Effect of targeted minimal sequence variations on the structure and biological activities of the human cathelicidin LL-37
Funding information: University of Trieste, Fondi Ricerca Ateneo
Abstract
LL-37 is an innate immune peptide derived from the human cathelicidin, which exerts pleiotropic roles in host defense and healing. These activities in part depend on its capacity to adopt an amphipathic helical structure in physiological solutions and then oligomerizing. Orthologues from other primates, such as rhesus RL-37, remain monomeric and disordered under the same conditions. Intramolecular salt-bridges, arising from appropriately spaced anionic and cationic residues in its sequence, may play a relevant role in determining the particular structure adopted by LL-37. To probe this, we have effected minimal, targeted residue variations such as replacement of a single residue (K15→G), or inversion of one or both sets of two residues (E10 K11→ K10 E11 or E16 K18→ K16 E18). This could alter the pattern of intramolecular salt bridging without affecting other functionally relevant parameters such as overall hydrophobicity, helix amphipathicity or charge. The structural and functional effects were analyzed using CD spectroscopy, surface plasmon resonance, antimicrobial activity assays, and bacterial membrane permeabilization to fluorescent probes of increasing sizes, using flow cytometry. Analogs were functionally different from both LL-37 and RL-37, so it was not possible to switch from the function of one to that of the other simply by altering the salt-bridging pattern in this manner. This indicates that the particular structure/function characteristics of LL-37 likely depend quite subtly, and in a precise and complex manner, on a complex pattern of intramolecular interactions.
Graphical Abstract
Abbreviations
-
- AMP
-
- antimicrobial peptide
-
- A/R
-
- attraction/repulsion
-
- CD
-
- circular dichroism
-
- CLD
-
- cathelin-like domain
-
- Ch
-
- cholesterol
-
- DOPC
-
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
-
- HDP
-
- host defense peptides
-
- LPS
-
- lipopolysaccharide
-
- LTA
-
- lipoteichoic acid
-
- PBS
-
- phosphate-buffered saline
-
- PI
-
- propidium iodide
-
- SDS
-
- sodium dodecylsulphate
-
- SPB
-
- sodium phosphate buffer (10 mM)
-
- SPR
-
- surface plasmon resonance
1 INTRODUCTION
The human host defense peptide LL-37 is an important component of our innate immune system, with pleiotropic roles in combating infections, modulating immune responses, mediating inflammation and stimulating angiogenesis, and cell proliferation in wound healing.1-6 It is a member of the cathelicidin family of antimicrobial peptides (AMPs),7 adopts an amphipathic helical active structure, and its central and ancient role in innate immunity is suggested by the fact that there is an orthologue in all other placental mammals.1
While studying the evolution of the CAMP gene, which codes for LL-37, in other primate species,8 we found that it is under positive pressure for sequence variation and that this principally affects its polar residues, while the peptide size and the distribution of hydrophobic residues are well conserved. The interchange of cationic, anionic, and neutral polar residues at certain positions results in a remarkable variation in the orthologues’ charge, a key parameter for antimicrobial activity, while other important parameters such as the size, overall hydrophobicity, and helix amphipathicity (hydrophobic moment) are less affected. On helix formation, charged residues cluster on one side of the helix, so that side-chains separated by 4 or 5 positions in the sequence (i→i + 3 or i→i + 4) are adjacent to each other on the helix face and could engage in electrostatic interactions. This seems to be a determining factor affecting the propensity for helix formation in LL-37 and driving its subsequent oligomerization,8-13 affecting its functional characteristics. The capacity for forming salt bridges seems to vary considerably among primate orthologues, as well as those in other mammalian orders,7, 8 suggesting it is a relevant aspect of their structure-function relationships.
In the past, we have investigated this relationship with reference mainly to two paradigm peptides, human LL-37, and rhesus RL-37. The former has a numerous residues potentially capable of forming intramolecular electrostatic attractions, and this appears to stabilize its amphipathic helical structure, as it does not need to interact with membranes to adopt it, but can do so in aqueous solution, in the presence of salt ions.8, 9, 14 This then drives oligomerization as a means of shielding the hydrophobic helix sector from the aqueous environment.12, 13 We indicate LL-37 and other primate orthologues behaving in this manner as A-form, as their functional form is “aggregated.” RL-37, on the other hand, has a disordered, coil-like structure in aqueous solution and remains monomeric until it interacts with biological membranes, so we indicate it as F-form (not aggregated or “free”).
