100%. Furthermore, CPS was calculated as PD-L1 positive tumor cells, lymphocytes, and macrophages/viable tumor cells × 100.4 For TPS, ≥1% was employed to define PD-L1 positive.5 For CPS, ≥1, ≥5 and ≥10 were chosen to define PD-L1 positive, respectively.6-8 Representative stainings for PD-L1 expression are shown in Figure 2.
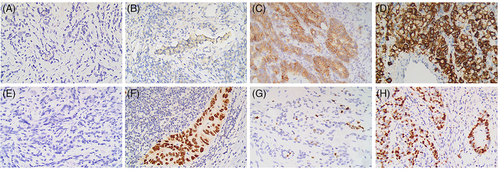
Molecular markers assessed by IHC in our study included HER2, p53, and Ki67. Positivity for HER2 was considered as IHC 2+ and 3+. For p53, tumors with distinct immunostaining in >25% of the cells were regarded as positive.9 Furthermore, cells showing nuclear staining were considered to be Ki67-positive. Ki67 index was calculated as Ki67-positive cells/cancer cells and ≥50% was considered to have a high expression of Ki67.10, 11 Representative examples of HER2, p53, and Ki67 status in GC are shown in Figure 3.
2.4 Inflammatory markers
Inflammatory markers reflect pro-inflammatory status and they were calculated as follows: platelet-to-lymphocyte ratio (PLR) = platelet/lymphocyte, neutrophil-to-lymphocyte ratio (NLR) = neutrophil/lymphocyte, monocyte-to-lymphocyte ratio (MLR) = monocyte/lymphocyte, and systemic immune-inflammation index (SII) = platelet × neutrophil/lymphocyte. The median values of PLR, NLR, MLR, and SII were used as the cutoff value, which were 125, 2.06, 0.28, and 429, respectively.
2.5 Statistical analysis
All data were arranged and analyzed with SPSS 24.0 software. All variables were evaluated by univariate analysis, and variables which p < .1 were analyzed with multivariate analysis to identify the independent correlative factors. p < .05 was considered statistically significant.
3 RESULTS
3.1 Patient characteristics
In these included patients, the median age was 66 years old in 242 (74.5%) males and 83 (25.5%) females. Among the tumor location, three groups were divided including upper, middle, and lower stomach and accounting for 148 cases (45.5%), 43 cases (13.2%) and 134 cases (41.2%), respectively. Concerning the histological type, 238 (73.2%) of patients were adenocarcinoma and the other 87 (26.8%) were signet ring cell/other carcinoma. Lauren classification was intestinal/mixed in 216 patients (66.5%) and diffuse in 109 (33.5%). In the differentiation of tumor, 298 (91.7%) of patients were poorly differentiation and 27 (8.3%) were well/moderate differentiation. There were 45 (13.8%) patients whose tumor size were <3 cm and the other 280 (86.2%) of cases had larger tumor mass (tumor size ≥3 cm). For tumor infiltration, there were 58 (17.8%) T1/T2 and 267 (82.2%) T3/T4 stage cases. For lymph node metastasis status, 260 (80.0%) of patients had lymph node metastasis. Regarding pTNM staging, I had 33 cases (10.2%), II had 99 cases (30.5%) and III had 193 cases (59.4%). In terms of molecular markers, HER2-positive was detected in 131 (40.3%) cases and HER2- negative was observed in 194 (59.7%) cases. p53 was positive in 200 (61.5%) patients and negative in 125 (38.5%) patients. 272 (83.7%) patients had high expression of Ki67 and 53 (16.3%) had low expression of Ki67. For inflammatory markers, PLR, NLR, MLR and SII were categorized into high PLR (≥125, 50.8%) and low PLR (<125, 49.2%); high NLR (≥2.06, 50.5%) and low NLR (<2.06, 49.5%); high MLR (≥0.28, 48.3%) and low MLR (<0.28, 51.7%); high SII (≥429, 50.2%) and low SII (<429, 49.8%) groups, respectively (Table 1).
