Positron Emission Tomography-Assisted Photothermal Therapy with Gold Nanorods
Abstract
Photothermal anticancer therapy based on plasmonic nanoparticles is proposed to enhance treatment efficacy while mitigating unintended side effects. However, most studies blindly rely on the accumulation of nanoparticles at the tumor site, which may result in inefficient treatment. In this study, the aim is to evaluate relevant parameters to improve plasmonic photothermal therapy. Gold nanorods (AuNRs) with an optimized aspect ratio and either amino or carboxylic acid surface functionalization are selected as photothermal agents. AuNR biocompatibility and photothermal activity in 2D and 3D human MDA-MB-231 triple-negative breast cancer cell models, evaluating localized hyperthermal cell death upon irradiation with resonant near-infrared (NIR) light, are analyzed first. To ensure reliable tracking of biodistribution in vivo, AuNRs are labeled with the positron emitter copper-64 (64Cu), and their distribution in a murine MDA-MB-231 tumor model is studied via positron emission tomography (PET) imaging. PET images reveal enhanced tumor accumulation of carboxylic acid-functionalized AuNRs compared to amino-functionalized AuNRs post-intravenous administration. Relatively low NIR laser power densities (0.5 W cm−2) are used for controlled heating – keeping local temperature below 50 °C – upon irradiation of intravenously and intratumorally administered AuNRs. As a result, tumor growth is significantly decelerated, even 9 days after application of photothermal therapy.
1 Introduction
Despite recent advancements, cancer remains a major contributor to global disease morbidity and mortality rates. Statistics indicate that ≈19.3 million cancer cases and 10 million cancer-related fatalities were documented worldwide in 2020, with incidence rates showing an upward trend.[1] Traditional cancer treatments such as surgery, radiation therapy, and chemotherapy invariably inflict harm on healthy tissues.[2] For instance, the therapeutic effectiveness of chemotherapy may fall short when considering the diversity among tumors and the risk of systemic toxicity due to inadequate targeting precision. Likewise, radiation therapy might adversely affect patients' immune function and blood systems, potentially leading to immune dysfunction and the rapid dissemination of cancer cells throughout the body. The persistent challenge of achieving specific tumor-targeting effects remains a complex issue, highlighting the need for innovative strategies. Binary approaches to cancer therapy, where two minimally toxic components of treatment require co-localization in the tumor to be effective, offer promising strategies to enhance patient outcomes while minimizing off-target side effects. One of such approaches is photothermal therapy (PTT), which involves injecting a naturally non-toxic photothermal agent (PTA), followed by targeted irradiation of the treated area with near-infrared (NIR) laser light. When laser irradiation is applied, photons are absorbed and rapidly decay back to the ground state, converting light energy into thermal energy to achieve sub-coagulative (43−55 °C) or coagulative (55−100 °C) temperatures to induce rapid cell death via protein denaturation and cell membrane damage that induces tumor necrosis.[3, 4]
As nanomaterials increasingly play a pivotal role in tumor prevention and treatment, nanomaterial-based PTT has gained significant attention owing to its superior photothermal properties and tumor-killing abilities.[5] Organic nanomaterials including porphyrins[6, 7] and polymer-based nanomaterials,[8, 9] as well as inorganic nanomaterials including noble metal nanoparticles (NPs),[10, 11] graphene-based materials,[12] and copper-sulfide NPs,[13] have been explored as PTAs.[14] Among these, gold nanoparticles (AuNPs) are particularly promising because they offer synthetic tunability, showing high absorption cross-sections in the NIR region due to localized surface plasmon resonance (LSPR) effects, resulting in high photothermal conversion efficiency.[15] AuNRs are increasingly used in biomedical applications due to well-established synthesis and surface functionalization methods, which result in highly monodisperse and biocompatible NPs, ideal for PTT applications.[16, 17] Such synthetic methods allow tuning the LSPR peak within the NIR biological transparency windows, in resonance with incident NIR light sources used for PTT.[18]
Apart from sufficient heating capacity to reach the desired temperature within the tumor and high biocompatibility and stability, the capacity to selectively (or preferentially) accumulate in the tumor tissue is particularly relevant. It has been reported that NPs within a specific size range passively accumulate in tumors due to the enhanced permeability and retention (EPR) effect.[19] This effect arises from the fenestrated vasculature formed during neo-angiogenesis, which, together with a deficient lymphatic drainage, facilitates traffic and retention of nanosized materials. Importantly, NP size also affects bioavailability and NP accumulation in off-target organs. For example, whereas NPs with sizes below 8 nm can be rapidly eliminated from the circulation through the kidneys, larger particles (>10 nm) accumulate in organs of the mononuclear phagocyte system (MPS) such as the liver and the spleen, thus decreasing bioavailability and compromising tumor uptake.[20] It is thus important to evaluate the biodistribution of nanometer-sized PTAs before studying therapeutic efficacy. In this context, in vivo noninvasive nuclear imaging techniques, such as positron emission tomography (PET) and single photon emission computed tomography (SPECT), are ideally suited to quantitatively determine the time-resolved biodistribution of NPs administered to living organisms.[21] This is of special importance for PTAs whereby the optimal time window for external stimulus application must be coordinated with the maximum dose at the site of interest.
