Ultraviolet curing of acrylic systems: Real-time Fourier transform infrared, mechanical, and fluorescence studies
Abstract
The photopolymerization of acrylic-based adhesives has been studied by Fourier transform infrared and fluorescence analysis in real time. Real-time infrared spectroscopy reveals the influence of the nature of the photoinitiator on the kinetics of the reaction. Furthermore, the incident light intensity dependence of the polymerization rate shows that primary radical termination is the predominant mechanism during the initial stages of the curing of the acrylic system with bis(2,4,6-trimethylbenzoyl) phenyl phosphine oxide (TMBAPO) as a photoinitiator. The fluorescence intensity of selected probes increases during the ultraviolet curing of the adhesive, sensing microenvironmental viscosity changes. Depending on the nature of the photoinitiator, different fluorescence–conversion curves are observed. For TMBAPO, the fluorescence increases more slowly during the initial stage because of the delay in the gel effect induced by primary radical termination. Mechanical tests have been carried out to determine the shear modulus over the course of the acrylic adhesive ultraviolet curing. In an attempt to extend the applications of the fluorescence probe method, we have undertaken comparisons between the fluorescence changes and shear modulus. Similar features in both curves confirm the feasibility of the fluorescence method for providing information about microstructural changes during network formation. © 2002 Wiley Periodicals, Inc. J Polym Sci Part A: Polym Chem 40: 4236–4244, 2002
INTRODUCTION
An adhesive is a complex mixture of substances that, when applied between two surfaces, provides material continuity for joining them and preventing their separation. In the last 50 years, the development of adhesives based on synthetic polymers has increased exponentially because of the high-performance demand for technical applications. During the formation of an adhesive joint, two stages can be distinguished at least. First, the liquid formulation spreads over the substrates and, second, this formulation hardens to bear and transfer the loads that may appear during its in-service life. The first stage is controlled by the thermodynamics and kinetics of the wetting process, which depends on the physicochemical nature of both the substrates and the liquid formulation. The hardening stage is controlled by a wide variety of different chemical processes, but for high-performance adhesives, the polymerization mechanism or curing is the preferred one. With regard to adhesive technology, the requirement for shortening curing times has brought a new approach to the use of formulations that will polymerize under ultraviolet (UV) or visible radiation. Photochemical polymerization has allowed the development of one-component adhesives that cure in a few minutes (or seconds) at room temperature. For the further development of adhesive technology, a knowledge of the polymerization processes is of great interest because the final (mechanical) properties of these materials are mainly related to the extent of cure along with the nature of the binder.
The online monitoring of polymerization reactions requires a determination of the degree of conversion in real time. Different techniques have been used in the past, such as differential scanning calorimetry and Fourier transform infrared (FTIR), which are based on the measurement of different macroscopic magnitudes. The fluorescence probe method1-7 has been shown to be very useful for following photopolymerization because the emission changes are related to the mutual rearrangements occurring in the microenvironment of the probe, more particularly, sensing the increase in rigidity during polymerization. Therefore, a nonlinear correlation should be expected between the fluorescence and the degree of conversion when the whole process is studied. Furthermore, fluorescence has found numerous applications in polymer science.8-10 Some examples include studies of (1) the molecular structure, first- and second-order transitions (the glass-transition, γ-transition, and β-transition temperatures), end-to-end distances, and crystallinity; (2) associations and aggregations in water solutions of polyelectrolytes and amphiphilic polymers; (3) polycomplex formation; (4) the miscibility of polymer blends and interpenetration of polymers; (5) the microheterogeneity of polymer solutions; (6) conformational transitions in solution, including local and segmental displacements; (7) energy transfer, including the antenna effect; (8) orientation and residual stresses; and (9) sol–gel transitions. The versatility of this technique shows that fluorescence-based methods will allow us to gain a deeper insight into several processes, including photopolymerization.
The monitoring of polymerization has also been carried out by means of rheometry and dynamomechanical analysis.11, 12 However, few efforts have been devoted to studying the mechanical properties over the course of curing. Recently, Smirnov et al.13 pointed out the internal stresses and shrinkage defects on the network formation process influencing the strength properties of adhesive bonds as well as the nature of its failure.