| Clinicopathological characteristics | Case, n (%) |
|---|---|
| Gender | |
| Male | 242 (74.5) |
| Female | 83 (25.5) |
| Age | |
| <65 | 147 (45.2) |
| ≥65 | 178 (54.8) |
| Tumor location | |
| Upper | 148 (45.5) |
| Middle | 43 (13.2) |
| Lower | 134 (41.2) |
| Histological type | |
| Adenocarcinoma | 238 (73.2) |
| Signet ring cell/other carcinoma | 87 (26.8) |
| Lauren classification | |
| Intestinal/mixed | 216 (66.5) |
| Diffuse | 109 (33.5) |
| Differentiation | |
| Poorly | 298 (91.7) |
| Well/moderate | 27 (8.3) |
| Tumor size (cm) | |
| <3.0 | 45 (13.8) |
| ≥3.0 | 280 (86.2) |
| Tumor infiltration | |
| T1/T2 | 58 (17.8) |
| T3/T4 | 267 (82.2) |
| Lymph node metastasis | |
| N0 | 65 (20.0) |
| ≥N1 | 260 (80.0) |
| pTNM staging | |
| I | 33 (10.2) |
| II | 99 (30.5) |
| III | 193 (59.4) |
| HER2 | |
| Negative | 194 (59.7) |
| Positive | 131 (40.3) |
| p53 | |
| Negative | 125 (38.5) |
| Positive | 200 (61.5) |
| Ki67 | |
| Low expression | 53 (16.3) |
| High expression | 272 (83.7) |
| PLR | |
| <125 | 160 (49.2) |
| ≥125 | 165 (50.8) |
| NLR | |
| <2.06 | 161 (49.5) |
| ≥2.06 | 164 (50.5) |
| MLR | |
| <0.28 | 168 (51.7) |
| ≥0.28 | 157 (48.3) |
| SII | |
| <429 | 162 (49.8) |
| ≥429 | 163 (50.2) |
- Abbreviations: GC, gastric cancer; HER2, human epidermal growth factor receptor 2; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
3.2 The expression of PD-L1
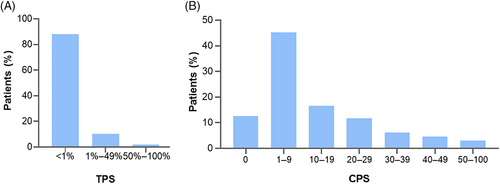
The percentage of PD-L1 TPS ≥1% was 12.0% (39/325). In addition, the positive rate of CPS with cutoff value of 1, 5 and 10 were 87.4% (284/325), 69.8% (227/325) and 42.2% (137/325), respectively. The distribution of PD-L1 expression evaluated by two scoring methods is presented in Figure 4.
3.3 Connections between PD-L1 expression of TPS/CPS and clinicopathological characteristics
Based on univariate analysis, when TPS ≥1% was chosen to evaluate PD-L1-positive, the association between PD-L1 and all factors above was no found including molecular markers and inflammatory markers (Table 2).
| Characteristics | TPS <1% | TPS ≥1% | Univariate analysis | |
|---|---|---|---|---|
| n = 286 (%) | n = 39 (%) | OR (95% CI) | p-value | |
| Gender | ||||
| Male | 215 (88.8) | 27 (11.2) | 1.346 (0.648–2.796) | .426 |
| Female | 71 (85.5) | 12 (14.5) | ||
| Age | ||||
| <65 | 132 (89.8) | 15 (10.2) | 1.371 (0.691–2.723) | .367 |
| ≥65 | 154 (86.5) | 24 (13.5) | ||
| Tumor location | ||||
| Upper | 135 (91.2) | 13 (8.8) | 1 | .267 |
| Middle | 37 (86.0) | 6 (14.0) | 0.549 (0.262–1.152) | |