Various studies have described the use of AuNPs as PTAs, with or without simultaneous drug delivery.[22-25] In all cases, monodisperse AuNRs with the appropriate dimensions/LSPR to match the laser irradiation wavelength were found to be required for efficient heat generation.[26] In this work, we employed monodisperse polyethylene glycol (PEG)-functionalized gold nanorods (AuNRs) with varying surface charge and evaluated for their potential use as PTAs. Real-time monitoring of photothermal effects was carried out using a thermal camera, to ensure low-density power irradiation. In our approach, the power density was maintained at a constant level of 0.5 W cm−2 by operating the laser in pulsed mode within second-long intervals. This ensures that the temperature in the irradiated area remains ≈45 °C, thereby preventing irreversible damage to the healthy tissue surrounding the tumor, which is often overlooked in PTT studies. The cytotoxicity and photothermal properties of AuNRs were evaluated in vitro, in 2D and 3D cell cultures. Subsequently, radiolabeling with the positron emitter copper-64 (64Cu) enabled the assessment of biodistribution and tumor accumulation capacity for different AuNRs, after intravenous administration in a mouse model of breast cancer. Finally, we assessed the therapeutic effect of PTT with selected AuNRs in the same mouse model, using both intravenous and intratumoral administration. Our results confirmed the effective heating performance and therapeutic potential of the AuNRs for tumor ablation, while mitigating undesirable side effects such as necrosis in the tumor and peritumoral areas, which often result in ulceration.
2 Results and Discussion
2.1 Synthesis and Functionalization of AuNRs
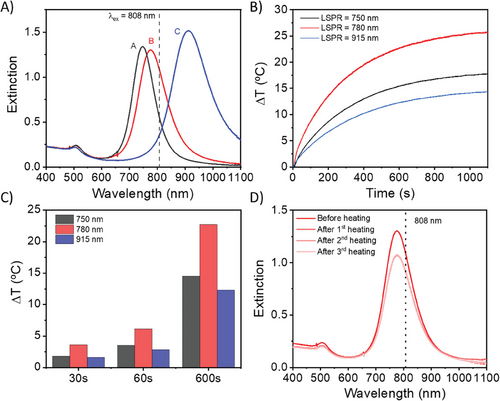
AuNRs were synthesized by the well-known seed-mediated growth method.[27-29] By employing hydroquinone as a reducing agent in the growth step,[30] monodisperse AuNRs were obtained with an aspect ratio greater than 5. Subsequently, and to precisely control the position of the longitudinal LSPR, partial oxidation of AuNRs along the longitudinal axis was carried out in a controlled manner by sequential addition of a solution containing cetyl trimethyl ammonium bromide (CTAB) and HAuCl4. Oxidation of Au0 by Au3+ is known to occur preferentially at AuNR tips,[31] so that the transverse axis remains unchanged, while the AuNR aspect ratio gradually decreases and the longitudinal LSPR blue-shifts accordingly. Oxidation conditions were tuned to achieve AuNRs with decreasing aspect ratio and LSPR wavelength, with longitudinal LSPR bands centered at 915 nm (AuNR915), 780 nm (AuNR780), and 750 nm (AuNR750), respectively (Figure 1A; Figure S1, Supporting Information). AuNRs were subsequently functionalized with thiolated polyethylene glycol (PEG-SH) carrying carboxylic acid or amine functional groups, to confer biocompatibility and variation in surface charge. Full details are given in the Methods section. Zeta potential measurements of carboxylic acid and amine PEG-functionalized AuNRs confirmed negative values for AuNR@PEG-COOH (≈ −30 mV) and slightly positive values for AuNR@PEG-NH2 (≈ +3 mV).
2.2 Photothermal Properties of AuNRs
It is well-known that anisotropic AuNPs, including AuNRs, undergo reshaping at high temperature.[32, 33] Therefore, we first tested the photothermal stability of AuNRs upon NIR laser irradiation, employing an 808 nm multimode diode laser with variable power output. By controlling the distance between the sample and the optical fiber, the spot size and power density could be finely tuned. Overall temperature changes in the solution were measured with an infrared (IR) camera, thus allowing real time data acquisition as a function of laser power density. We aimed to achieve rapid heating of the samples, up to ≈45 °C, followed by regulation of the temperature within the range of 42–50 °C to achieve cell damage characterized by inhibition of deoxyribonucleic acid (DNA) replication and metabolic processes.[34] Above this temperature, coagulative necrosis may occur, leading to irreversible protein denaturation and tissue damage. Using the carboxy-PEG functionalized AuNRs, we first evaluated the heating characteristics of AuNRs of different aspect ratios under different power densities (Figure 1B,C; Figure S2, Supporting Information). AuNR780@PEG-COOH were found to induce the fastest and highest heating, due to better resonance between the AuNR LSPR maximum and the laser excitation wavelength, reaching an increase of 6 °C within 1 min, and 24 °C within 10 min. With regard to AuNR750@PEG-COOH and AuNR915@PEG-COOH, the former shows a slightly higher extinction coefficient at 808 nm, thereby leading to slightly higher heating than the latter (Figure 1A–C). In both cases, the heating efficiency is significantly lower than that for AuNR780@PEG-COOH. Considering the requirement to work within a defined temperature range in vivo, we evaluated the use of cyclic on/off irradiations. This is especially important because of the risk of AuNR reshaping at high temperatures, which would result in LSPR blue-shift and thus loss of resonance with the laser beam (Figure S3A, Supporting Information). Indeed, we observed that excessive irradiation in the form of high-power densities led to a blueshift of the LSPR for AuNR915@PEG-COOH (Figure S3B, Supporting Information). In the case of AuNR780@PEG-COOH, repeated laser irradiation using a power density of 0.5 W cm−2 (Figure 1D) did not cause significant changes in the UV–vis–NIR spectra after three consecutive cycles of irradiation, but still reached temperatures expected to inhibit cell proliferation.