Oligomerization appears to correlate with a less potent and more medium sensitive antimicrobial activity in vitro, as the A-form LL-37 seems to interact more with medium, serum or outer bacterial wall components, reducing its capacity to penetrate through the Gram-positive peptidoglycan or Gram-negative outer membrane LPS layer.8-15 Conversely, the F-form RL-37 has a generally more potent, broader spectrum antibacterial activity that is less sensitive to medium conditions or the presence of serum components.11 Furthermore, the mechanism of bacterial membrane lysis appears to be quite different. LL-37 approaches the membrane as an oligomeric bundle which seems to favor formation of relatively large toroidal pores, while RL-37 approaches it as a compact monomeric coil and structures only on membrane interaction, favoring extensive formation of small lesions, likely due to a carpet-like effect.10, 12
The decreased efficacy of the A-form LL-37 as a directly membranolytic antibiotic may be balanced by an increased efficacy in modulating host cells. For example, LL-37 and other A-form orthologues activate the purinergic P2X7 receptor in fibroblasts, which promotes their proliferation, while RL-37 and other F-form orthologs apparently do not.16 Furthermore, LL-37 is processed in the extracellular medium by secreted proteases,17 and the shortened peptides revert to the F-form, having increased antimicrobial activity while losing the capacity to stimulate host cell processes.18, 19 This allows for the best of both worlds.
In this context, it is useful to understand how salt-bridging in LL-37 affects helical structuring, which drives oligomerization and favors particular types of membrane interactions with either bacteria or host cells. The effect may not be easy to discern, as studies with covalent dimers or photo-induced oligomers of LL-37, as well as selectively fluorescently labeled analogs of LL-37 and RL-37, have indicated that enhanced induction of the secondary structure is insufficient on its own to completely explain their different modes of action.12, 13 Furthermore, when altering the salt bridging capacity it is necessary to avoid alterations in other functionally important parameters, such as the amphipathicity, overall charge and hydrophobicity.
For this reason, we have set out to investigate the possibility of introducing very limited alterations in the primary structure of LL-37 that may disrupt salt bridging but have little effect on the other relevant parameters, to then determine how this affects structuring and interaction with model biological membranes, and how this in turn affects functional aspects. Results indicate a very subtle relationship between the primary and secondary structures, so that while limited alterations of the salt bridging definitely affect the functioning of LL-37, underlining their importance as a component of its complex intramolecular interactions, they do not result in a switch to an F-form behavior.
2 MATERIALS AND METHODS
2.1 Design of the LL-37 analogs
LL-37 analogs were designed by introducing the sequence of LL-37 and RL-37 in an ad hoc created Excel file capable of counting Arg, Lys, Asp, and Glu residues 4 or 5 positions from each other (i→ i + 3 or i→ i + 4). As these would be on the same side of a helix, they could result in intramolecular electrostatic interactions. LL-37 analogs were then designed by either mutating just one residue in a strategic position that potentially engages in a number of possible attraction interactions, or by interchanging pairs of oppositely charged residues. This can alter their capacity to form salt-bridges, with little or no effect on the overall charge and a minimal effect on physico-chemical parameters such as the overall hydrophobicity or helix amphipathicity (hydrophobic moment).
In particular, considering the sequence of LL-37, we identified the following numbered residues as potentially involved in salt bridging:
LLGDFFRKSK10E11KIGK15E16FK18RIVQRIKDFLRNLVPRTES.
We therefore designed one analog in which K10 and E11 were interchanged. In a second one E16 and K18 were interchanged. In a third analog, both these interchanges were effected. In the fourth analog, K15 was replaced by Gly, which occurs in this position in the RL-37 sequence (see Table 1).
| % helixd | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peptide | Sequencea | MW | q | A/Rb | Hc | μHrelc | H2O | SPB | PIL | TFE |
| RL-37 | + ++ +-+ ++ + +- + RLGNFFRKVKEKIGGGLKKVGQKIKDFLGNLVPRTAS |
4101.0 | +8 | 3/3 | −1.66 | 0.56 | <5 | 5 | 5 | 55 |
| LL-37 | - ++ +-+ +- ++ + +- + + - LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
4493.4 | +6 | 9/7 | −1.83 | 0.59 | 5 | 25 | 40 | 60 |
| LL-37(E10K11) | - ++ -++ +- ++ + +- + + - LLGDFFRKS EK KIGKEFKRIVQRIKDFLRNLVPRTES |
4493.4 | +6 | 7/9 | −1.83 | 0.6 | 5 | 25 | 35 | 60 |
| LL-37(K16E18) | - ++ +-+ ++ -+ + +- + + - LLGDFFRKSKEKIGK K F E RIVQRIKDFLRNLVPRTES |
4493.3 | +6 | 8/8 | −1.83 | 0.59 | 5 | 15 | 35 | 55 |
| LL-37(E10K11K16E18) | - ++ -++ ++ -+ + +- + + - LLGDFFRKS EK KIGK K F E RIVQRIKDFLRNLVPRTES |
4493.2 | +6 | 6/10 | −1.83 | 0.59 | 5 | 15 | 40 | 60 |
| LL-37(G15) | - ++ +-+ - ++ + +- + + - LLGDFFRKSKEKIG G EFKRIVQRIKDFLRNLVPRTES |
4422.1 | +5 | 8/4 | −1.63 | 0.56 | 5 | 20 | 35 | 60 |
- a Charged residues are indicated. Residues that were altered in the analogs are bold and shaded.
