Thermodynamics of adsorption of uranyl ions onto amidoximated poly(acrylonitrile)/poly(N-vinyl 2-pyrrolidone) interpenetrating polymer networks
Abstract
The interaction of uranyl ions (UO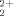 ) with interpenetrating polymer networks (IPNs) based on amidoximated poly(acrylonitrile)/poly(N-vinyl 2-pyrrolidone) was examined. The adsorption capacity of IPN hydrogels as well as the adsorption kinetics and the effect of temperature on UO
) with interpenetrating polymer networks (IPNs) based on amidoximated poly(acrylonitrile)/poly(N-vinyl 2-pyrrolidone) was examined. The adsorption capacity of IPN hydrogels as well as the adsorption kinetics and the effect of temperature on UO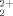 ion adsorption were investigated. Thermodynamic quantities and kinetic parameters were calculated with adsorption isotherm data. The initial adsorption-rate values for each temperature were calculated, and the corresponding rate constants decreased with increasing temperature. The adsorption enthalpy, entropy, and free energy of the UO
ion adsorption were investigated. Thermodynamic quantities and kinetic parameters were calculated with adsorption isotherm data. The initial adsorption-rate values for each temperature were calculated, and the corresponding rate constants decreased with increasing temperature. The adsorption enthalpy, entropy, and free energy of the UO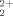 ion with amidoximated IPN hydrogels were calculated from basic thermodynamic relations. It was assessed that adsorption occurred by strong electrostatic interactions with an adsorption enthalpy of −31.5 kJ/mol. © 2004 Wiley Periodicals, Inc. J Polym Sci Part B: Polym Phys 42: 986–993, 2004
ion with amidoximated IPN hydrogels were calculated from basic thermodynamic relations. It was assessed that adsorption occurred by strong electrostatic interactions with an adsorption enthalpy of −31.5 kJ/mol. © 2004 Wiley Periodicals, Inc. J Polym Sci Part B: Polym Phys 42: 986–993, 2004