2.3 In Vitro Cytotoxicity Studies
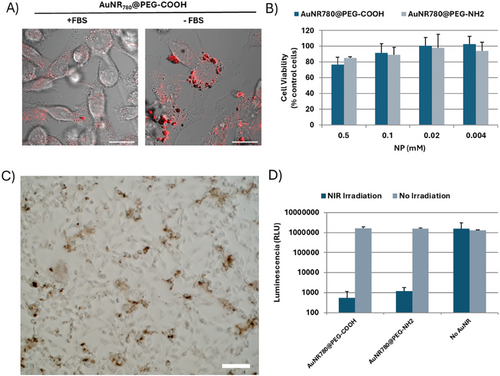
We subsequently tested the biocompatibility and uptake of AuNR780@PEG AuNRs with the human breast cancer cell line MDA-MB-231, which was later used for the formation of a xenograft tumor model. The MDA-MB-231 cell line is an adherent epithelial cell line that has been shown to have variable levels of AuNR uptake, highly dependent on surface coating and charge.[35-37] Therefore, we first determined uptake as a function of AuNR surface charge (Figure S4, Supporting Information), adding AuNRs at a concentration of 0.5 mm [Au0]. Via reflection microscopy, no apparent NP uptake was observed, presumably due to the formation of a protein corona, masking any changes in AuNP surface functionalization. Thus, we proceeded to incubate the AuNRs in the absence of FBS, which resulted in a significant increase in AuNR-cell association, marked by AuNR endocytosis and/or accumulation with cells (Figure 2A; Figure S5, Supporting Information). Indeed, UV–vis–NIR measurements of AuNRs incubated with and without fetal bovine serum (FBS) at 37 °C suggest that FBS stabilizes AuNRs during prolonged storage (Figure S6, Supporting Information). Considering the changes in cell morphology observed and the reduction in cell viability at high (0.5 mm) AuNR concentrations, we reduced the working concentration to 0.1 mm [Au0], allowing high uptake in the absence of FBS (Figure 2B,C; Figure S7, Supporting Information).
2.4 3D Spheroid Model
Like MCF7 and T-47D cells, MDA-MB-231 cell primary tumors are epithelial in nature; however, MDA-MB-231 cells express low levels of cell-cell adhesion molecules EpCAM and E-Cadherin.[38] Thus, in vitro, MDA-MB-231 cell aggregates or spheroids generally show poor boundaries between the core and outer spheroidal edge, as well as low reproducibility in size and roundness.[39] In vivo, this translates into tumors with invasive and metastatic phenotypes. This phenotype is not optimal for spheroid studies in which samples are repeatedly handled because the spheroids have high variability in size and may fall-apart, affecting the reproducibility of the measurements. Thus, we optimized spheroid formation and NP incubation conditions, with the aim of achieving spheroids with maximum roundness and large diameters, the latter being especially important for subsequent irradiation studies where a single spheroid/condition was desired. As can be observed in Figure S8 (Supporting Information), large (880 µm), round (roundness; 0.93) spheroids with clear boundaries were formed after 72 h in cell media supplemented with 10% (v/v) FBS and commercial extracellular matrix (ECM; to improve spheroid boundary roundness). Subsequent removal of FBS, chosen to mimic NP-incubation conditions based on above 2D results, led to an increase in spheroid diameter of ≈100 µm over 3 days; however, roundness and clear borders were retained. We continued to explore the effect of FBS removal upon incubation of spheroids with NPs, observing the presence of AuNR agglomerates in and around the spheroid, forming a halo suggestive of an area of ECM surrounding the spheroid (Figure S9, Supporting Information). At noncytotoxic concentrations (0.1 mm, as determined in 2D studies), no significant changes could be observed in the area or diameter of the spheroids (Figure S10, Supporting Information). We subsequently proceeded to irradiate spheroids that had been exposed to AuNRs to determine the PTT-induced cytotoxicity. As shown in Figure 2D, a significant reduction in spheroid cell viability was observed 3 days after exposure to NIR irradiation in resonance with the LSPR of the AuNRs. In situ measurements (Table S1, Supporting Information) confirmed an increase in temperature of ≈14 °C, sufficient to sub-coagulative levels of cell death marked by inhibition of cell proliferation.
2.5 Radiolabeling of AuNRs
The biodistribution of AuNRs and the capacity to accumulate in the tumor were investigated by PET imaging to select the AuNRs with optimal pharmacokinetic properties to undergo therapeutic experiments, as described in previous works.[25, 40] For this study, AuNR780@PEG-COOH and AuNR780@PEG-NH2 were used, thus determining the effect of surface functionalization on biodistribution. We chose the radionucleotide copper-64 due to its relatively long half-life of 12.7 h, which, contrary to other short-lived positron emitters (e.g., 18F, with a half-life of 109.8 min) enables the tracking of the AuNRs for at least 24 h, sufficient to determine maximal tumor accumulation. Additionally, previous studies by our group and others have demonstrated stable labeling of AuNRs.[25, 40] Labeling was addressed through a process involving the reduction of a copper salt on the AuNR surface, using hydrazine as a reducing agent. Following this process, radiochemical yields (non-decay corrected) of 35 ± 4% and 47 ± 3% for AuNR780@PEG-COOH and AuNR780@PEG-NH2, respectively, were obtained, with radiochemical purity values above 95% in all cases, as determined by radio-thin layer chromatography (see Figure S11, Supporting Information, for representative chromatograms). To confirm that the labeling process does not affect the morphological properties of AuNRs, transmission electron microscopy (TEM) analysis was carried out after complete radioactive decay of the samples (6 days). The results showed that both the size and aspect ratio remained intact during the labeling process (Figure S12, Supporting Information).