- b Ratio of residues spaced 4 or 5 apart that could engage in electrostatic attractions A or would lead to repulsions R.
- c The mean per residue hydrophobicity H and relative amphipathicity (μHrel) were determined as described previously.20 The hydrophobic moment is relative to a perfectly amphipathic peptide of 36 residues (two full helix turns) composed of an equal number of the most hydrophobic and hydrophilic residues in the hydrophobicity scale that was used, in this case Phe and Arg.
- d % helix formation was determined relative to a perfectly formed helix of the same length according to Chen et al.24 Conditions were: SPB (10 mM Na2HPO4/NaH2PO4, pH 7.4); PIL (130 mM NaCl, 24 mM NaHCO3, 0.6 mM MgCl2, 1.3 mM CaCl2 and 3.9 mM KCl, pH 7.3); TFE (50% TFE in SPB).
To determine how these variations could affect salt bridging, the sequences of RL-37, LL-37 and the four analogs were submitted to the QUARK ab initio protein structure prediction server,21 and the resulting predicted structures visualized with the DS Visualizer (Accelrys Software) to determine which salt-bridging interaction could occur, in terms of oppositely charged residues spaced <4 Å apart.
2.2 Peptide synthesis and purification
All peptides were synthesized with a CEM Liberty automated microwave assisted peptide synthesizer, using Fmoc solid-phase chemistry and the Fmoc-Ser(tBu)-Novasyn-TGA resin (substitution 0.24 mmol/g, Novabiochem). All protected amino acids were from Novabiochem (Merck Millipore). Couplings were performed with a 4-fold excess of incoming amino acids in NMP (N-methyl pyrrolidone) and were activated with either PyBOP or HCTU. Double couplings were performed as appropriate, having used the Peptide Companion software (Coshi Soft) to predict difficult couplings. The deprotection of the Fmoc group was performed using a solution of 20% piperidine in DMF. On synthesis completion, peptides were cleaved from the resin using a version of the reagent K composed of 87% TFA (trifluoroacetic acid), 2% TIPS (triisopropylsilane), 3% water and 8% DODT (3,6-dioxa-1,8-octanedithiol) for 4 h at room temperature (22°C), under continuous stirring, and then precipitating in ice-cold TBME (methyl t-butyl ether).
The correct sequence of the crude peptides was confirmed by ESI-MS (Bruker Daltonics Esquire 4000) before purifying by RP-HPLC using a Phenomenex Jupiter Proteo C18 column (20 × 250 mm, 10 μm, 90 Å) using a gradient of 30%-60% acetonitrile in 60 min with a 8 mL/min flow. Peptide purity (> 95%) was confirmed by ESI–MS and analytical RP-HPLC. The peptides were lyophilized three times from 10 mM HCl suspensions to remove TFA counterions, and resuspended in water. The concentration of peptide stock solutions was quantified by (i) accurately weighing the peptide used to prepare solutions, (ii) spectrophotometric determination of the peptide bonds using ɛ214 calculated as described22 and (iii) by measuring the differential absorbance at 215 nm and 225 nm,23 taking the mean value of the three.
The structure and biological activities of the peptides were compared to those of the parent peptide LL-37, its rhesus orthologue RL-37, and a dimeric version of LL-37 linked via an intermolecular S-S bridge involving an added C-terminal cysteine residues, to produce the covalent dimer [LL-37(C38)]2 (C-terminal dimer or CTD in the text). The preparation of these peptides has been described previously.12
2.3 Circular dichroism
CD spectroscopy was performed on a J-715 spectropolarimeter (Jasco) using 2-mm path length quartz cells and 20 μM peptides in water, 10 mM SPB (10 mM Na2HPO4/NaH2PO4, pH = 7.4), PIL buffer (Physiologic ion like; 130 mM NaCl, 24 mM NaHCO3, 0.6 mM MgCl2, 1.3 mM CaCl2, and 3.9 mM KCl, pH 7.3), and at increasing concentrations of trifluoroethanol (TFE) in SPB. The helical content (percentage helix) was estimated from the molar ellipticity at 222 nm.24 CD spectra are the accumulation of at least three scans.