| Lower | 114 (85.1) | 20 (14.9) | 0.924 (0.345–2.475) | |
| Histological type | ||||
| Adenocarcinoma | 208 (87.4) | 30 (12.6) | 0.800 (0.363–1.761) | .579 |
| Signet ring cell/other carcinoma | 78 (89.7) | 9 (10.3) | ||
| Lauren classification | ||||
| Intestinal/mixed | 194 (89.8) | 22 (10.2) | 1.629 (0.826–3.216) | .159 |
| Diffuse | 92 (84.4) | 17 (15.6) | ||
| Differentiation | ||||
| Poorly | 260 (87.2) | 38 (12.8) | 0.263 (0.035–1.996) | .197 |
| Well/moderate | 26 (96.3) | 1 (3.7) | ||
| Tumor size (cm) | ||||
| <3.0 | 42 (93.3) | 3 (6.7) | 2.066 (0.608–7.014) | .245 |
| ≥3.0 | 244 (87.1) | 36 (12.9) | ||
| Tumor infiltration | ||||
| T1/T2 | 49 (84.5) | 9 (15.5) | 0.689 (0.308–1.543) | .365 |
| T3/T4 | 237 (88.8) | 30 (11.2) | ||
| Lymph node | ||||
| N0 | 59 (90.8) | 6 (9.2) | 1.430 (0.572–3.572) | .444 |
| ≥N1 | 227 (87.3) | 33 (12.7) | ||
| pTNM staging | ||||
| I | 29 (87.9) | 4 (12.1) | 1 | .911 |
| II | 86 (86.9) | 13 (13.1) | 1.072 (0.344–3.338) | |
| III | 171 (88.6) | 22 (11.4) | 1.175 (0.565–2.445) | |
| HER2 | ||||
| Negative | 169 (87.1) | 25 (12.9) | 0.809 (0.404–1.621) | .550 |
| Positive | 117 (89.3) | 14 (10.7) | ||
| p53 | ||||
| Negative | 110 (88.0) | 15 (12.0) | 1.000 (0.503–1.989) | 1.000 |
| Positive | 176 (88.0) | 24 (12.0) | ||
| Ki67 | ||||
| Low expression | 48 (90.6) | 5 (9.4) | 1.371 (0.510–3.686) | .531 |
| High expression | 238 (87.5) | 34 (12.5) | ||
| PLR | ||||
| <125 | 142 (88.8) | 18 (11.3) | 1.150 (0.588–2.250) | .682 |
| ≥125 | 144 (87.3) | 21 (12.7) | ||
| NLR | ||||
| <2.06 | 141 (87.6) | 20 (12.4) | 0.924 (0.473–1.804) | .816 |
| ≥2.06 | 145 (88.4) | 19 (11.6) | ||
| MLR | ||||
| <0.28 | 150 (89.3) | 18 (10.7) | 1.287 (0.658–2.517) | .461 |
| ≥0.28 | 136 (86.6) | 21 (13.4) | ||
| SII | ||||
| <429 | 145 (89.5) | 17 (10.5) | 1.331 (0.678–2.611) | .406 |
| ≥429 | 141 (86.5) | 22 (13.5) | ||
- Abbreviations: CI, confidence interval; HER2, human epidermal growth factor receptor 2; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PD-L1, programmed death ligand-1; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; TPS, tumor proportion score.
There were insignificant association between PD-L1 CPS ≥1 and gender, age, tumor location, pTNM staging, Ki67, p53, PLR, NLR, MLR, SII and so on. In addition, PD-L1 CPS ≥1 was related to HER2 positivity (p = .030). However, multivariate analysis revealed that PD-L1 CPS ≥1 had no relation to all clinicopathological characteristics (Table 3).
