Incidence rate, basic characteristics, and survival outcomes of bladder squamous cell carcinoma
Feng Qi, Wenbo Xu, and Xiao Li contributed equally to this study.
Funding information: The Research Project of Jiangsu Cancer Hospital, Grant/Award Number: ZM202015
Abstract
To explore the incidence rate (IR), clinicopathological characteristics, and prognostic factors of bladder squamous cell carcinoma (BSCC) based on surveillance, epidemiology, and end results (SEER) database. We extracted the IRs of BSCC from 1975 to 2016 in the SEER database, and plotted the trending curves. Then, the clinicopathological characteristics of BSCC patients diagnosed from 2010 to 2015 were selected and compared with those of patients with urothelial carcinoma (UC) in the same period. Furthermore, differences in overall survival (OS) and cancer-specific survival (CSS) of BSCC and UC patients were compared. Finally, COX regression models were constructed to explore the risk factors affecting OS and CSS in BSCC patients. The IR of BSCC showed a downward trend from 1975 to 2000 and stabilized at about 0.3/100 000 after 2000. BSCC patients had a later stage at diagnosis and worse prognosis when compared with those with UC. Older age, higher TNM stage, no surgical treatment, and unmarried status were significantly related to worse prognosis of BSCC patients. This study explored the IR trends, clinicopathological characteristics, and prognostic factors of BSCC. In the future, prospective, large sample, and well-designed clinical studies are needed to verify our results.
1 INTRODUCTION
Bladder cancer is one of the most common malignant tumors of the urinary system, and its incidence ranked 12th among all malignant tumors in 2018.1 There are various histological types of bladder cancer, of which urothelial carcinoma (UC) is the most common subtype, accounting for about 90% of all cases, while only 2%–5% of cases are squamous cell carcinomas.2 There are multiple risk factors for the occurrence of bladder squamous cell carcinoma (BSCC), such as chronic inflammation/irritation, long-term catheterization, neurogenic bladder, urinary tract stones, and chronic schistosomiasis infection.3-5 Compared with UC patients, BSCC had a more advanced stage at diagnosis and was more aggressive, which also led to significantly worse overall survival (OS) of BSCC than UC.6, 7 Previous study3 has found that the 5-year cancer-specific survival (CSS) rate of BSCC patients after surgery was only 58%. However, due to the relative rarity of BSCC, most of the previous studies were single-center and with small samples. As one of the largest cancer databases in the world, SEER database is valuable for exploring the epidemiology, clinical features, and prognosis of BSCC. This study analyzed the incidence rate (IR) trends, clinicopathological characteristics, and prognosis of BSCC based on SEER database, to provide a reliable basis for the clinical diagnosis and treatment of this rare subtype of bladder cancer.
2 MATERIALS AND METHODS
2.1 Database
All data in this study were obtained from the SEER database. The SEER database is one of the largest and most authoritative cancer databases in the world. The SEER database records the epidemiological data, clinicopathological characteristics, treatment modalities, and long-term follow-up of cancer patients in the United States since 1975. At present, SEER database has covered about 30% of the whole population in the United States.
2.2 Incidence rate
The “Rate Session” function in SEER*Stat software was used to extract the incidence data of BSCC from 1975 to 2016 to investigate the IR trend, and further stratified by sex, age, and ethnicity.
2.3 Clinical features and survival outcomes
This part included patients diagnosed with BSCC and UC from the SEER database. The inclusion criteria were as follows: (1) pathologically diagnosed with BSCC and UC; (2) year of diagnosis was between 2010 and 2015; (3) complete follow-up data were available. Exclusion criteria included: (1) patients with other malignancies; (2) data were from autopsy and death certificates; (3) missing data in important variables, including race, TNM stage, treatment modality, and so on.
The “Case Listing Session” function in SEER*Stat software was used to extract the basic information, clinicopathological characteristics, treatment methods, and long-term follow-up of the enrolled patients, variables including: age at diagnosis, sex, race, tumor differentiation, AJCC 7th TNM stage, the administration of surgery, radiotherapy, chemotherapy, marital status, survival time, and cause of death. Age was divided into three groups: <45, 45–65, and >65 years old. Race was divided into White, Black, and other. Tumor differentiation was classified into well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated. Furthermore, marital status was grouped into: unmarried, married, and separated/divorced/widowed. Lastly, basic data and clinicopathological characteristics of BSCC patients were compared with UC patients at the same period.