2.4 Antibacterial activity and membrane permeabilization
Antimicrobial activity was evaluated against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923. MIC values were determined using the serial 2-fold dilution assay for each peptide using 20% Mueller-Hinton broth (MHB) in 10 mM SPB, pH 7.3 (as LL-37 is medium sensitive) in 96-well polypropylene microtitre plates (Sarstedt). Bacterial growth was monitored also in the absence of peptide as a control. Bacterial suspension (50 μL) with 5 × 105 cells/mL in 20% MHB was added in each well containing 50 μL peptide solution in 20% MHB. The MIC was taken as the lowest concentration where no visible bacterial growth was observable after 24 h of incubation at 37°C. Bactericidal activity was determined by incubating 1 x 106 cells/mL in 20% MHB with concentrations near the MIC for 60 min, serially diluting in PBS and then plating on MH agar. Colony counts were made after overnight incubation of the plates.
To determine the effect of peptides on bacterial growth kinetics, 106 cells/mL were exposed to different peptide concentrations in 20% or 100% MHB (pH 7.2). Bacterial growth curves were obtained by monitoring the optical density at 600 nm for 4 h at 37°C, using a Tecan Sunrise microtiter plate reader. Quantification of growth inhibition (I, expressed in percentage) was calculated as %I = 1 – (AP/A0) × 100, where A0 is the measured absorbance at 210 min in the absence of peptide and AP the absorbance at a given peptide concentration at that time. The IC50 value for growth inhibition was calculated as the mean peptide concentration that reduced growth to 50%, at 210 min.
2.5 Flow cytometry assays
Flow cytometric analyzes of membrane permeabilization were performed on a mid-exponential culture of S. aureus (106 cells/mL) in 20% MHB in 10 mM SPB, treated with 2–8 μM peptide concentrations for 15–60 min at RT. Propidium iodide (PI, Sigma–Aldrich) or FITC-labeled dextran of 4, 10, 20, or 40 kDa (FD4, FD10, FD20, or FD40, Sigma–Aldrich) were then added to final concentrations of 10 μg/mL or 0.25 mg/mL, respectively.
Measurements were performed on a CYTOMICSTM FC500 (Beckman Coulter Inc. Fullerton, California), equipped with an Argon laser (488 nm, 5 mV) and standard configuration with photomultiplier tube fluorescence detector for green (525 nm, FL1), orange (575 nm, FL2) or red (610 nm, FL3) filtered light. After acquisition of at least 10,000 events per each run, data were stored as list mode files and analyzed with the FCS Express V3 software.
2.6 Surface plasmon resonance studies
Inter-peptide interactions and peptide-membrane interactions were studied using an X100 instrument (Biacore, GE Lifesciences). Conditions for anchoring LL-37 molecules have been described previously13 and were used also for anchoring a selected analog. Briefly LL-37(K16E18), suspended in sodium acetate buffer (pH 5), was anchored to a CM5 chip following the protocol provided with the Biacore EDC/NHS coupling kit. The acidic conditions and peptide concentration used (50 μg/mL) were such that the peptide was most likely to deposit on the surface as monomeric molecules.13 The level of the substitution reached after immobilization was equivalent to 200 response units (RU). At these peptide concentrations, the anchored molecules on the chip should be well separated (∼4 nm apart), given that 100 RU corresponds to 0.1 ng/mm2 of the surface.25 Peptide-peptide interaction studies were performed either in mildly basic conditions using HBS-EP (0.01 M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% v/v Surfactant P20, GE Lifesciences), which favor helical structuring and oligomerization for LL-37. Free LL-37(K16E18) solutions with concentrations ranging from 4 to 64 μM were then flowed in successive cycles for 180 s of contact time and 300 of dissociation time across the surface of the LL-37(K16E18)-modified chip to collect sensorgrams. The analyte was then washed away after each cycle by injecting 50 mM NaOH for 60 s.
For studies with immobilized membrane, large unilamellar vesicles (LUVs) were prepared using 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC). Dry lipids were dissolved in a chloroform/methanol mixture (2:1 v/v), the solvent was removed by evaporation under a stream of nitrogen, and resulting cake vacuum-dried for 3 h. The dry lipid cake was re-suspended to a concentration of 1 mM in PBS by spinning the flask at a temperature above the transition temperature. The resulting multilamellar vesicle suspensions were then disrupted by several freeze-thaw cycles prior to extrusion through polycarbonate filters with 100 nm pores using a Mini-Extruder (Avanti Polar Lipids, Inc.). LUVs were used within a day to prepare the sensor chip surface. The surface of an L1 sensor chip was preconditioned with 40 mM of the nonionic detergent octylglucopyranoside (OGP). The DOPC liposomes were immobilized on the sensor surface by injecting three times for 10 min with flowrate of 5μl/min, until about 8000 RU was reached. Subsequently a 10 mM sodium hydroxide solution was injected (30 µL/min for 30 sec) to remove poorly anchored liposomes. BSA at a concentration of 0.1 mg/mL, was then injected at 5 µL/min for 60 sec for complete coverage of the nonspecific binding sites for polypeptides. This procedure results in integral liposomes on the chip surface, without fusion.26 To detect the binding of peptides with the liposomes, solutions with increasing peptide concentrations (0.5 to 16 μM) were injected at a constant flow rate of 10 µL/min for a contact time of 540 s, followed by a dissociation time of 1200 s with PBS buffer, to allow the peptides to return to bulk solution.