The aim of this work is to find a correlation between fluorescence and conversion during the UV curing of an acrylic adhesive with two different photoinitiators. Real-time FTIR has been used to measure the acrylate double-bond conversion at different irradiation times, and the changes in the fluorescence intensity have been measured in real time. Furthermore, we have attempted to correlate our results with mechanical tests. In this regard, the shear moduli of the adhesive joints have been measured during UV curing.
EXPERIMENTAL
Fluorescent Probes and Acrylic Adhesives
4-Dimethylamino-4′-nitrostilbene (DMANS), 2-hydroxy-4-dimethylamino-4′-nitrostilbene (2OHDEANS), and 4-dimethylaminophenyl-4′-nitrophenylbutadiene (DMANBu) were synthesized to be used as fluorescent probes as previously described.14 The synthesis of 4-(N,N′-diethyl)amino-7-nitrobenz-2-oxa-1,3-diazole (NBD–NEt2) and a methacrylic derivative (NBD–NAcr) have been earlier described.15 Molecular formulas of stilbene derivatives and the NBD fluorescent probes are shown in Figure 1.
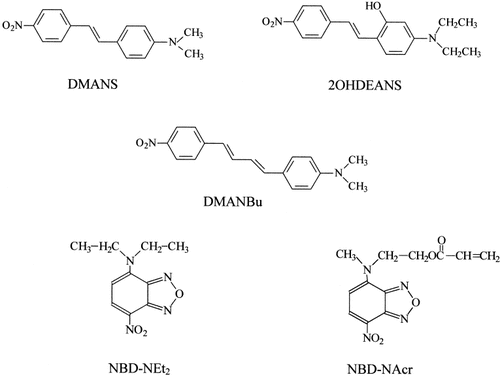
Chemical structures and abbreviations of the fluorescent probes used in this work.
The adhesive formulation L312 was provided by Loctite España. The fractionation and analysis of the components of L312 give the following composition. This adhesive comprises a mixture of 50% polyurethane–methacrylate resin, 35–40% hydroxyalkyl methacrylate, 5–10% acrylic acid, 1–3% cumene hydroperoxide, 0.1–1% tributylamine, and 0.1–1% trimethoxy[3-(oxiranylmethoxy)propyl]silane as an adhesion promoter.
The commercial photoinitiators 2-benzyl-2-N,N-dimethylamino-1-(4-morpholinophenyl)-1-butanone (DBMP; Irgacure 369) and bis(2,4,6-trimethylbenzoyl) phenyl phosphine oxide (TMBAPO; Irgacure 819) were provided by Ciba Specialty Chemicals. The mechanisms of fragmentation of both photoinitiators under UV-light exposure are shown in Scheme 1.
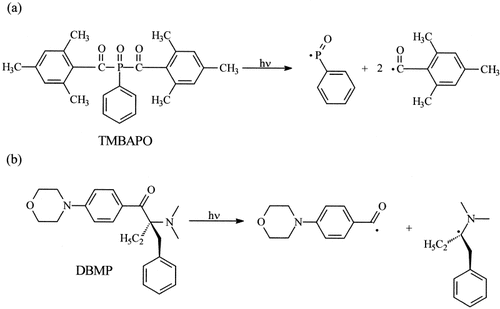
Photofragmentation mechanisms of the photoinitiators DBMP and TMBAPO under UV irradiation.
Sample Preparation
The fluorescent probe (ca. 0.03% w/w) and the photoinitiator (1% w/w) were dissolved in the adhesive formulation. The photocurable formulations were applied as a uniform layer coating on a polyethylene film or aluminum foil and then were covered with a low-density polyethylene (LDPE) film (40 μm thick) that did not absorb at the infrared (IR) frequencies selected to follow the photopolymerization reaction nor at the excitation/emission and UV-irradiation wavelengths. The function of the LDPE film was to prevent oxygen diffusion from the atmosphere into the sample during the irradiation at room temperature. The photosensitive coatings (15 μm) were obtained by controlled pressing.
Both photoinitiators also absorb excitation light (355 nm); however, fluorescence from the photoinitiator does not interfere the fluorescence spectra of the probe because of their low fluorescence quantum yield.