2.6 Radiochemical Stability of AuNRs
The stability of the radiochemical label is essential for conducting in vivo experiments because detachment of the radioactive atom would lead to incorrect interpretation of imaging results. Evaluating the stability of radiolabeling in vivo is extremely complex, and typically in vitro models are used to simulate in vivo behavior. For NPs labeled with radiometals, one of the best strategies to determine labeling stability is to incubate these NPs in different media, usually in the presence of a chelating agent capable of sequestering the radionuclide, and to determine the percentage of radioactivity released at different incubation times. We thus investigated the radiochemical stability of 64Cu-labeled AuNRs by incubating them in three different media at 37 °C: i) physiological saline solution to which ethylenediaminetetraacetic acid (EDTA) was added as a chelating agent (0.9% NaCl + 2.5 mm EDTA); ii) sodium phosphate-buffered saline to which EDTA was also added, at a concentration of 2.5 mm (PBS + 2.5 mm EDTA); and iii) mouse serum. At different time points, the amount of 64Cu remaining within the AuNRs was analyzed using radio-thin layer chromatography (TLC). Regardless of the incubation media, more than 90% of the initial 64Cu remained bound to the AuNRs after 48 h of incubation (Figure S13, Supporting Information), thus demonstrating the radiochemical stability and suitability of AuNR780@PEG-COOH and AuNR780@PEG-NH2 for in vivo studies.
2.7 Biodistribution Studies
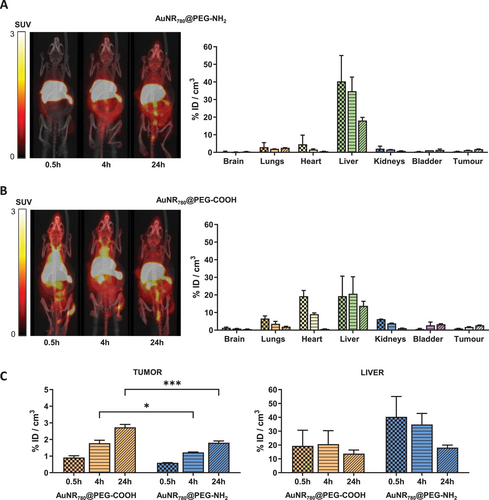
Biodistribution studies were carried out in a xenograft mouse model, generated by subcutaneous inoculation of MDA-MB-231 cells in immunocompromised female mice. Injection of 2 × 106 cells per animal using Matrigel/phosphate buffered saline (PBS) 1/1 (v/v) resulted in a quasi-linear tumor growth curve, with average tumor volumes of 200–400 mm3 at day 30 after inoculation, optimal for subsequent biodistribution studies. Animals (n = 4) were randomly distributed in 2 groups (n = 2 per group) and received intravenous (IV) injection (100 µL) of 64Cu-labeled AuNRs (see Table S2, Supporting Information, for experimental details), corresponding to 100 µg Au per animal (AuNR stock 5 mm [Au0] (1 mg mL−1)). PET images were obtained 30 min, 4 h, and 24 h after injection. The results show a significant accumulation of AuNR780@PEG-NH2 in the liver (Figure 3A), reaching values of ≈40% of injected dose per cubic centimeter of tissue (ID cm−3) at 30 min post administration, followed by a progressive decrease till the end of the study, reaching values of ≈20% ID cm−3 at 24 h post administration. The significant accumulation of AuNRs in the liver likely stems from their aggregation triggered by interactions with negatively charged biomolecules in biological media, such as proteins. Consequently, this aggregation leads to a markedly reduced concentration of AuNRs in the bloodstream, as evidenced by the minimal radioactivity detected within the volume of interest (VOI) delineated in the heart. The heart's VOI serves as a proxy for the radioactivity concentration in the left ventricle, thereby reflecting the bloodstream's radioactivity level. This ultimately results in poor bioavailability, and tumor accumulation values of ≈2% ID cm−3 at 24 h post administration (Figure 3C). For AuNR780@PEG-COOH, the initial accumulation in the liver was much lower (≈20%), probably due to lower aggregation rates, although it remained practically constant throughout the study, with a slight decreasing trend (non-statistically significant) at 24 h post administration. When analyzing AuNR780@PEG-COOH accumulation in the tumor (Figure 3B), we observed significantly higher amounts than the value achieved for AuNR780@PEG-NH2 (p = 0.0008) making them the candidate of choice for PTT studies. Considering the amount of AuNRs administered, the concentration in the tumor can be estimated as ≈3 µg mL−1 (equivalent to 15 nmol Au/cm3) at 24 h post injection. Values of tumor accumulation achieved in our experimental scenario are slightly lower than those previously reported for 64Cu-labeled and PEG-NH2 functionalized AuNRs.[25] Importantly, AuNRs used in this previous study were notably smaller in size (25.1 ± 2 nm x 8.0 ± 0.5 nm) and were evaluated in a different tumor model generated by subcutaneous inoculation of U-87 MG cells (glioma model). Overall, the results highlight the relevance of both particle size, surface functionalization, and tumor model when evaluating the biodistribution and tumor accumulation capacity of nanoparticles.
2.8 In Vivo Photothermal Therapy Studies
Thanks to their optimal heating properties and relatively high tumor accumulation, AuNR780@PEG-COOH were selected for subsequent in vivo PTT experiments. We first determined the PTT efficiency in model tissue, using commercial chicken breast to perform trial experiments. Chicken muscle tissue (commercial chicken breast) was chosen because of its similar density and transparency to murine muscle tissue. Based on the data obtained from PET biodistribution studies in which ≈3 µg Au was observed in the tumor 24 h after IV injection, we injected 5 µL (5 µg of AuNRs when at a concentration of 5 mm of Au0) at a depth of ≈3 mm, and subsequently irradiated the tissue using various laser power densities (0.544, 0.978, and 1.412 W cm−2) at a fixed spot size (1 cm2). A considerable increase in temperature above the control (no laser irradiation) could be observed after 2 min, irrespective of the laser power (Figure S14, Supporting Information). To maintain the final temperature reached within the range of 42–50 °C, we chose 0.5 W cm−2 with cyclic on/off laser irradiation to avoid under- or over- heating of the tissue.