Sensorgrams for each peptide-peptide or peptide-liposomes interaction were obtained using BIAevaluation software and then elaborated using GraphPad.
2.7 Statistical analysis
Data obtained from repeated experiments were subjected to computer-assisted analysis using GraphPad Instat 3, and statistical significance was assumed at P ≤ .05 (ANOVA, Student-Newman-Keuls post test); EC50 values were extrapolated by regression-correlation analysis performed by GraphPad Instat 3 from experimental concentration-effect curves (r2 ≥0.9).
3 RESULTS
3.1 Design of the LL-37 analogs and potential effect on salt-bridge formation
The role of salt bridges in inducing LL-37 to fold as a stable helix, driving its oligomerization in aqueous solution, was studied by designing LL-37 analogs in which intramolecular electrostatic interactions (salt bridges) that could potentially stabilize the structure were altered. It is well known that ion pairs of oppositely charged residues spaced four or five positions apart in a helix can significantly stabilize its structure.27-29 The LL-37 variants were rationally designed to affect salt-bridging while causing minimal variations in the primary sequence, and were limited to either a single residue substitution (K15 → G in LL-37G15, see Table 1), to the inversion of a pair of Lys and Glu residues (e.g., K10E11 → E10K11 or E16K18 → K16E18) or to the inversion of both pairs of Lys and Glu residues (K10E11E16K18 → E10K11K16E18). Apart from LL-37(G15), which has a decreased charge and slightly altered hydrophobicity/amphipathicity characteristics, the other three have the same amino acid composition, charge and overall hydrophobicity/amphipathicity as the parent LL-37.
The inversion of Glu and Lys residues alters the possible salt bridges that can be formed with residues up or downstream. A first parameter taken into account was the ratio of oppositely charged residues in the peptides that could result in potential attractions, to that of similarly charged residues that could only result in repulsions (A/R, see Table 1). This is altered in favor of repulsions in the inversion variants, with respect to LL-37, and the overall number of potential attractions is decreased. For LL-37(G15), the A/R ratio is favorable but the overall number of possible attractions is in any case decreased. This preliminary assessment would thus suggest that all analogs could have decreased salt bridging.
Considering what salt-bridges may actually occur, several studies on helical peptides have indicated that the opposite charges on side-chains need to be spaced less than 4Å apart for these to be stabilizing.27, 30, 31 Furthermore, salt-bridges involving certain residues are reported to be more stabilizing than others.30-33 These considerations suggest that it is quite unlikely for all of the possible salt-bridges in LL-37 to occur simultaneously (e.g., a charged residue may not be able to form bridges in both N- and C-terminal directions). To try and assess what bridges could occur, the predicted helical structure of LL-37 and RL-37 were obtained using the Quark server34 (see Figure 1), and inter-charge distances measured using DS Visualizer.
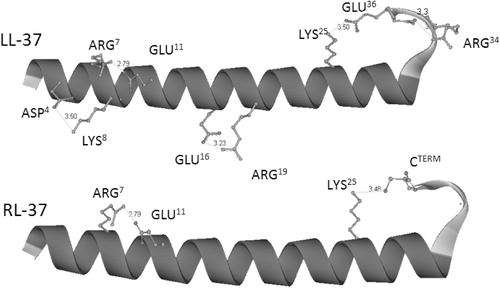
Structures and salt bridging for LL-37 and RL-37. Structures were predicted from the sequence using the Quark server,34 and coordinates visualized using DS Visualizer, which was also used to measure distances between charged atoms. Inly the side-chains of residues potentially involved in salt-bridges (distance < 4Å) are shown
The figure shows how LL-37 can form three salt bridges involving D4↔K8, R7↔E11, and E16↔R19. Furthermore, there is a noncanonical bridge between K25 and E36 involving a C-terminal loop in the peptide. For RL-37, only the R7↔E11 bridge was observed, as well as a non canonical one involving K25 and the C terminus. This study would seem to confirm that residues selected far sequence inversion (E11 and E16) may indeed be involved in stabilizing salt-bridges, while that selected for replacement (K15) may not.