Monitoring the UV Curing of Acrylic Systems
The UV curing of the adhesive formulations was carried out by irradiation with a 400-W mercury lamp (Macam-Flexicure irradiation system) at room temperature under an air atmosphere until limiting conversion. The incident intensity was measured with a Scientech H310D photocalorimeter and was varied with different interference filters in front of the sample. For all samples, the disappearance of double bonds and the fluorescence changes of the probes were monitored by FTIR and luminescence spectroscopy, both under real-time conditions. Experimental details have been described previously.16
FTIR in Real Time
Real-time infrared (RTIR) measurements were carried out in a Nicolet 520 IR spectrophotometer provided with a Fourier transform algorithm. The samples were placed over a specular reflection accessory in the IR spectrophotometer, in which they were simultaneously exposed to the UV light and to the IR analyzing beam. The decrease in the absorbance at 817 cm−1 (corresponding to the disappearance of the acrylate double bond) was annualized by means of a software program to record data in real time (acquisition time = 0.3 s).
Fluorescence Spectroscopy in Real Time
The fluorescence emission was collected with a monochromator (Oriel MS257) during UV irradiation of the acrylic adhesives. The spectra were recorded with an intensified charge coupling device (CCD) camera (Andor ICCD-408) with a camera exposure time of 1 μs. The excitation wavelength was fixed at 355 nm from the Nd–YAG laser (Quanta-Ray, Spectra Physics). It was checked that the excitation laser beam used for analysis did not contribute to polymerization initiation, and for this purpose, an attenuator was placed in front of the sample to diminish the incident light.
Mechanical Properties
An MTS Synergie 200 (100N) computer-aided dynamometer was used for measuring the shear strength of overlapped adhesive joints. The following conditions were fixed: a deformation rate of 0.5 mm/min and a maximum load of 100 N.
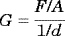 (1)
(1)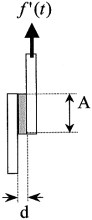
Geometrical disposition of the specimens for mechanical tests.
RESULTS AND DISCUSSION
The polymerization rate, fluorescence intensity changes, and shear modulus have been in situ monitored over the course of the UV curing of the acrylic commercial adhesive L312 with different photoinitiators, DBMP and TMBAPO.
Kinetic Study by RTIR
First, the reactions between the photoinitiator and fluorescent compound were examined. No reaction was observed, and this indicated that they should not modify the properties of the final material. Therefore, the kinetic profiles measured by FTIR in real time are the same in the absence and presence of the fluorescent probes. Figure 3 plots the polymerization rate versus conversion. The initial polymerization rate for TMBAPO is higher than that for DBMP. The irradiation of the TMBAPO photoinitiator gives rise to phosphonyl radicals, which are more reactive than the carbon-centered radicals, photogenerated by the photolysis of DBMP (Scheme 1). Therefore, higher polymerization rates are achieved with the acyl phosphide photoinitiator.
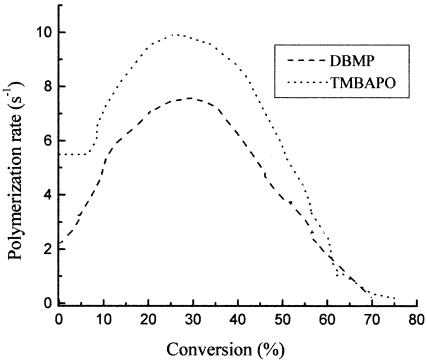
Plot of the polymerization rate as a function of conversion for the UV curing of L312 with the two photoinitiators DBMP and TMBAPO. I0 = 1.84 mW · cm−2.
As expected for diffusion-controlled kinetics, a dramatic increase in the polymerization rate is observed at low conversions (ca. 6% for TMBAPO). This autoacceleration effect is due to the decreased mobility of the growing macroradicals as the viscosity increases, which causes a reduction in the termination rate constant, and as a result, the macroradical concentration increases (and, therefore, so does the polymerization rate).
The magnitude and onset of the gel effect have been shown to depend strongly on the monomer structure for various multifunctional (meth)acrylates. Goodner and Bowman17 studied the influence of primary radical termination on the gel effect, showing that as the primary radical termination rate constant increases, the autoacceleration rate is diminished. Furthermore, the increase in the primary radical concentration (e.g., increasing irradiation light intensities) drives the primary radical termination to compete with bimolecular termination, becoming the dominant mechanism earlier in the reaction under certain conditions.