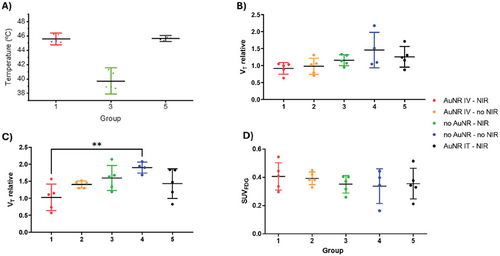
We next proceeded to study PTT in mice, using the tumor model described in Section 2.7. A total of 25 mice were inoculated, and 50 days after tumor inoculation, the animals (n = 24; one animal did not show tumor development) were divided into 5 groups, maintaining as homogeneous as possible the average tumor volume among groups (see Table S3, Supporting Information, for group distribution and individual tumor volumes). The last group, in which animals received an intratumoral (IT) injection of AuNRs, was included as “positive control”, to ensure as close as possible maximal dose at the site of irradiation. The amount of AuNRs administered to this group was selected to be equivalent to the amount of AuNRs detected in the tumor of IV administered animals, based on PET biodistribution results. In all instances, real-time temperature monitoring was conducted using an IR camera (Figure S15A, Supporting Information). The maximum temperature set to be attained within the tumor was established at 45 ± 2 °C, maintained by manually toggling the laser on and off whenever temperatures surpassed or fell below the threshold. We observed a slight temperature increase (3–5 °C) in the negative control group (Group 3, -AuNP +NIR irradiation) during irradiation, presumably due to the absorbance of hemoglobin in this biological window.[41] With regard to Groups 1 and 5, in which mice were injected IV or IT with AuNRs, respectively, and subsequently irradiated, significantly higher levels of heating were observed when compared to the control group, reaching the established temperature range within less than one minute (Figure 4A; Figure S15B,C, Supporting Information).
Following irradiation, tumor volume was continuously monitored to assess PTT therapeutic effects. Additionally, animals were closely monitored to evaluate their progression, particularly in the tumor area. Visual inspection of the animals revealed non-severe burn-like wounds in those subjected to AuNRs IV and IT administration and irradiation (Groups 1 and 5, respectively). These burns completely healed within a few days with appropriate treatment (Vetramil ointment, twice daily). In agreement with the biocompatible nature of NIR light sources for PTT therapy, animals that were not administered AuNRs did not suffer any lesions in the tumor area after being subjected to laser irradiation under the same conditions.
Tumor volumes relative to the day of irradiation, measured at 9 days post-irradiation, revealed significantly lower values in animals from Group 1 (+AuNR IV, +NIR) relative to the control Group 4 (no AuNR, no NIR) (p value of 0.0062) suggesting a therapeutic effect halting tumor progression (Figure 4C). Values obtained for Group 1 already show a decreasing trend with respect to the control group at 4 days after treatment (Figure 4B), although not significant (p value of 0.086). At both time points, relative tumor volumes obtained for Groups 2 (+AuNR IV, no NIR) and 3 (no AuNR, +NIR) were not statistically different from those found for the control Group 4, although average values show an increasing trend with respect to Group 1, particularly at t = 9 days.
The lack of therapeutic response in the group administered intratumorally (IT, Group 5) is surprising, especially given the observed temperature increase in the tumor tissue, which was similar to that in Group 1, which showed tumor volume reduction. This difference in therapeutic response might be due to the local distribution of the particles within the tumor. After intravenous (IV) administration, the particles reach the tumor via the enhanced permeability and retention (EPR) effect. Although the distribution might not be perfectly homogeneous, PET images indicate a relatively uniform distribution of radioactivity, suggesting a consistent spread of AuNRs. In contrast, IT administration may result in a focal concentration of nanoparticles in specific tumor areas (injection site), causing a temperature increase and localized cell death in that spot while leaving other regions unaffected. These results support the idea that a homogeneous distribution of photothermal agents throughout the tumor is crucial for an effective therapeutic response.
In order to evaluate the suitability of the well-established radiotracer 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG) as a tool to evaluate therapeutic response, animals were subjected to PET scans at day 7 post irradiation (Figure 4D). Despite therapeutic PTT effects observed through tumor volume measurements, we did not observe any significant differences in the standard uptake values (SUV) between groups. Contrary to our results, previous studies have demonstrated that therapeutic response at short times (1 day) after PTT can be successfully monitored using PET-[18F]FDG.[42, 43] Indeed, this approach has been proposed as a prognostic tool to predict survival. The lack of differences in [18F]FDG tumor uptake in our study might be due to different reasons such as the slow metabolic nature of MDA-MB-231-based tumors, or the presence of an augmented immune response causing infiltration of phagocytic cells such as macrophages, which are known to uptake [18F]FDG resulting in hypercaptation. This would negate the expected decrease in glycolytic metabolism derived from reduced tumor activity, thus explaining the lack of differences between experimental groups.
While the SUV analysis did not yield a difference between groups, it is worth mentioning the trends observed when correlating tumor volume with the SUV value obtained in the PET-[18F]FDG studies between treated Groups 1 and 5, and Control Groups 2, 3 and 4 (Figure S16, Supporting Information). Animals in Groups 1 and 5 showed a negative correlation between SUV values and tumor volume (i.e., higher tumor volume corresponded to lower SUV values), whereas animals in Groups 2, 3, and 4 exhibited the opposite trend. An explanation for this correlation has not been found but considering the low number of animals in each group, these values should be treated with caution, and definitive conclusions cannot be drawn. However, it is a phenomenon worth exploring in future studies.