The sequences of all the analogs were also submitted to the Quark server and visualized, and the predicted salt-bridges are shown in supplementary materials Supporting Information Figure S1. LL-37(E10K11) and LL-37(K16E18) each show a disrupted bridge (respectively R7↔E11 and E16↔R19), while in LL-37(E10K11 K16E18) both are disrupted, although formation of a new, noncanonical E18-R19 bridge is observed. As expected, LL-37G15 does not present significant bridge disruption, although the pattern is subtly different from that of LL-37, with a bipartite bridge involving R7. Furthermore, all the analogs show the additional noncanonical bridge involving a residue in the N-terminal part of the helix (either K25 or R29) and E36 in the C-terminal loop or the C-terminus itself. Overall, these models suggest that the dynamic nature of the helix can allow for compensatory salt-bridging to occur if specific salt bridges are disrupted.
The analysis indicates that all three inversion variants LL-37(E10K11), LL-37(K16E18) and LL-37(E10K11 K16E18) could have a destabilized salt bridging pattern that should result in lower propensity to adopt a helical structure in solution, which could in turn affect oligomerization. In other words, they might behave more like F-form peptides. The LL-37(G15) variant could instead maintain a similar structuring propensity to LL-37, although the region in the center of the peptide would be more flexible.
3.2 Structure stability
The secondary structure of the four LL-37 variants was characterized by CD spectroscopy and compared with that of the parent peptide (Figure 2). Spectra were collected in the presence of water, where LL-37 does not adopt a helical structure; in low salt concentration buffer (10 mM SPB, pH 7), where it starts to acquire this conformation, and a in buffer with plasma-like ionic concentrations (PIL), which strongly stabilizes it. They were also measured in the presence of increasing concentrations of TFE (up to 50% in SPB), a solvent known to induce helix formation in peptides.
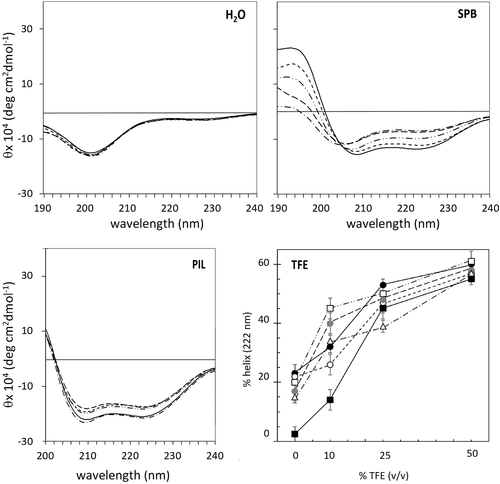
CD spectra of LL-37 and its analogs in different environments. Spectra of LL-37(—), LL-37(E10K11) (—–), LL-37(K16E18) (— — —), LL-37(E10K11 K16E18) (— · — · —), and LL-37(G15) (— ·· — ·· —) in H20, SPB (10 mM phosphate buffer, pH = 7.4) and PIL buffer (130 mM NaCl, 24 mM NaHCO3, 0.6 mM MgCl2, 1.3 mM CaCl2 and 3.9 mM KCl, pH 7.3), and effect of increasing %TFE (v/v) on the helix content of analogs in SPB. %Helix content was determined according to Chen et al.24 from the ellipticity at 222 nm. LL-37 (•), RL-37 (▪), LL-37(G15) (□), LL-37(E10K11) (○), LL-37(K16E18) ( ), LL-37(E10K11 K16E18) (Δ) Peptide concentration was 20 μM. Spectra are the average of two measurements, each averaging four scans
), LL-37(E10K11 K16E18) (Δ) Peptide concentration was 20 μM. Spectra are the average of two measurements, each averaging four scans
All peptides were effectively random coil in pure water, as expected, and showed a very similar helical content in the presence of 50% TFE (see Table 1), suggesting that the slight variations in sequence did not intrinsically affect helix stability in the presence of this strongly helix-inducing solvent. Under these conditions, the determined helix content of ∼ 60% can be considered to be the maximum achievable, consistent with end effects in peptides of this length. The behavior at lower concentrations of TFE (e.g., 10%) is more varied (see Figure 2 and Supporting Information Figure S2 for spectra). The different extent of helix formation and the shape of the spectra suggest that altering the salt bridging pattern may result in different folding pathways. In any case, the analogs behaved more like A-form LL-37 than the F-form RL-37, suggesting either that the residual salt bridging was sufficient to stabilize the helix, or the dynamic secondary structure was capable of forming compensating bridges.