During the UV curing of L312 with two different photoinitiators, a certain reduction of the gel effect is observed when TMBAPO is compared to DBMP (Fig. 3). This behavior indicates that primary radical termination plays a role with the acyl phosphide photoinitiator and would reduce the kinetic chain length and, therefore, the molecular weight. Furthermore, at a 30% conversion, the polymerization rate is reduced because of a decrease in the propagation rate constant associated with the low diffusivity of the monomer into the polymer matrix.
The influence of the incident light intensity on the polymerization rate was determined to investigate the mechanism of the termination reactions. Plots of conversion versus irradiation time during the UV curing of L312 under different irradiation conditions are shown in Figure 4 with DBMP and TMBAPO as photoinitiators. The reaction rate is proportional to the intensity of the incident light (I0), and the final conversion (0.75) is independent of the light intensity with TMBAPO. However, the limiting conversion increases at a high value of I0 in comparison with those measured at low intensities (from 0.67 at 0.24 mW/cm2 to 0.85 at 1.84 mW/cm2) when DBMP is used as a photoinitiator.
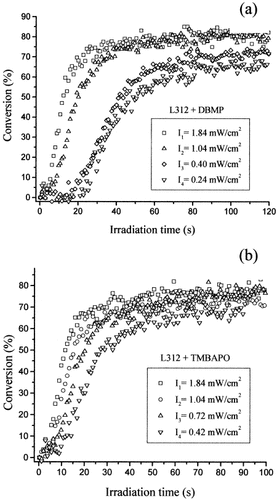
Time dependence of the double-bond conversion for the UV curing of L312 initiated by various light intensities with the photoinitiators (a) DBMP and (b) TMBAPO.
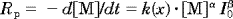 (2)
(2)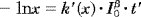 (3)
(3)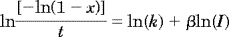 (4)
(4)| Conversion (%) | TMBAPO β | DBMP |
|---|---|---|
| 0.15 | 0.33 | 0.64 |
| 0.20 | 0.30 | 0.52 |
| 0.25 | 0.39 | 0.53 |
| 0.30 | 0.36 | 0.56 |
Fluorescence Monitoring
The photocuring of L312, with TMBAPO and DBMP as photoinitiators, was followed by FTIR and fluorescence in real time. Figure 5 shows the plot of the fluorescence integrated intensity as a function of the acrylate double-bond conversion with two families of fluorescent probes: stilbene derivatives and NBD probes.
Plots of the fluorescence versus the conversion of the UV curing of the adhesive L312 with DBMP and TMBAPO as photoinitiators. The fluorescent probes were (a) DMANS, DMANBu, and 2OHDEANS and (b) NBD derivatives. I0 = 1.84 mW · cm−2.
DMANS shows the highest sensitivity, whereas the NBD derivatives (NBD–NEt2 and NBD–NAcr) show a slightly lower sensitivity, as does DMANBu. Moreover, the fluorescence changes during the whole process, especially during the last stage, and this is more interesting from an applied viewpoint. Surprisingly, the curves show different profiles depending on the photoinitiator used for the UV curing of L312. In this sense, it is interesting to compare fluorescence curves during the photopolymerizations of L312 with DBMP and of L312 with TMBAPO. During the network formation with DBMP, higher fluorescence changes are measured at the first stage of conversion (up to approximately 15%), whereas further progress of the reaction modifies more slowly the fluorescence parameters. However, when TMBAPO is used as a photoinitiator, small variations of the fluorescence intensity are observed up to 20–30% conversion, and then the fluorescence intensity increases sharply with conversion. Fluorescence changes are induced by variations in the viscosity and polarity in the microenvironment of the probe. Therefore, this feature indicates that the local rigidity of the probe microenvironment increases more rapidly in L312 with DBMP than in L312 with TMBAPO. This effect is an indication of microstructural changes during the network photogeneration, induced with different photoinitiators. Furthermore, this proposal agrees with FTIR results, which show that the kinetics of the curing reaction are influenced by the nature of the photoinitiator. During the initial stages of UV curing of L312 with TMBAPO, primary radical termination plays an important role. As a result of this termination mechanism, the kinetic chain length diminishes, and so does the molecular weight; this brings a delay of the gel effect. This behavior can explain why the fluorescence increases more slowly during the initial stages of curing with TMBAPO.