2.9 Histological and TEM Studies of Excised Tissue
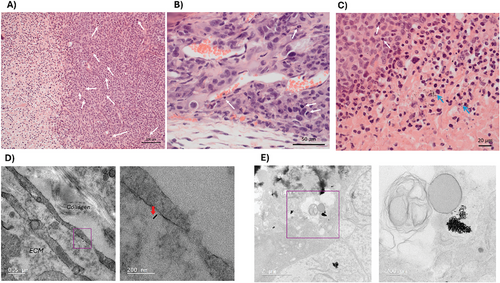
In order to evaluate the effects derived from the irradiation process on tumors, as well as the distribution of AuNRs within the tumor, histological studies were carried out on mice subjected to the same experimental groups. Two weeks after irradiation, animals were perfused with formalin solution for sacrifice, and subsequently tumors were excised and subjected to hematoxylin-eosin (H&E) staining or OsO4 staining followed by resin embedding for histological and TEM evaluation, respectively. Histological analysis of tumors revealed encapsulated solid tumors abundant in atypical and mitotic cells, all of which presented fibrotic areas in the interior necrotic core. A synergistic effect of AuNR administration and NIR irradiation in tumor grading was observed, with those from experimental Groups 1 and 5 (IV and IT injection, respectively, followed by NIR irradiation) developing necrosis in ≥ 60% of the tumor (Grade 3), whilst those only exposed to AuNRs without NIR irradiation led to necrosis in 30–60% of the tumor (Grade 2). All tumors showed the presence of inflammatory cell infiltration including macrophages, lymphocytes, neutrophils, and plasma cells (Figure 5A–C). These results support the hypothesis of [18F]FDG hypercaptation by phagocytes such as macrophages. We were even able to observe the presence of AuNR deposits in zones of tumor necrosis (Figure 5C). Analysis of the skin of irradiated and non-irradiated mice showed damage to skin epidermis and hair follicles in mice from Groups 1 and 5, in agreement with increases in temperatures observed upon NIR irradiation (Figure S17, Supporting Information). However, hyperkeratosis and epidermal hyperplasia were not significantly different to control groups. Finally, we conducted TEM imaging of tumor samples, motivated by the observation of AuNRs in histological sections from mice injected with AuNRs. Individual AuNRs were clearly observed intracellularly and in the ECM of mice submitted to IV injection and sacrificed after a period of 2 weeks (Figure 5D; Figure S18, Supporting Information). In mice exposed to AuNRs and irradiation, massive accumulation of AuNRs was observed (Figure 5E).
3 Conclusion
Our work presents a comprehensive investigation into the preparation and characterization of AuNRs and their evaluation as PTAs for PTT. AuNRs showed efficient heating capabilities suitable for PTT, along with high biocompatibility and uptake in both 2D and 3D cultures. Radiolabeling and biodistribution studies highlighted the tumor accumulation potential, which was superior for AuNRs functionalized with PEGylated carboxylic acid groups, probably due to lower liver uptake and consequent higher bioavailability at short time-points after IV administration. In vivo PTT experiments demonstrated significant tumor growth inhibition following NIR irradiation of AuNR-treated tumors both after IV and IT administration, corroborated by histological and TEM analyses indicating tumor necrosis and immune cell infiltration, thus validating the efficacy of AuNR-based PTT. Due to the strong resonance between AuNR780 and the laser wavelength of choice for irradiation (808 nm), and the relatively high levels of tumor AuNR uptake, we observed that relatively low NIR laser power densities (0.5 W cm−2) together with laser pulsation within the seconds range could be applied to achieve moderate levels of intratumoral heating, sufficient to achieve significant therapeutic effects when compared to control groups. With regard to PET imaging using [18F]FDG as a tracer, we observed potential limitations in assessing the therapeutic response at long time-points post-PTT application, possibly due to differences in the metabolic demands within the tumor microenvironment, potentially masking reductions in uptake attributable to decreased cell proliferation. These results suggest a window of opportunity for alternative imaging approaches or radiotracers such as the direct proliferation marker 18F-fluorothymidine ([18F]FLT), or the amino acid metabolism marker [11C]methionine. Overall, our study underscores the promising role of AuNRs in cancer therapy and provides valuable insights for future research in nanomedicine and PTT optimization.
4 Experimental Section
Materials
Gold (III) chloride trihydrate (HAuCl4, ≥ 99.9%), sodium borohydride (NaBH4, 99%), hexadecyltrimethylammonium bromide (CTAB, ≥ 99%), hydroquinone (HQ, ≥ 99%), and silver nitrate (AgNO3, ≥ 99%) were purchased from Sigma–Aldrich–Merck. Mercapto-poly (ethylene glycol) with carboxylic acid or amine functional groups (α-mercapto-ω-carboxy poly (ethylene glycol), SH-PEG-COOH, MW: 5K and α-mercapto-ω-Amino poly (ethylene glycol), SH-PEG-NH2, MW: 5K) were purchased from Rapp Polymere. All chemicals were used without further purification. Milli-Q water (resistivity 18.2 MΩ∙cm at 25 °C) was used in all experiments. All glassware and stirrer bars were washed with aqua regia.
Synthesis of Gold Seeds
The gold seeds were prepared by reduction of HAuCl4 with NaBH4 in the presence of a cationic surfactant (CTAB) in a 20 mL scintillation vial. To an aqueous mixture containing CTAB (100 mm, 4.7 mL) and HAuCl4 (50 mm, 0.025 mL), freshly prepared NaBH4 (10 mm, 0.3 mL) was added under vigorous stirring at room temperature. The solution turned from yellow to brownish immediately and the stirring was stopped after 2 min. The seed solution was aged at room temperature for 30 min before use to promote the decomposition of excess sodium borohydride.