In 10 mM SPB, the CD spectrum for LL-37 indicates a helix content estimated at about 25%, suggesting that the peptide is in equilibrium between coil and helical forms (see Figure 2). The helix content is reduced to 15% for LL-37(K16E18) and LL-37(E10K11K16E18), whereas it is unaltered for LL-37(E10K11) (25%, see Table 1), suggesting that the central E16-R19 bridge in LL-37 is more relevant for structuring. The stability of the LL-37(G15) analog is also slightly decreased. On increasing the concentration of salt ions, in PIL buffer, the structural differences between LL-37 and its analogs are attenuated, as both inter- and intramolecular interactions are favored. Note that RL-37 is still random coil under these conditions.8, 10, 11 Taken together, these data would suggest that intramolecular salt bridges do contribute to helix formation in an aqueous environment, but are not the only relevant factors.
3.3 Surface plasmon resonance studies of peptide self-association
Surface plasmon resonance (SPR) is a useful technique for studying interactions between molecules, or between molecules and supported membranes. We have previously reported the use of a Biacore setup to observe self-assembly of free flowing LL-37 (analyte) with LL-37 molecules anchored on the Biacore sensor surface (ligand).13 Here we report the same experiment, but using its analog LL-37(K16E18) as both analyte and ligand, as it has the least stabilized structure in CD studies.
Peptide molecules were anchored to the carboxydextran moieties covering the surface of a CM5 chip by formation of amide bonds with the N-terminal amines. Anchoring conditions were chosen so that covalently bound peptide molecules were likely to be spread out and monomeric.13 Peptide association was then investigated by flowing a peptide solution over the chip surface, at increasing concentrations (4–64 μM). When increasing concentrations of LL-37 were flowed over the LL-37-modified chip surface under conditions that induce helix formation (HBS-EP buffer, pH 7.4, as verified by CD), a moderate, linear increase in response units was observed up to 16 μM. It then increased sharply, nonlinearly and reversibly, suggesting a high level of reversible aggregation onto the surface-bound LL-37 molecules (Figure 3A). This behavior was not observed under acidic conditions (acetate buffer, pH 5), in which the peptide remains unstructured.13
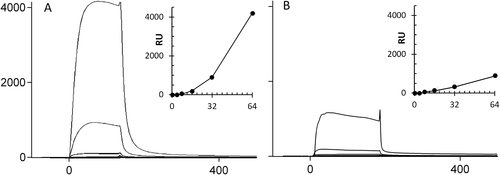
Binding sensorgrams for (A) LL-37 and (B) LL-37(K16E18) injected onto an LL-37-modified and LL-37(K16E18)-modified CM5 chip, respectively. Peptides were immobilized on the sensorchip under conditions that result in well-separated, monomeric anchored peptide molecules. Solutions with increasing peptide concentrations (4, 8, 16, 32, and 64 μM) were then injected under conditions (HBS-EP buffer, pH 7.4) that favor helix formation, as verified by CD. Analyte was injected at 10 μL/min for 180 s followed by a 300 wash and 50 mM NaOH solution was used as regeneration solution before injecting the next higher concentration. The baseline is adjusted to zero for each injection. The insets show the variation of response units with increasing peptide concentration
The sensorgrams obtained by flowing LL-37(K16E18) analyte over an LL-37(K16E18)-modified CM5 chip in HBS-EP buffer are shown for comparison in Figure 3B. Despite having a higher intrinsic loading than LL-37 (RUmax = 200 and 50 respectively), LL-37(K16E18) reaches a signal which is only a quarter that for LL-37 at the highest concentration, suggesting that it does have a decreased capacity for oligomerization. Nonetheless, the trend is the same as for LL-37, so that there is a significant jump in the signal at >16 μM, suggesting that self-aggregation may still occur, in a similar manner to the parent peptide, but to a reduced extent.
