Salivary and plasmatic levels of tumor necrosis factor-alpha do not correlate with the clinicopathological profile in breast cancer patients
Carolina Panis and Carolina Panis equally contributed to the study.
Funding information: Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Number: -; Fundação Araucária
Abstract
Herein, we evaluated the salivary and plasmatic levels of tumor necrosis factor-alpha (TNF-α) in women diagnosed with breast cancer (BC; n = 20) versus women with benign breast conditions (Control; n = 29) and correlated the TNF-α findings with BC clinicopathological parameters. TNF-α was higher in the saliva samples from both groups than in plasma levels. BC and Control patients presented similar plasmatic and salivary values of TNF-α. The salivary and plasmatic values of TNF-α did not correlate with tumor features (estrogen receptor; progestogen receptor; Ki67, and HER2), indicating that its salivary content does not correlate with the parameters of disease prognosis. Therefore, TNF-α is not helpful as a salivary marker in breast cancer patients.
1 INTRODUCTION
Cancer is the second leading cause of death worldwide, and breast cancer (BC) is the most frequent cancer that affects women worldwide.1, 2 Its early detection, in association with better diagnostic tools, its early detection is a central element that determines a more favorable prognosis for BC.3, 4 In this regard, increasing attention has been drawn to the relationship between BC and oral health.5 Sawczuk et al.6 reported a more than two-fold higher incidence of BC in women with periodontal disease than those without it. Moreover, changes in salivary pH and oxidative agents also have been found in unstimulated saliva from women with BC.
Saliva has been studied as an alternative biological fluid in the non-invasive diagnostics of several systemic diseases, such as endocrine, cardiovascular, autoimmune, or infectious illnesses.7 This fluid constitutes an aqueous component in which different proteins (e.g., enzymes, immunoglobulins) and electrolytes (e.g., sodium, calcium, bicarbonate) are dissolved. The majority of such substances are provided to saliva by the three main salivary glands, parotid, submandibular, and sublingual.8 After being released into the oral cavity, saliva is mixed with gingival crevicular fluid, bronchial and nasal secretions, serum, and blood derivatives from oral wounds, microorganisms, leukocytes, desquamated epithelial cells, and food debris.9 Systemic inflammatory conditions may influence the levels of specific salivary biomarkers since saliva contains serum-derived components.10
BC is associated with cytokine production,11 but its reflection on the cytokine levels in the saliva is unclear. In this sense, salivary cytokines present potential promising features to be employed as biomarkers for disease screening, as demonstrated in several pathologies, such as Sjögren's syndrome, diabetes mellitus, and Cushing's disease.12 Saliva has also been suggested for BC screening since several studies have demonstrated the presence of important tumor markers in the saliva of patients with BC.7 These findings indicate that saliva can mirror the plasmatic variation in the content of different substances, being influenced by systemic events as chemotherapy and surgical procedures.13
Tumor necrosis factor-α (TNF-α) is an important pro-inflammatory cytokine involved in all stages of BC development. High levels of TNF-α have been found in tumor microenvironment as well as in serum and plasma from BC patients being correlated with increased mortality risk.14 TNF-α serum levels also were correlated with clinical disease stage, lymph node metastasis, estrogen receptor (ER), and human epidermal growth factor receptor 2 (HER2) antigen expression in BC patients.15 Moreover, increased salivary concentrations of TNF-α and IL-6 seem to reflect the cancerous tissue's local production of these cytokines.16 However, the relationship between the plasmatic and salivary levels of TNF-α with some traits of clinicopathological BC is still unknown. Thus, in the present study, we compared the plasmatic and salivary levels of TNF-α between matched samples from women diagnosed with benign breast disease and women with BC. In addition, we related plasmatic and salivary levels of TNF-α with anthropometric and clinicopathological parameters of women with BC.
2 METHODS
2.1 Type of study and experimental design
It is an exploratory pilot study approved by the Ethics Committee of the Universidade Estadual do Oeste do Paraná under the number 35524814.4.0000.0107. All women signed a free and informed consent form.
2.2 Inclusion and exclusion criteria and experimental design
Between November (2017) and October (2018), women (n = 82) with lesions suspicious of breast cancer (BIRADIS IV or V) that attended a public Oncology Hospital from Southwest Paraná state were selected. After a biopsy, 20 women that confirmed the diagnosis of breast cancer (BC) and 29 with benign breast conditions (Control) were included. We excluded 32 women with no matched saliva and peripheral blood samples.