| Characteristics | CPS <1 | CPS ≥1 | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| n = 41 (%) | n = 284 (%) | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Gender | ||||||
| Male | 33 (13.6) | 209 (86.4) | 1.480 (0.654–3.348) | .346 | ||
| Female | 8 (9.6) | 75 (90.4) | ||||
| Age | ||||||
| <65 | 21 (14.3) | 126 (85.7) | 1.317 (0.684–2.536) | .411 | ||
| ≥65 | 20 (11.2) | 158 (88.8) | ||||
| Tumor location | ||||||
| Upper | 16 (10.8) | 132 (89.2) | 1 | .572 | ||
| Middle | 5 (11.6) | 38 (88.4) | 1.447 (0.716–2.925) | |||
| Lower | 20 (14.9) | 114 (85.1) | 1.333 (0.468–3.797) | |||
| Histological type | ||||||
| Adenocarcinoma | 26 (10.9) | 212 (89.1) | 0.589 (0.295–1.173) | .132 | ||
| Signet ring cell/other carcinoma | 15 (17.2) | 72 (82.8) | ||||
| Lauren classification | ||||||
| Intestinal/mixed | 22 (10.2) | 194 (89.8) | 0.537 (0.277–1.042) | .066 | 0.655 (0.329–1.305) | .229 |
| Diffuse | 19 (17.4) | 90 (82.6) | ||||
| Differentiation | ||||||
| Poorly | 39 (13.1) | 259 (86.9) | 1.882 (0.429–8.261) | .402 | ||
| Well/moderate | 2 (7.4) | 25 (92.6) | ||||
| Tumor size (cm) | ||||||
| <3.0 | 6 (13.3) | 39 (86.7) | 1.077 (0.425–2.728) | .876 | ||
| ≥3.0 | 35 (12.5) | 245 (87.5) | ||||
| Tumor infiltration | ||||||
| T1/T2 | 7 (12.1) | 51 (87.9) | 0.941 (0.395–2.241) | .890 | ||
| T3/T4 | 34 (12.7) | 233 (87.3) | ||||
| Lymph node | ||||||
| N0 | 9 (13.8) | 56 (86.2) | 1.145 (0.517–2.536) | .738 | ||
| ≥N1 | 32 (12.3) | 228 (87.7) | ||||
| pTNM staging | ||||||
| I | 6 (18.2) | 27 (81.8) | 1 | .345 | ||
| II | 9 (9.1) | 90 (90.9) | 0.701 (0.264–1.860) | |||
| III | 26 (13.5) | 167 (86.5) | 1.557 (0.699–3.465) | |||
| HER2 | ||||||
| Negative | 31 (16.0) | 163 (84.0) | 2.301 (1.086–4.875) | .030 | 1.994 (0.918–4.332) | .081 |
| Positive | 10 (7.6) | 121 (92.4) | ||||
| p53 | ||||||
| Negative | 11 (8.8) | 114 (91.2) | 0.547 (0.263–1.135) | .105 | ||
| Positive | 30 (15.0) | 170 (85.0) | ||||
| Ki67 | ||||||
| Low expression | 11 (20.8) | 42 (79.2) | 2.113 (0.984–4.538) | .055 | 1.917 (0.880–4.173) | .101 |
| High expression | 30 (11.0) | 242 (89.0) | ||||
| PLR | ||||||
| <125 | 23 (14.4) | 137 (85.6) | 1.371 (0.709–2.651) | .348 | ||
| ≥125 | 18 (10.9) | 147 (89.1) | ||||
| NLR | ||||||
| <2.06 | 17 (10.6) | 144 (89.4) | 0.689 (0.355–1.337) | .270 | ||
| ≥2.06 | 24 (14.6) | 140 (85.4) | ||||
| MLR | ||||||
| <0.28 | 19 (11.3) | 149 (88.7) | 0.782 (0.406–1.509) | .464 | ||
| ≥0.28 | 22 (14.0) | 135 (86.0) | ||||
| SII | ||||||
| <429 | 22 (13.6) | 140 (86.4) | 1.191 (0.618–2.296) | .602 | ||
| ≥429 | 19 (11.7) | 144 (88.3) | ||||
- Abbreviations: CI, confidence interval; CPS, combined positive score; HER2, human epidermal growth factor receptor 2; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PD-L1, programmed death ligand-1; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
In univariate analysis, PD-L1 CPS ≥5 was related to high expression of Ki67 (p = .001), adenocarcinoma (p = .002), Lauren intestinal/mixed type (p = .001). A multivariate analysis was performed, which demonstrated that PD-L1 CPS ≥5 was more frequently found in patients with high expression of Ki67 (OR = 2.658, 95% CI: 1.401–5.045, p = .003) and with the increase of pTNM staging, the rate of PD-L1 CPS ≥5 was increased (p = .033) (Table 4).