Differences in OS and CSS between BSCC and UC patients were compared. Subsequently, COX regression models were constructed to explore the risk factors affecting OS and CSS in BSCC patients.
2.4 Statistical analysis
Statistical analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, USA) software. Categorical variables were presented as n (%) and compared by χ2 test. Differences in OS and CSS between BSCC and UC patients were explored using Kaplan–Meier analysis and log-rank test. Univariate COX regression model was used to preliminarily pick out potential risk factors that may affect the prognosis of BSCC, and then those potential variables were incorporated into the multivariate COX regression model to determine the independent risk factors of OS and CSS in BSCC patients. All p values were two-sided, and p < .05 was considered statistically significant.
3 RESULTS
3.1 Incidence rate
As shown in Figure 1A, the incidence of BSCC decreased year by year between 1975 and 2000 and stabilized at about 0.3/100 000 after 2000. In the age group, the IR increased with age gradually. In people less than 45 years old, the IR was extremely low and maintained at about 0–0.1/100 000. Moreover, the IR in the 45–65 and >65-year-old population were consistent with the general population, remaining at around 0.3/100 000 and 1.9/100 000 after 2000, respectively (Figure 1B). In the racial subgroup, IR in all ethnic groups has been decreasing since 1975 and leveled off after 2000 (Figure 1C). Moreover, the IR trend in males and females was consistent with that in the general population and remained at about 0.45/100 000 and 0.25/100 000 respectively after 2000 (Figure 1D).

3.2 Clinicopathological features
A total of 696 BSCC patients and 41 697 UC patients diagnosed between 2010 and 2015 were included in this part. As shown in Table 1, compared with UC patients, BSCC patients were diagnosed with a younger age (>65 years old: 64.51% vs. 67.93%, p < .001), higher proportion of female (51.87% vs. 24.85%, p < .001), higher tumor differentiation (moderately well differentiated: 50.29% vs. 40.61%, p < .001), and higher T stage (T2-4: 38.07% vs. 23.92%, p < .001), N stage (N1-3: 15.80% vs. 4.87%, p < .001), M stage (M1: 10.49% vs. 3.41%, p < .001). Moreover, the proportion of married patients was significantly lower in BSCC patients (44.68% vs. 62.46%, p < .001).
| Variables | BSCC | UC | p value |
|---|---|---|---|
| N | 696 (100) | 41 697 (100) | |
| Age, years | <.001 | ||
| <45 | 32 (4.60) | 986 (2.36) | |
| 45–65 | 215 (30.89) | 12 385 (29.70) | |
| >65 | 449 (64.51) | 28 326 (67.93) | |
| Race | <.001 | ||
| White | 581 (83.48) | 37 101 (88.98) | |
| Black | 83 (11.93) | 2476 (5.94) | |
| Other | 32 (4.60) | 2120 (5.08) | |
| Sex | <.001 | ||
| Male | 335 (48.13) | 31 335 (75.15) | |
| Female | 361 (51.87) | 10 362 (24.85) | |
| Grade | <.001 | ||
| I–II | 350 (50.29) | 16 932 (40.61) | |
| III–IV | 346 (49.71) | 24 765 (59.39) | |
| T stage | <.001 | ||
| Ta, Tis, T1 | 431 (61.93) | 31 721 (76.08) | |
| T2-4 | 265 (38.07) | 9976 (23.92) | |
| N stage | <.001 | ||
| N0 | 586 (84.20) | 39 668 (95.13) | |
| N1-3 | 110 (15.80) | 2029 (4.87) | |
| M stage | <.001 | ||
| M0 | 623 (89.51) | 40 277 (96.59) | |
| M1 | 73 (10.49) | 1420 (3.41) | |
| Surgery | <.001 | ||
| No | 52 (7.47) | 1171 (2.81) | |
| Yes | 644 (92.53) | 40 526 (97.19) | |
| Radiotherapy | <.001 | ||
| No/unknown | 604 (86.78) | 39 456 (94.63) | |
| Yes | 92 (13.22) | 2241 (5.37) | |
| Chemotherapy | .044 | ||
| No/unknown | 517 (74.28) | 29 511 (70.77) | |
| Yes | 179 (25.72) | 12 186 (29.23) | |
| Marital status | <.001 | ||
| Unmarried | 141 (20.26) | 5385 (12.91) | |
| Married | 311 (44.68) | 26 042 (62.46) | |
| SDW | 244 (35.06) | 10 270 (24.63) |
- Note: Data were presented in the form of n (%).