Synthesis of AuNRs
AuNRs were prepared as described elsewhere[30] with some modification. AuNRs were synthesized by adding an aliquot of gold seeds (1.4 mL, 0.25 mm) under vigorous stirring to a growth solution comprising CTAB (100 mL, 100 mm), HAuCl4 (1 mL, 0.05 mm), AgNO3 (1.10 mL, 100 mm), and HQ (5 mL, 100 mm) at 30 °C. The stirring was stopped after 5 min, and the mixture was left for 6 h at 30 °C. AuNRs were washed by two centrifugation rounds (8000 rpm, 30 min) to remove excess reagents. Oxidative etching of AuNRs (LSPR = 1063 nm) was performed by means of the Au+3-CTAB complex. Different samples of etched gold nanorods were prepared using the nanoparticles obtained as described above (Table S4, Supporting Information, for size details of the AuNRs). To a solution containing AuNRs (30 mL, 0.5 mm) in CTAB (100 mm), an aliquot of Au+3-CTAB complex ([HAuCl4] = 1 mm, [CTAB] = 100 mm) was added dropwise at 30 °C under vigorous stirring for 30 min. Subsequently, the solutions were centrifuged twice (9000 rpm, 30 min) and after the second centrifugation step, the solution was redispersed in CTAB 0.5 mm. The final concentration of metallic gold was 0.5 mm for all samples. Size and aspect ratio characteristics are shown in Table S3 (Supporting Information).
Functionalization of AuNRs with Thiolated PEG[44]
An aqueous solution of freshly prepared thiolated PEG with carboxylic acid or amine functional groups (200 molecules / nm2) was added dropwise under vigorous stirring to a solution of gold nanorods (10 mL, 0.5 mm) in CTAB 0.5 mm. The mixture was allowed to react for 2 h at room temperature. Finally, PEG-functionalized gold nanorods were centrifuged (8000 rpm, 30 min) twice to remove excess of PEG and redispersed in water (see Figure S19, Supporting Information, for an example UV–vis–NIR spectra). Negative zeta potential values were obtained for AuNRs@PEG5K-COOH (−28.2 ± 0.2 mV) while slightly positive values were determined for AuNRs@PEG5K-NH2 (3.3 ± 0.2 mV).
AuNR Characterization
Optical extinction spectra were recorded using an Agilent 8453 UV–vis spectrophotometer. TEM images were obtained with a JEOL JEM-1400PLUS TEM operating at an acceleration voltage of 120 kV. The dimensions of the AuNRs were determined by TEM images analysis by measuring more than 200 AuNRs randomly chosen. Z-Potential measurements were performed using a NanoSizer (Zeta-Sizer, Malvern Instrument, UK).
Photothermal Studies
The heating performance of AuNRs was performed by irradiating samples with a multimode diode fiber coupled laser system (Lumics, LU808T040) operating at a fixed wavelength (808 nm) with modulable power. Spot size was adjusted to 1 cm in diameter by changing the distance between the laser output (optic fiber) and the sample. The power was measured using a Thorlabs S405C power meter connected to the computer through the PM100USB. For the thermal characterization, 1 mL of 0.1 mm of Au° colloidal suspensions were placed in a PS absorbance cuvette and irradiated with a 1 cm2 spot size at different powers. Changes in temperature were measured with an IR camera FLIR (AX35), drawing a region of interest (ROI) and ensuring baseline or nonirradiated areas were also measured.
2D Cell Culture
MDA-MB-231 cells were cultured in complete Dulbecco's Modified Eagle Medium (cDMEM) media containing fetal bovine serum (FBS, 10% v/v) and penicillin-streptomycin (PS, 1% v/v). For 2D cell viability studies, cells were seeded at 1.5 × 10^5 cells mL−1, 100 µL per well. After overnight adhesion, cell media was removed, and AuNRs diluted in DMEM or cDMEM added (100 µL per well). The cell viability of MDA-MB-231 cells was tested using the MTT and LDH assays after 24 h following the manufacturer's instructions. AuNR uptake was characterized using reflection confocal microscopy (Zeiss 880) using 633 nm laser irradiation and detection at the same wavelength. Brightfield microscopy was also conducted using a Cell Axio Observer microscope (Zeiss) equipped with color camera.
3D Cell Culture
MDA.MB.231 cells were seeded at 5 × 104 cells per well in round-bottomed 96-well plates (Nunclon Sphera) in DMEM, with or without FBS (10% v/v), and with or without Geltrex ECM (2% v/v) and left for 96 h to form spheroids. Spheroids were characterized using brightfield microscopy (Cell Axio Observer microscope, Zeiss) and ImageJ to analyze size and diameter.
Radiolabeling of AuNRs
64CuCl2 was produced by proton irradiation of 64Ni as previously described[40] and obtained as a solution in 0.1 m HCl. A fraction of this solution containing ≈40 MBq was evaporated to dryness, and the residue was dissolved in 0.4 m ammonium acetate buffer (pH 5.5). The resulting solution was added dropwise into a solution containing AuNRs. After stirring for 5 min, hydrazine hydrate (3 µmol) was added, and the solution was allowed to react at room temperature for 1 h and then washed by centrifugation. The radiochemical yield was calculated as the ratio between the amount of radioactivity present in the AuNRs after washing and the initial amount of radioactivity. One fraction of the sample was allowed to decay completely (≈8 half-lives) and subsequently analyzed by TEM to confirm stability under labeling conditions. The radiochemical stability of 64Cu-labeled AuNRs was investigated by incubating in three different media at 37 °C: i) physiological saline solution (0.9% NaCl) + 2.5 mm EDTA; ii) sodium phosphate-buffered saline + 2.5 mm EDTA; and iii) mouse serum. At different time points, the nanorods were separated by centrifugation and the amounts of radioactivity in the pellet and in the supernatant were determined. The fraction of 64Cu attached to the nanorods was calculated as the ratio between the amount of radioactivity in the pellet and the total amount of radioactivity (pellet + supernatant).