Mechanical Measurements
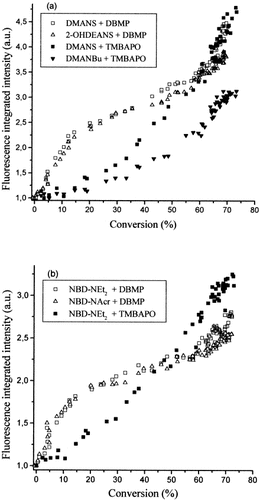
Figure 6 gives kinetic curves of the change in the shear modulus over the course of the UV curing of the acrylic adhesive L312 with different photoinitiators. A rapid increase in the value of the modulus can be seen with DBMP. However, the shear modulus of the adhesive formulation containing TMBAPO shows an inhibition time before its value increases as the polymerization progresses. This feature has been correlated with the delay of the autoacceleration effect. Because the composition formulation only changes the photoinitiator, the delay of the gel effect with TMBAPO has been attributed to a decrease in the primary chain length due to the primary radical termination.
Time dependence of the shear modulus during joint adhesive L312 formation with DBMP and TMBAPO as photoinitiators. I0 = 1.84 mW · cm−2.
The shear modulus is a measurement of the stiffness of the material and increases from a soft material to a hard material. From a macroscopic point of view, photocuring can be considered, therefore, a hardening of a material. Moreover, fluorescent probes are sensitive to changes in the local rigidity of the medium. In a medium in which molecular mobility is restricted, the fluorescence emission increases because of the associated restriction in the nonradiative relaxation pathway of the excited state of the probe. This nonradiative process normally involves some kind of vibrational coupling between the excited state and the environment, which behaves as a thermal bath. For these reasons, a correlation should be found between fluorescence and modulus during a cure process.
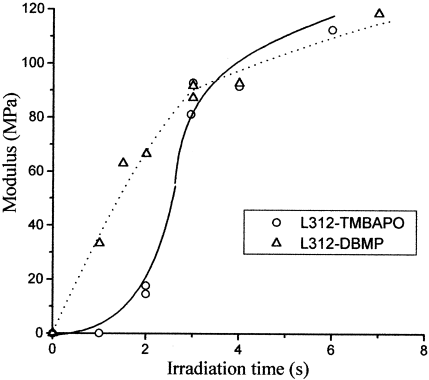
Figure 7 shows a comparison of fluorescence and shear modulus changes during the UV curing of the acrylic adhesive L312 with DBMP as a photoinitiator. The fluorescence increase follows the same trend as that for the modulus changes with conversion. As a result of the crosslinking, the rigidity of the material increases, as does the fluorescence emission, because the nonradiative relaxation pathway is significantly restrained. A similar behavior is observed for the system L312 and TMBAPO. A comparison between Figures 5 and 6 shows that, in this case, both fluorescence and shear modulus increase after an inhibition time (not observed with DBMP). These new results corroborate our proposal that the fluorescence method can be useful for the analysis of microstructural changes during UV curing.
Plot of the fluorescence changes versus the conversion during the UV curing of L312 with DBMP. The fluorescent probes were DMANS and 2OHDEANS. On the right axis, the shear modulus of the adhesives formulations as a function of conversion is plotted. I0 = 1.84 mW · cm−2.
CONCLUSIONS
The combination of RTIR, mechanical tests, and fluorescence experimental data have allowed us to clarify the influence of the photoinitiator nature on the UV curing of an acrylic-based adhesive. The high reactivity of phosphonyl oxide primary radicals photogenerated from TMBAPO results in a primary radical termination mechanism. As a result of this predominant termination mechanism, the primary kinetic chain length is shortened, the molecular weight is reduced, and the gel effect is diminished.
For first time, the fluorescence has been correlated with microstructural changes occurring over the course of polymerization. Therefore, the fluorescence method is presented here as a powerful tool for studying deep photopolymerization reactions because it can provide information about the process in a more sophisticated fashion than a simply correlation of the fluorescence extent of cure.
Acknowledgements
The authors thank the Union European Commission for funding through the BRITE-Euram Project (BE97-4472). Gratitude is also extended to Plan Nacional I+D+I (Ministerio de Ciencia y Tecnología) for its financial support (MAT2000-1671) and to Comunidad Autónoma de Madrid (07N/0002/1998). The authors thank K. Dietliker (Ciba Specialty Chemicals) for providing the photoinitiators and Loctite España for providing the adhesives.