- Abbreviations: BSCC, bladder squamous cell carcinoma; Grade I, well differentiated; Grade II, moderately differentiated; Grade III, poorly differentiated; Grade IV, undifferentiated; SDW, separated, divorced, and widowed; UC, urothelial carcinoma.
In terms of treatment options, the proportions of receiving surgery (92.53% vs. 97.19%, p < .001) and chemotherapy in BSCC patients (25.72% vs. 29.23%, p < .001) were significantly lower than that of UC patients. The proportion of patients receiving radiotherapy was significantly higher in BSCC patients (13.22% vs. 5.37%, p < .001).
3.3 Survival analysis
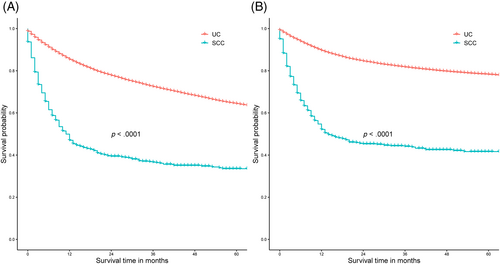
As shown in Figure 2, BSCC patients had worse OS (Figure 2A) and CSS (Figure 2B) than UC patients at the same period (p < .0001). Univariate COX regression model found that age, race, sex, tumor differentiation, T stage, N stage, M stage, the administration of surgery, radiotherapy, and marital status were significantly associated with OS and CSS in patients with BSCC, while multivariate COX regression model showed that higher age at diagnosis, higher TNM stage, no surgical treatment, and unmarried status were independent risk factors for poor prognosis (OS and CSS) (Tables 2 and 3).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p | HR (95% CI) | p |
| Age | <.001 | <.001 | ||
| <45 | Reference | Reference | ||
| 45–65 | 1.070 (0.634–1.806) | .800 | 1.045 (0.610–1.793) | .872 |
| >65 | 1.708 (1.033–2.823) | .037 | 2.104 (1.240–3.570) | .006 |
| Race | .002 | .108 | ||
| White | Reference | Reference | ||
| Black | 1.455 (1.115–1.898) | .006 | 1.203 (0.915–1.581) | .185 |
| Other | 0.594 (0.354–0.996) | .048 | 0.664 (0.394–1.119) | .124 |
| Sex | <.001 | .129 | ||
| Male | Reference | Reference | ||
| Female | 1.469 (1.215–1.776) | <.001 | 1.166 (0.956–1.421) | .129 |
| Grade | .003 | .107 | ||
| I–II | Reference | Reference | ||
| III–IV | 1.329 (1.101–1.604) | .003 | 1.173 (0.966–1.423) | .107 |
| T stage | <.001 | <.001 | ||
| Ta, Tis, T1 | Reference | Reference | ||
| T2-4 | 2.481 (1.945–3.166) | <.001 | 2.351 (1.814–3.047) | <.001 |
| N stage | <.001 | .042 | ||
| N0 | Reference | Reference | ||
| N1-3 | 1.967 (1.566–2.470) | <.001 | 1.302 (1.010–1.678) | .042 |
| M stage | <.001 | <.001 | ||
| M0 | Reference | Reference | ||
| M1 | 3.990 (3.071–5.184) | <.001 | 2.849 (2.127–3.817) | <.001 |
| Surgery | <.001 | <.001 | ||
| No | Reference | Reference | ||
| Yes | 0.355 (0.261–0.484) | <.001 | 0.353 (0.254–0.490) | <.001 |
| Radiotherapy | .005 | .777 | ||
| No/unknown | Reference | Reference | ||
| Yes | 1.432 (1.117–1.836) | .005 | 1.037 (0.805–1.337) | .777 |
| Chemotherapy | .574 | |||
| No/unknown | Reference | |||
| Yes | 0.942 (0.764–1.161) | .574 | ||
| Marital status | <.001 | <.001 | ||
| Unmarried | Reference | Reference | ||
| Married | 0.598 (0.465–0.769) | <.001 | 0.584 (0.448–0.760) | <.001 |
| SDW | 1.101 (0.861–1.407) | .444 | 0.968 (0.743–1.262) | .810 |
- Abbreviations: BSCC, bladder squamous cell carcinoma; CI, confidence interval; Grade I, well differentiated; Grade II, moderately differentiated; Grade III, poorly differentiated; Grade IV, undifferentiated; HR, hazard ratio; OS, overall survival; SDW, separated, divorced, and widowed.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p | HR (95% CI) | p |
| Age | .011 | <.001 | ||
| <45 | Reference | Reference | ||
| 45–65 | 1.127 (0.645–1.970) | .674 | 1.159 (0.652–2.060) | .616 |
| >65 | 1.548 (0.904–2.654) | .112 | 2.134 (1.210–3.764) | <.001 |
| Race | .004 | .196 | ||
| White | Reference | Reference | ||
| Black | 1.495 (1.121–1.995) | .006 | 1.173 (0.872–1.578) | .291 |
| Other | 0.625 (0.359–1.089) | .097 | 0.675 (0.385–1.183) | .170 |