2.3 Data collection from medical records
Clinicopathological data were collected from the medical records of the patients, including their age at diagnosis (years), body weight (kg), and height (m), from which the body mass index (BMI) was calculated and categorized as recommended by the World Health Organization (WHO).17 Moreover, the profiles for tumor estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), ki67 proliferation index (ki67%), and information regarding tumor molecular subtype, disease aggressiveness (Luminal A or HER2 amplified as less aggressive disease, Luminal B and triple negatives as a more aggressive disease), histological tumor grade, and the presence of intratumoral angiolymphatic emboli were also recorded.
2.4 Blood and saliva samples collection and TNF-α levels measurement
Peripheral blood samples (10 ml) were collected after 12 h of fasting by venipuncture into heparinized tubes and immediately centrifuged (4000 rpm for 5 min). Plasma aliquots were stored at −20°C until analysis. Saliva was obtained by spontaneous elimination and transferred to microtubes in variable volumes (0.2–2 ml), held at −20°C. To avoid saliva contamination with blood, patients did not brush their teeth before sample collection or had any dental procedure 24 h before. Patients with bleeding oral injuries did not collect saliva for the study, and any sample visibly containing blood was excluded.
To measure TNF levels, saliva and plasma aliquots were analyzed by enzyme-linked immunoassay using a commercial human sandwich kit (eBioscience, USA, batch E12414-1630) and following all the recommendations of the manufacturer. Briefly, 100 μl of plasma or saliva were incubated in a plate previously sensitized with anti-TNF-α capture antibody for 4 h, washed, and then incubated with a peroxidase-labeled detection antibody for 2 h. After adding substrate, the reactions were read at 450 nm in a microplate reader (Celler, Brazil). The results were compared with a standard TNF curve and expressed in picogram per milliliter (pg/ml).
2.5 Statistical analysis
Data were submitted to a t-test or Mann–Whitney U nonparametric test according to a previous normality analysis (Shapiro–Wilk test) and homogeneity of variances (Test F). Linear Pearson's correlation was applied to evaluate relationships between age; anthropometric variables (body weight, height, and BMI); tumor markers (RE; PR; HER2, KI67%), molecular subtype; and histological grade; with plasmatic and salivary TNF-α values. All tests were performed using the R (R Core Team, 2019) software, with a p < .05 level of significance.
3 RESULTS
In Table 1, we describe the clinicopathological parameters directly associated with the diagnosis in BC patients. In this sense, 85% of the sample were ≥50 years, more than half (60%) presented the Luminal A molecular subtype, 85% expressed positivity for RE, and 50% for PR. It was also observed that HER2 was negative in 85% of the sample, with less disease aggressiveness in 60% of the patients. Regarding histological tumor grade, 47.37% of the tumors were degree 1, and 42.10% were degree 2. Intratumoral angiolymphatic emboli were not observed in 68.75% of the samples. The BMI indicated that 29.41% of the participants were overweight and 29.41% were obese.
| Parameter | Categories | Frequency (%) | p-value |
|---|---|---|---|
| Age (years) | ≥50 | 85 | .006* |
| <50 | 15 | ||
| Molecular subtype | Luminal A | 60 | .0029* |
| Luminal B | 20 | ||
| Luminal-HER2 | 0 | ||
| HER2 | 5 | ||
| Triple negative | 15 | ||
| RE | Positive | 85 | .0017* |
| Negative | 15 | ||
| PR | Positive | 50 | 1 |
| Negative | 50 | ||
| HER | Positive | 5 | <.0001* |
| Negative | 85 | ||
| No data | 10 | ||
| Histological degree | 1 | 47.37 | .1419 |
| 2 | 42.10 | ||
| 3 | 10.53 | ||
| Angiolymphatic emboli | Yes | 31.25 | .1336 |
| No | 68.75 | ||
| Ki67 index | ≤20% | 80 | .0073 |
| >20% | 20 |
- Abbreviations: HER, human epidermal growth factor receptor 2; PR, progesterone receptor; RE, estrogen receptor.
- * p < .05.
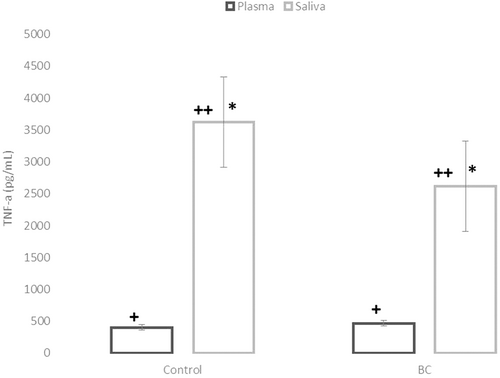
Age and anthropometric characteristics in women with BC were compared to the Control group (Table 2). BC patients presented higher age at diagnosis than those in the Control group (p < .0001), without statistical difference regarding body weight, height, and BMI between the groups (p > .05). The plasmatic and salivary levels of TNF-α were compared between the Control group and BC patients (Figure 1). Salivary levels of TNF-α were significantly higher in plasma concentration in both groups (p < .001). However, when we compared the Control group versus BC, neither plasmatic nor salivary levels of TNF-α were different (p > .05).