| Characteristics | CPS <5 | CPS ≥5 | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| n = 98 (%) | n = 227 (%) | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Gender | ||||||
| Male | 70 (28.9) | 172 (71.1) | 0.799 (0.469–1.362) | .411 | ||
| Female | 28 (33.7) | 55 (66.3) | ||||
| Age | ||||||
| <65 | 51 (34.7) | 96 (65.3) | 1.481 (0.920–2.383) | .106 | ||
| ≥65 | 47 (26.4) | 131 (73.6) | ||||
| Tumor location | ||||||
| Upper | 37 (25.0) | 111 (75.0) | 1 | .182 | ||
| Middle | 15 (34.9) | 28 (65.1) | 1.568 (0.937–2.626) | |||
| Lower | 46 (34.3) | 88 (65.7) | 0.976 (0.474–2.007) | |||
| Histological type | ||||||
| Adenocarcinoma | 60 (25.2) | 178 (74.8) | 0.435 (0.260–0.727) | .002 | 0.572 (0.319–1.027) | .062 |
| Signet ring cell/other carcinoma | 38 (43.7) | 49 (56.3) | ||||
| Lauren classification | ||||||
| Intestinal/mixed | 52 (24.1) | 164 (75.9) | 0.434 (0.266–0.710) | .001 | 0.573 (0.322–1.020) | .058 |
| Diffuse | 46 (42.2) | 63 (57.8) | ||||
| Differentiation | ||||||
| Poorly | 92 (30.9) | 206 (69.1) | 1.563 (0.611–4.001) | .352 | ||
| Well/moderate | 6 (22.2) | 21 (77.8) | ||||
| Tumor size (cm) | ||||||
| <3.0 | 18 (40.0) | 27 (60.0) | 1.667 (0.870–3.194) | .124 | ||
| ≥3.0 | 80 (28.6) | 200 (71.4) | ||||
| Tumor infiltration | ||||||
| T1/T2 | 19 (32.8) | 39 (67.2) | 1.159 (0.631–2.130) | .634 | ||
| T3/T4 | 79 (29.6) | 188 (70.4) | ||||
| Lymph node | ||||||
| N0 | 21 (32.3) | 44 (67.7) | 1.134 (0.633–2.034) | .672 | ||
| ≥N1 | 77 (29.6) | 183 (70.4) | ||||
| pTNM staging | ||||||
| I | 14 (42.4) | 19 (57.6) | 1 | .062 | 1 | .033 |
| II | 22 (22.2) | 77 (77.8) | 0.642 (0.302–1.365) | 0.473 (0.213–1.052) | ||
| III | 62 (32.1) | 131 (67.9) | 1.656 (0.944–2.906) | 1.527 (0.838–2.783) | ||
| HER2 | ||||||
| Negative | 66 (34.0) | 128 (66.0) | 1.595 (0.970–2.622) | .066 | 1.206 (0.707–2.057) | .492 |
| Positive | 32 (24.4) | 99 (75.6) | ||||
| p53 | ||||||
| Negative | 35 (28.0) | 90 (72.0) | 0.846 (0.517–1.382) | .504 | ||
| Positive | 63 (31.5) | 137 (68.5) | ||||
| Ki67 | ||||||
| Low expression | 26 (49.1) | 27 (50.9) | 2.675 (1.465–4.884) | .001 | 2.658 (1.401–5.045) | .003 |
| High expression | 72 (26.5) | 200 (73.5) | ||||
| PLR | ||||||
| <125 | 48 (30.0) | 112 (70.0) | 0.986 (0.614–1.583) | .953 | ||
| ≥125 | 50 (30.3) | 115 (69.7) | ||||
| NLR | ||||||
| <2.06 | 45 (28.0) | 116 (72.0) | 0.812 (0.505–1.306) | .391 | ||
| ≥2.06 | 53 (32.3) | 111 (67.7) | ||||
| MLR | ||||||
| <0.28 | 51 (30.4) | 117 (69.6) | 1.020 (0.635–1.639) | .934 | ||
| ≥0.28 | 47 (29.9) | 110 (70.1) | ||||
| SII | ||||||
| <429 | 50 (30.9) | 112 (69.1) | 1.070 (0.666–1.718) | .781 | ||
| ≥429 | 48 (29.4) | 115 (70.6) | ||||
- Abbreviations: CI, confidence interval; CPS, combined positive score; HER2, human epidermal growth factor receptor 2; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PD-L1, programmed death ligand-1; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
A significant connection was shown among PD-L1 CPS ≥10 and a higher percentage of elderly (aged ≥65 years, p = .044), adenocarcinoma (p = .015), Lauren intestinal/mixed type (p = .034), lymph node metastasis (p = .004) and larger tumor mass (tumor size ≥3 cm, p = .005). Moreover, multivariate analysis suggested that PD-L1 CPS ≥10 was more often detected in GC patients with lymph node metastasis (OR = 2.495, 95% CI: 1.293–4.814, p = .006) and larger tumor size (OR = 2.322, 95% CI: 1.052–5.127, p = .037) (Table 5).