| Sex | <.001 | .077 | ||
| Male | Reference | Reference | ||
| Female | 1.505 (1.221–1.854) | <.001 | 1.216 (0.979–1.512) | .077 |
| Grade | .015 | .331 | ||
| I–II | Reference | Reference | ||
| III–IV | 1.290 (1.050–1.585) | .015 | 1.111 (0.899–1.373) | .331 |
| T stage | <.001 | <.001 | ||
| Ta, Tis, T1 | Reference | Reference | ||
| T2-4 | 2.991 (2.247–3.981) | <.001 | 2.815 (2.080–3.808) | <.001 |
| N stage | <.001 | .011 | ||
| N0 | Reference | Reference | ||
| N1-3 | 2.281 (1.795–2.898) | <.001 | 1.414 (1.084–1.846) | .011 |
| M stage | <.001 | <.001 | ||
| M0 | Reference | Reference | ||
| M1 | 4.638 (3.530–6.094) | <.001 | 3.140 (2.317–4.255) | <.001 |
| Surgery | <.001 | <.001 | ||
| No | Reference | Reference | ||
| Yes | 0.338 (0.242–0.470) | <.001 | 0.325 (0.229–0.463) | <.001 |
| Radiotherapy | .018 | .874 | ||
| No/unknown | Reference | Reference | ||
| Yes | 1.390 (1.059–1.826) | .018 | 0.978 (0.741–1.291) | .874 |
| Chemotherapy | .879 | |||
| No/unknown | Reference | |||
| Yes | 1.018 (0.812–1.275) | .879 | ||
| Marital status | <.001 | <.001 | ||
| Unmarried | Reference | Reference | ||
| Married | 0.534 (0.408–0.698) | <.001 | 0.521 (0.394–0.691) | <.001 |
| SDW | 0.950 (0.731–1.236) | .704 | 0.831 (0.627–1.103) | .201 |
- Abbreviations: BSCC, bladder squamous cell carcinoma; CI, confidence interval; CSS, cancer-specific survival; Grade I, well differentiated; Grade II, moderately differentiated; Grade III, poorly differentiated; Grade IV, undifferentiated; HR, hazard ratio; SDW, separated, divorced, and widowed.
4 DISCUSSION
BSCC is a rare histological type, accounting for only 2%–5% of all bladder cancer cases.2 Hence, previous related studies were mostly case reports or single-center, small-sample retrospective studies. This study found that the IR of BSCC decreased year by year between 1975 and 2000 and stabilized at about 0.3/100 000 after 2000. Abdel-Rahman et al.8 found that the IR of bladder cancer increased year by year from 1973 to 2013, while the IR of BSCC showed a downward trend from 1975 to 2000, and it has been stable at about 0.3/100 000 after 2000. However, no previous study has further investigated the IR of BSCC stratified by different subgroups. In this study, we found that the IR of BSCC in each subgroup remained to be similar with the general population and gradually increased with age. In addition, the IR in the male population was higher than that in the female population. In bladder cancer, similar distributions were detected in different subgroup populations.9
BSCC patients had a later disease stage at diagnosis and worse prognosis when compared with UC patients. In addition, the proportion of receiving chemotherapy in BSCC patients was lower than that in UC patients. Matulay et al.2 analyzed the differences between BSCC and UC patients between 2004 and 2015 in the NCDB database, and found that BSCC patients had higher T, N, and M stages at diagnosis. BSCC patients had a worse prognosis than UC patients in all tumor stages. Furthermore, in patients with BSCC, neoadjuvant chemotherapy prior to radical cystectomy did not confer a survival benefit. Previous studies8, 10-12 found that BSCC patients were less sensitive to chemotherapy and more prone to chemotherapy resistance when compared with UC patients. This may also explain that the proportion of receiving chemotherapy in BSCC patients is lower than that in UC patients. Rosiello et al.13 explored the effect of perioperative chemotherapy on survival outcomes in patients with locally advanced or metastatic BSCC. They found the use of perioperative chemotherapy in those high-risk patient groups (T4b stage, lymph node, or distant metastases).