| Control (n = 29) | BC (n = 20) | p-value | ||
|---|---|---|---|---|
| Age (years) | 32.6 ± 2.9 | 61.3 ± 2.9 | <.0001* | |
| Body weight (kg) | 64.1 ± 2.9 | 68.6 ± 2.9 | .302 | |
| Height (m2) | 1.63 ± 0.02 | 1.59 ± 0.02 | .144 | |
| BMI (kg/m2) | Eutrophic | 15 (65%) | 7 (41%) | .131 |
| Overweight/obese | 8 (35%) | 10 (59%) |
- Abbreviations: BC, breast cancer; BMI, body mass index.
- * p < .05.
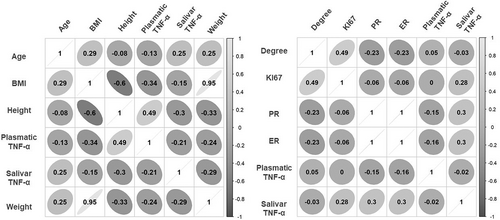
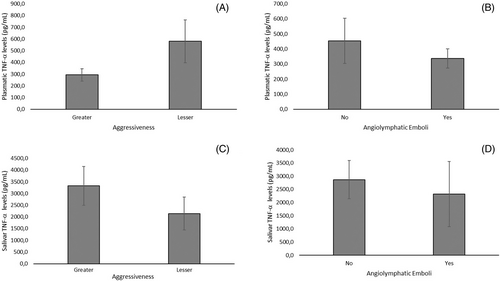
A correlation of the anthropometric and clinicopathological profiles of BC patients with their plasmatic and salivary levels of TNF-α is shown in Figure 2. Neither salivary nor plasmatic levels of TNF-α were significantly associated with age, body weight, height, or BMI in BC patients (Figure 2A). Moreover, we found that salivary and TNF-α plasmatic levels were not associated with clinicopathological tumor markers (ER; PR; HER, and Ki-67; Figure 2B). Similarly, as shown in Figure 3, the aggressiveness (categorized as high = Luminal B, HER2, and triple-negative subtypes and low = Luminal B subtypes) of the diseases (A and B) and the presence of intratumoral clots (C and D) were not significantly correlated neither with salivary nor with the TNF-α plasma level in BC patients.
4 DISCUSSION
As far as we know, this is the first report concerning the measurement of TNF levels in matched samples of plasma and saliva from BC patients, aiming to evaluate the potential correlation of these variables with their clinicopathological parameters.
In our sample, women with BC were more than 60 years old, older than the Control women. These data confirm literature findings that the incidence of BC increases with age and is usually diagnosed in the 50–60 age group.3 We also characterized several BC markers routinely used for prognosis, including the ER, PR, HER2, and Ki-67 index.13 We reported that women with BC presented a higher frequency of Luminal-A tumors with a low expression of the protein Ki-67, parameters that indicate slow growth and a better prognosis.18
The relationship between cancer occurrence and abnormalities in the oral cavity has been frequently reported in the literature.16 A study by Sfreddo et al.19 evaluated the association between periodontitis and BC in a sample of adult Brazilian women, showing that women diagnosed with periodontitis had two to three times higher rates of BC than women without periodontitis. Thus, several studies have considered saliva an essential biological fluid to screen putative biomarkers for BC diagnosis and staging.7 Moreover, changes in the salivary composition, such as volume, protein content, and ion concentration, have been identified in the saliva of cancer patients.4 Thus, women without the BRCA1 mutation presented the highest salivary buffer capacity in comparison with healthy individuals, and those with the BRCA1 mutation exhibited a much lower buffer capacity.6
Altered cytokine secretion is frequently observed in BC patients, suggesting that these inflammatory substances circulating in the blood could also be present in the saliva.13 Molecules such as C-erbB2, CA 15–3, Cathepsin D, sialic acid, EGF, VEGF, and CEA, are promising salivary markers, perhaps useful for the diagnosis of breast carcinomas.12 However, in contrast with the literature that suggests that TNF is frequently altered in BC patients, in the present work, we did not find any positive correlation between anthropometric and clinicopathological BC profiles, neither with plasma nor with salivary TNF-α levels. We did not observe any correlation between the matched plasma samples and salivary TNF-α levels in BC patients. In this context, Laidi et al.20 investigated if serum and salivary auto-antibodies against HER2 and MUC1 tandem repeat fragments could play BC screening. These authors reported that for some genes found in the saliva, such as anti-MUC1 and anti-HER2, the correlation between autoantibodies was more critical than in serum. Moreover, the authors emphasized that the correlation between serum and saliva values for all antibodies was weak.