| Characteristics | CPS <10 | CPS ≥10 | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| n = 188 (%) | n = 137 (%) | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Gender | ||||||
| Male | 145 (59.9) | 97 (40.1) | 1.391 (0.842–2.296) | .198 | ||
| Female | 43 (51.8) | 40 (48.2) | ||||
| Age | ||||||
| <65 | 94 (63.9) | 53 (36.1) | 1.585 (1.013–2.479) | .044 | 1.465 (0.912–2.354) | .114 |
| ≥65 | 94 (52.8) | 84 (47.2) | ||||
| Tumor location | ||||||
| Upper | 84 (56.8) | 64 (43.2) | 1 | .905 | ||
| Middle | 26 (60.5) | 17 (39.5) | 1.061 (0.661–1.703) | |||
| Lower | 78 (58.2) | 56 (41.8) | 0.911 (0.452–1.836) | |||
| Histological type | ||||||
| Adenocarcinoma | 128 (53.8) | 110 (46.2) | 0.524 (0.311–0.881) | .015 | 0.561 (0.313–1.006) | .052 |
| Signet ring cell/other carcinoma | 60 (69.0) | 27 (31.0) | ||||
| Lauren classification | ||||||
| Intestinal/mixed | 116 (53.7) | 100 (46.3) | 0.596 (0.370–0.962) | .034 | 0.657 (0.383–1.128) | .127 |
| Diffuse | 72 (66.1) | 37 (33.9) | ||||
| Differentiation | ||||||
| Poorly | 173 (58.1) | 125 (41.9) | 1.107 (0.501–2.447) | .801 | ||
| Well/moderate | 15 (55.6) | 12 (44.4) | ||||
| Tumor size (cm) | ||||||
| <3.0 | 35 (77.8) | 10 (22.2) | 2.905 (1.385–6.096) | .005 | 2.322 (1.052–5.127) | .037 |
| ≥3.0 | 153 (54.6) | 127 (45.4) | ||||
| Tumor infiltration | ||||||
| T1/T2 | 36 (62.1) | 22 (37.9) | 1.238 (0.691–2.218) | .473 | ||
| T3/T4 | 152 (56.9) | 115 (43.1) | ||||
| Lymph node | ||||||
| N0 | 48 (73.8) | 17 (26.2) | 2.420 (1.322–4.430) | .004 | 2.495 (1.293–4.814) | .006 |
| ≥N1 | 140 (53.8) | 120 (46.2) | ||||
| pTNM staging | ||||||
| I | 24 (72.7) | 9 (27.3) | 1 | .184 | ||
| II | 54 (54.5) | 45 (45.5) | 0.497 (0.219–1.125) | |||
| III | 110 (57.0) | 83 (43.0) | 1.104 (0.678–1.798) | |||
| HER2 | ||||||
| Negative | 115 (59.3) | 79 (40.7) | 1.157 (0.739–1.811) | .525 | ||
| Positive | 73 (55.7) | 58 (44.3) | ||||
| p53 | ||||||
| Negative | 68 (54.4) | 57 (45.6) | 0.795 (0.506–1.249) | .320 | ||
| Positive | 120 (60.0) | 80 (40.0) | ||||
| Ki67 | ||||||
| Low expression | 36 (67.9) | 17 (32.1) | 1.672 (0.895–3.122) | .107 | ||
| High expression | 152 (55.9) | 120 (44.1) | ||||
| PLR | ||||||
| <125 | 92 (57.5) | 68 (42.5) | 0.972 (0.626–1.510) | .901 | ||
| ≥125 | 96 (58.2) | 69 (41.8) | ||||
| NLR | ||||||
| <2.06 | 93 (57.8) | 68 (42.2) | 0.993 (0.640–1.543) | .976 | ||
| ≥2.06 | 95 (57.9) | 69 (42.1) | ||||
| MLR | ||||||
| <0.28 | 99 (58.9) | 69 (41.1) | 1.096 (0.706–1.703) | .683 | ||
| ≥0.28 | 89 (56.7) | 68 (43.3) | ||||
| SII | ||||||
| <429 | 95 (58.6) | 67 (41.4) | 1.067 (0.687–1.658) | .772 | ||
| ≥429 | 93 (57.1) | 70 (42.9) | ||||
- Abbreviations: CI, confidence interval; CPS, combined positive score; HER2, human epidermal growth factor receptor 2; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PD-L1, programmed death ligand-1; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index.