Animal Experiments – General Aspects
Animals were maintained and handled in accordance with the Guidelines for Accommodation and Care of Animals (European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes) and internal guidelines. All experimental procedures were approved by the internal ethical committee and the authorities from the Provincial Council of Gipuzkoa (Diputación foral de Gipuzkoa), procedure PRO-AE-SS-183, before conducting experimental work.
Tumor Model
The MDA-MB-231 cells were cultured in DMEM Low Glucose (#D6046 Sigma) supplemented with 10% fetal bovine serum (FBS) and 1x Penicillin-Streptomycin for two weeks before proceeding with their injection into animals. The cells were cultured in T-75 flasks, with passages every 2–3 days. Upon reaching 80–90% confluence, the cell count per flask was ≈9–10 × 106. The tumor model was generated by subcutaneous inoculation of 2 × 106 MDA.MB.231 cells into FOXnu female mice. Experimentally, 3 × 106 suspended in 75 µL of medium were mixed with 75 µL of Matrigel HC diluted 1:1 in PBS. Of the resulting 150 µL, Hundred microliters were finally injected into the right flank of each mouse. Tumor growth was monitored using a caliper, determining the tumor volume according to the formula: V = (W2 x L)/2, where L is the major diameter, and W is the minor diameter.
Imaging Studies
Tumor-bearing animals (n = 4, 2 per group) were anesthetized by inhalation of 5% isoflurane in pure O2 and maintained by 1.5–2% isoflurane in 100% O2. With the animal under anesthesia, ≈100 µL of the corresponding radiolabeled AuNR was administered intravenously via one of the lateral tail veins (see Table S3, Supporting Information, for experimental details per group). At 30 min, 4 h, and 24 h after administration, 10-min static whole-body PET scans were acquired using a β-CUBE PET scanner (Molecubes, Ghent, Belgium). After each PET scan, whole-body computed tomography (CT) acquisitions were performed using a X-CUBE CT scanner (Molecubes, Ghent, Belgium) to provide anatomical information about each animal as well as the attenuation map for the later PET image reconstruction. PET images were reconstructed using the 3D OSEM reconstruction algorithm and applying random, scatter, and attenuation corrections. PET-CT images of the same mouse were co-registered and analyzed using the PMOD image processing tool (PMOD Technologies LLC, Switzerland; version 3.4). Volumes of interest (VOIs) were manually delineated in those organs clearly visualized on CT images (brain, heart, lungs, liver, kidneys, bladder, and tumor). Activity values (decay-corrected) were obtained and converted into KBq/cm3 by applying a calibration factor obtained from previous scans on a phantom (micro-deluxe, Data Spectrum Corp.) under the same experimental conditions. The concentration of radioactivity in each organ and time-point was expressed as percentage of injected dose per cm3 (%ID/cm3).
For [18F]FDG studies, animals were fasted for 6–8 h prior to radiotracer injection. Animals were anesthetized as above, and with the animal under anesthesia, ≈100 µL of the radiotracer (3.7–7.4 MBq; kindly provided by CuriumPharma Spain) was administered intravenously via one of the lateral tail veins. The animals were recovered from anesthesia and were submitted to 10 min PET imaging followed by a CT scan after 40 min of radiotracer incorporation.
Therapeutic Studies
Tumor-bearing animals (n = 24) were randomized according to tumor volume in five different groups (Groups 1–5; 5 animals per group except for group 4, n = 4), and were treated as follows: Group 1: The animals received intravenous administration of AuNRs, and after 24 h, they were subjected to laser irradiation (AuNR IV – NIR); Group 2: Animals received intravenous administration of AuNRs but were not subjected to laser irradiation (AuNR IV – no NIR); Group 3: Animals did not receive administration of AuNRs but were subjected to laser irradiation (no AuNRs – NIR); Group 4: Animals did not receive administration of AuNRs and were not subjected to laser irradiation (no AuNRs – no NIR); and Group 5: Animals received intratumoral administration of AuNRs and were subjected to laser irradiation (AuNRs IT – NIR). The amount of AuNRs administered intravenously (groups A and B) was 100 µL at a concentration of 5 mm in gold. For the intratumoral route, it was decided to administer a quantity of 5 µL of AuNRs at a concentration of 5 mm in gold in order to achieve a similar concentration of AuNRs in the tumor to that achieved for intravenous administration. Tumor irradiations was conducted 24 h after intravenous administration of the AuNRs, optimal time to maximize the concentration of AuNRs in the tumor according to biodistribution studies, while the concentration in the blood should already be sufficiently low so as not to interfere with the assays. However, irradiation following intratumoral administration was carried out immediately, to ensure that the majority of AuNRs were still in the tumor. Therapeutic response was assessed by measuring tumor volume over time.
Histology and TEM of Tissues
Tumors with skin attached (where possible) were exercised and perfused with 10% formalin. Samples were processed for traditional histological analysis with H&E staining, and samples of tumor tissue were reserved and stained with OsO4 followed by resin embedding for imaging with the TEM.
Acknowledgements
J.L. and V.G.V. thank Grant PID2020-117656RB-I00 funded by MCIN/AEI/ 10.13039/501100011033. L.L.M. thanks Spanish State Research Agency, MCIN/AEI/10.13039/501100011033 (Grant PID2020-117779RB-I00). This work was performed under the Maria de Maeztu Units of Excellence Program from the Spanish State Research Agency – Grant no. MDM-2017-0720. The in vivo work was carried out at the ICTS ReDIB, CIC biomaGUNE. Ana Sánchez-Iglesias is acknowledged for AuNR synthesis.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