TNF-α is an important cytokine related to the obesity inflammatory state, reported at high levels.11 Here, we noted a significant presence of BC women with overweight or obesity and wondered if this condition was associated with salivary or plasmatic TNF-α. Elevated adipose mass, particularly when associated with hyperinsulinemia, can raise the BC risk.21 As demonstrated by Martínez-Reza et al.11 it is possible to estimate, not only in the blood but also in the saliva, the concentrations of cytokines produced in cells from adipose tissue. In this work, obese participants presented higher salivary TNFα-R1 and R2 concentrations than healthy ones. Despite the significant presence of altered BMI (overweight/obesity) in BC patients in the present work, we did not find a significant correlation between BMI with the plasmatic or salivary TNF-α level. A more advanced degree of obesity may be required to exhibit some correlation with the TNF-α levels.
As mentioned above, ER, PR, HER2, and Ki-67 indexes are routinely used to diagnose and screen tumors in BC patients.12 None of these tumor markers evaluated in the present study was associated significantly with salivary or with plasmatic levels of TNF-α in BC patients. These results indicate that TNF-α is not a reliable marker related to clinicopathological parameters in BC patients. Sahebjamee et al.16 found a weak correlation of TNF-α salivary with oral squamous cell carcinoma patients. Similarly, the study of Gleber-Netto et al.22 analyzed salivary proteins in patients with head and neck cancer, showing elevated levels of EGF, interleukin (IL) 6 and 8, and, to a lesser extent, other NFκB-derived members such as TNFα or IL 1β, IL4, and 10, and VEGF. The study performed by Juretić et al.23 showed that TNF-α and IL-6 cytokines were detected in the whole saliva of 57 patients with oral premalignant lesions. These findings suggest that the TNF-α level can vary according to cancer topography and, therefore, this cytokine could not be an excellent marker to correlate with BC features. This hypothesis was confirmed by a review conducted by Parameswaran and Patial.24
Moreover, other salivary markers may be more critical in BC. In this sense, an important study identified 18 salivary biomarkers in BC, but only three up-regulated metabolites displayed the area under the curve (AUC) values higher than 0.920, indicating the high accuracy in predicting BC.25 Similarly, a recent review from Meleti et al.12 demonstrated that 40 studies (78%) reported a statistically significant association between the presence of BC and the presence of one or more markers in the saliva of the patients. Among the several molecules found in the saliva of BC patients, C-erbB2, CA 15–3, cathepsin D, sialic acid, P53, EGF, VEGF, and the CEA were frequently present, but not cytokines as the TNF-α. The present work shows some limitations, such as the reduced sample size, a factor that might have affected the statistical analysis. Some issues related to handling (due to mucins and other components that destroy the relevant biomarkers) have also been reported.26, 27 In addition, the saliva composition could change during stimulatory conditions.28 Also, a validation study could help to further explore TNF-α meaning in BC context.
In conclusion, plasmatic or salivary levels of TNF-α were not altered in BC patients. In none of these sites, the TNF-α levels significantly correlate with the clinicopathological BC parameters. Therefore, TNF-α is not helpful as a salivary marker in BC patients. These data reinforce several studies that failed to prove the effectiveness of recombinant TNF-α to treatment of different cancer types in humans.29-33 Thus, the TNF-α activity and its biological effects in cancer needed further studies, in special in the salivary fluid.
AUTHOR CONTRIBUTION
Marcelo Marcos Opolski: data collection and analysis, draft manuscript. Vitor Teixeira Maito: data collection and analysis, draft manuscript. Aedra Carla Bufalo Kawassaki: data collection and analysis, draft manuscript. Janaína Carla da Silva: data collection and analysis, draft manuscript. Rodrigo Kern: data collection and analysis, draft manuscript. Daniel Rech: recruitment of subjects and sample obtention, draft manuscript. Stefania Tagliari de Oliveira: data collection and analysis, draft manuscript. Pâmela Lonardoni Micheletti: data collection and analysis, draft manuscript. Carolina Panis and Sabrina Grassiolli: designed and supervised the conduct of the study, data analysis, and draft manuscript.
ETHICAL STATEMENT
The Institutional Ethics Committee approved this proposal under the number CAAE 35524814.4.0000.0107, and only patients who signed an informed consent form were con-sidered for this study.
CONFLICT OF INTEREST
The authors declare no conflict to interest.