BSCC has a high degree of malignancy, rapid tumor growth, and poor response to adjuvant therapy such as radiotherapy and chemotherapy. Therefore, the prognosis of patients with BSCC is very poor. Previous studies reported that the 1-, 3-, and 5-year OS rates of BSCC were 82.5%, 54.9%, and 34.2%, respectively, and the median survival time was only 38.0 months. Therefore, it is essential to explore the risk factors affecting the prognosis of BSCC patients. Due to the low incidence of BSCC, there is currently no authoritative prognostic model for the disease. The TNM staging system established by the AJCC is the most used method for predicting prognosis for BSCC patients. However, TNM stage does not take into account factors such as demographic information and treatment, and there are still some shortcomings in individualized prediction. In this study, age, TNM stage, surgical treatment, and marital status were picked out by COX regression model as independent risk factors affecting the prognosis of patients with BSCC.
Ren et al.13 analyzed the clinical characteristics and prognostic factors of 63 BSCC patients and found that pathological T stage, distant metastasis, and surgical method were the risk factors affecting the prognosis. Hu et al.14 constructed a nomogram model to predict the prognosis of BSCC patients, in which clinical variables included in the model included age, TNM stage, the administration of surgery, and tumor size. Spradling et al.15 believed that lymphovascular invasion was associated with the oncological outcome of BSCC. In addition, several molecular biomarkers have been explored to predict the survival of BSCC patients, such as fibroblast growth factor 2 (FGF-2), COX-2, p53, Bax, and epidermal growth factor receptor (EGFR).16-18 The emergence of these molecular biomarkers also greatly helps to predict the prognosis and explore potential therapeutic targets for BSCC patients.
Although this study comprehensively analyzed the IR, clinicopathological features, and survival status of BSCC, there were still some deficiencies that cannot be ignored. First, some important variables, such as past history and comorbidities, are lacking in the SEER database. Second, survival outcomes may be affected by the purpose of treatment (curative or palliative), the modality and duration of adjuvant therapy, and such data are missing in the SEER database. Furthermore, the follow-up of patients in SEER database may be terminated due to the migration of patients in different regions. Finally, this study is a retrospective study based on public database analysis, and prospective, multicenter clinical trials are needed in the future to provide reliable evidence-based support for the diagnosis and treatment guidelines for BSCC.
5 CONCLUSION
To sum up, during 1975–2000, the incidence of BSCC showed a downward trend year by year and remained at about 0.3/100 000 after 2000. Compared with UC patients, BSCC patients had later stages at diagnosis and worse prognosis. Additionally, age, TNM stage, the administration of surgery, and marital status were independent risk factors affecting the prognosis of BSCC patients.
AUTHOR CONTRIBUTIONS
Zicheng Xu conceived and designed the study; Feng Qi, Ting Xu, and Wenbo Xu collected the data; Feng Qi, Wenbo Xu, and Xiao Li analyzed the data; Feng Qi, Wenbo Xu, and Xiao Li drafted the paper; Zicheng Xu and Qing Zou reviewed the manuscript. All authors interpreted the data, edited, or commented, and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank the NCI for providing the SEER data set. This study was supported by the Research Project of Jiangsu Cancer Hospital (ZM202015 to Feng Qi).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was exempt by Institutional Review Board (IRB) approval because the original data were from a public database.
Open Research
DATA AVAILABILITY STATEMENT
All data included in this study are available on reasonable request from the corresponding author.