4 DISCUSSION
ICT has established a stable status in multiple cancers and now is also emerging as a viable option for GC patients.12-14 In immunotherapy, PD-L1 is the most widely predictive biomarker. Programmed death-1 (PD-1)/PD-L1 interacts in the tumor immune microenvironment, induces T lymphocyte apoptosis, decreases the activity of cytotoxic T cells, and induces the tolerance to tumor cells, causing tumor immune escape and progression.15 Immune checkpoint inhibitors can block this pathway, so compared with traditional treatment, immunotherapy significantly improves the survival rate of GC patients.
Several researches have explored the relevance of PD-L1 with clinicopathological features of GC, and the results of different studies are discrepant. Chang and colleagues16 demonstrated that PD-L1 overexpression was more often detected in higher T-stage, diffuse Lauren classification, and lymphatic invasion. A meta-analysis used by Zhang and colleagues17 suggested that patients with larger tumor size were more likely to express PD-L1, the same went for patients with lymph node metastasis. Conversely, another study indicated that PD-L1 was connected with less advanced stage, intestinal type, and well/moderately differentiation and PD-L1 was a favorable prognostic marker.18 The discrepancy of results in these studies may be related to the selection of different scoring methods and cutoff values. In addition, several significant clinical trials also chose different scoring methods and different cutoff values.2, 3, 8 However, the cutoff values selection of the PD-L1 scoring system is inconclusive.19 Therefore, we aimed to analyze the associations of PD-L1 expression with clinicopathological characteristics in GC patients by different scoring methods and different cutoff values.
Yu and colleagues20 have shown that PD-L1 will be changed after receiving preoperative adjuvant therapy, which may be related to changes in tumor immune microenvironment during chemotherapy for GC. Further, samples used for IHC in some studies were obtained from either tissue biopsy or surgical resection. Research has shown that compared with resection tissues, local biopsies could not represent the entire tumor biology and intratumoral heterogeneity.21 In consequence, patients included in this study did not receive preoperative adjuvant therapy and samples used for IHC were surgically resected tissues.
Hagi et al.22 studied 200 patients with GC and found that positive rate of TPS ≥1% was 25%, and the positive rate of CPS with cutoff value of 1, 5 and 10 were 58.5%, 37.0% and 19.5%, respectively. Fassan et al.23 included a study of 155 patients and found that the positive rate of CPS ≥1, CPS ≥5 and CPS ≥10 were 31.0%, 16.8% and 12.9%, respectively. Compared with the positive rate in this study, the reason for the difference may be: (1) patient population were different; (2) the treatment histories were different; (3) tumor heterogeneity.
Studies have noted positive relationship between PD-L1 and Ki67 in non-small cell lung cancer (NSCLC) and breast cancer.24, 25 In our study, the same results also discovered in patients who had PD-L1 CPS ≥5, but the specific mechanism was not yet clear, and whether Ki67 could improve the efficiency of predicting the efficacy of immunotherapy in GC remained to be explored. PD-L1 CPS ≥10 was correlated with larger tumor size and lymph node metastasis. This might be related to tumor cells escaping immunological surveillance in the complicated tumor microenvironment.26 The increased PD-L1 expression promoted tumor growth and infiltration, meanwhile with the increase of tumor invasion, the number of lymphocytes and macrophages around the tumor increased and the expression of PD-L1 increased accordingly. When CPS ≥5 and CPS ≥10 were chosen to evaluate PD-L1-positive, it was correlated with several adverse prognostic factors, including Ki67, pTNM staging, tumor size and lymph node metastasis. Therefore, we conjecture that PD-L1 is an adverse prognosticator from the perspective of clinicopathological characteristics which is in accordance with previous studies.16, 17, 27
Currently, the HER2 receptor is recognized as an important therapeutic target for targeted therapy. Several preclinical studies have shown that targeted therapy for HER2 and immunotherapy have a synergistic effect on tumor reduction.28 In addition, KEYNOTE-811 demonstrated that anti-PD-1 combined with anti-HER2 and chemotherapy significantly improved the efficacy of patients with GC.29 However, there are conflicting reports on the relationship between PD-L1 and HER2 expression. Oki et al.30 found that inhibition of HER2 expression by siRNA could downregulate PD-L1 expression. In contrast, Wang et al.31 believed that HER2-negative GC had higher PD-L1 expression. Herein, though PD-L1 CPS ≥1 was positively correlated with HER2 by univariate analysis, multivariate analysis revealed that HER2 status had no specific influence on PD-L1. This result is concordant with Chen et al.32 Therefore, the clinical synergistic effect described above may be attributed to other mechanisms. One of potential mechanisms was that trastuzumab can increase PD-L1 expression by engaging immune effector cells.33
As is well-known, inflammation is closely relevant to tumorigenesis and tumor development.34 The systemic inflammatory response can result in changes in peripheral blood cell count, and such changes can be reflected by inflammatory markers. Recent studies have shown that inflammatory markers have been used to predict response to immunotherapy in several tumors, particularly in NSCLC and melanoma.35-37 The relevance of PD-1/PD-L1 pathway with inflammatory pathway remains unclear. We did not confirm a significant association among PD-L1 and inflammatory markers, suggesting that they reflected different aspects of immunity.
Clinical studies on the treatment of advanced GC emerge one after another. In CheckMate-649, compared with chemotherapy, the survival benefits of first-line nivolumab combined with chemotherapy increased with the increase of PD-L1 cutoff value.8 In KEYNOTE-590, the survival time of first-line pembrolizumab combined with chemotherapy was significantly better than chemotherapy in patients who had PD-L1 CPS ≥10.7 In KEYNOTE-061 study, second-line pembrolizumab versus paclitaxel did not reach statistical difference in survival rate of overall population, but subgroup analyses showed a trend of survival benefit was found in patients with PD-L1 CPS ≥10.38 These significant clinical trials have confirmed that advanced GC patients with high CPS scores were more likely to have better outcomes in immunotherapy. We hope that results in our study can provide reference for screening patients with high CPS scores.
This study still has certain limitations. The inherent biases could not be avoided due to this is a retrospective study. Moreover, prognostic analysis was not conducted as a result of the short follow-up period of cases enrolled in the present study. We will follow up these cases continually to obtain survival data and treatment protocols for further research.
To sum up, PD-L1 CPS ≥5 was associated with high expression of Ki67 and pTNM staging. PD-L1 CPS ≥10 was more often observed in GC patients with larger tumor size or lymph node metastasis. It is expected that these results can provide a reference for screening GC patients with high PD-L1 expression level.
AUTHOR CONTRIBUTIONS
Study design: Jianwei Lu, Xinyu Xu; Collection and assembly of data: Lixiang Si, XiaoHua Pan, Kang He, Ling Sun, Yajing Wang; Data analysis and interpretation: Lixiang Si, XiaoHua Pan; Manuscript writing: Lixiang Si, XiaoHua Pan; Final approval of manuscript: All authors.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICS STATEMENT
This research was performed and approved by the clinical research ethics committee of Jiangsu Cancer Hospital and was conducted in accordance with the Declaration of Helsinki.