3.4 Interaction with model membranes
Sensorgrams for increasing concentrations of the LL-37 analogs flowed onto LUVs immobilized on an L1 chip are shown in Figure 4, with those for LL-37, its covalent dimer CTD12 and RL-37 also shown for comparison. All sensorgrams were obtained under the same conditions, and we chose to use DOPC LUVs as the membranes are neutral, and should not be affected by the peptide's charge or reflect transient electrostatic interactions. The conditions under which the chip was prepared each time, and the phospholipid composition of the LUVs, were such that these were likely to remain intact on anchoring.25
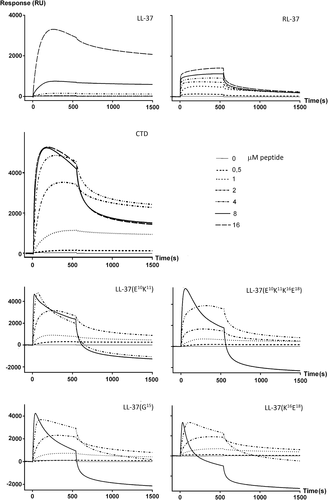
Binding sensorgrams for LL-37, its orthologue RL-37 and analogs to DOPC LUVs. LUVs were immobilized on an L1 sensorchip and peptides were injected at increasing concentrations (see inset legend) at 10 μL/min for 540 s followed by a 1200 s wash with buffer before injecting the next higher concentration. The baseline is adjusted to zero for each injection
The sensorgrams for LL-37 and RL-37 indicate that the two peptides have different modes of interaction with the membrane. Response units increase gradually with concentration for RL-37, reaching a maximum of about 1500 RU, whereas they increase more slowly for LL-37 and then dramatically to over 4000 RU above a threshold concentration of 8 μM. This behavior is similar to that previously observed with both neutral DPPC LUVs, and anionic DPPC/DPPG (4:1) LUVs.13 These results suggest that both peptides can interact with and likely insert into the membrane, but that at higher concentrations only LL-37 undergoes significant self-association at the membrane, or inserts into it as an oligomer. The behavior of CTD would seem to confirm this, as its obligate dimeric structure shifts the threshold for the RU jump to 1 μM, and also results in a decreased off rate, which suggests membrane insertion. At >4 μM, saturation seaems to set in, as the signal no longer increases while the off rate does, suggesting a transient surface binding.
The behavior of the LL-37 analogs did not conform to the behavior of either RL-37 or LL-37, but was in fact somewhat surprising. It seems intermediate to that of the two reference peptides up to about 2 μM, but then the shape of the sensorgram changes dramatically, with a marked loss of RU during both injection and washing, going to negative values. This is consistent with a disruptive effect of the peptides on the LUVs, and consequent loss of material.
3.5 Antibacterial activity of the LL37 analogs
The capacity of the analogs to inhibit bacteria in vitro was evaluated by determining the MIC and the growth inhibition IC50 values against two reference strains (Table 2). A potent antibacterial activity was observed against the gram-negative E. coli in 20% MHB, for all peptides with the exception of the covalently linked dimer CTD (likely due to its decreased capacity to penetrate the bacterial outer membrane12). It was not possible to observe significant differences between the activity of the variants and LL-37 or RL-37 under these conditions.
| E. coli | S. aureus | |||
|---|---|---|---|---|
| (μM) | (μM) | |||
| Peptide | MIC | aIC50 | MIC | aIC50 |
| LL-37 | 2 | 0.3 ( 1.9 )b | 8 | 3.6 |
| RL-37 | 1 | 0.34 ( 1.5 ) | 1 | 0.7 |
| LL-37(K16E18) | 1 | 0.38 ( 0.9 ) | 4 | 2.2 |
| LL-37(E10K11) | 2 | 0.37 ( 1.4 ) | 8 | 3.7 |
| LL-37(E10K11K16E18) | 2 | 0.32 (1.3 ) | 8 | 3.0 |
| LL-37(G15) | 1 | 0.43 ( 1.6) | 8 | 5.8 |
| CTDc | 8 | 1.5 | >16 | 4.2 |
- a IC50 is the peptide concentration causing 50% bacterial growth inhibition at 210 min in 20% MHB, extrapolated from the inhibition curves shown in Supporting Information Figure S3A.
- b IC50 values in parentheses are derived from the growth inhibition curves shown in Supporting Information Figure S4, and performed in 100% MHB.
- c C-terminal covalent dimer of LL-37.12
The effect on bacterial growth kinetics is a more sensitive method than MIC determination to observe activity differences. Furthermore, the antimicrobial activity of LL-37 is known to depend strongly on medium interactions, so that the difference in activity with respect to RL-37 is more marked in full medium.11 For this reason, the IC50 values for inhibition of bacterial growth kinetics was determined for both 20% and 100% MHB (values in parentheses, Table 2). Under these conditions, variants were more active than LL-37, and activity was comparable to that of RL-37. This indicates that the medium sensitivity of LL-37 depends quite specifically on its structural characteristics.
The antimicrobial activity is more diversified against the Gram-positive S. aureus, with RL-37 showing a significantly more potent activity than LL-37 and its analogs, while CTD is effectively inactive, even in 20% MHB. It has been proposed that the A-form of LL-37 reduces its capacity to reach the bacterial membrane through peptidoglycan layer, and this is particularly evident for the obligate dimer CTD.11, 12 Among the analogs, only LL-37(K16E18) showed a slightly improved MIC value with respect to LL-37, and a correspondingly lower IC50 value for S. aureus growth inhibition. The other analogs show a similar activity to that of LL-37, or a reduced activity in the case of LL-37(G15) (likely due to its lower charge of +5). These results suggests that while limited disruption of salt bridging in LL-37 can affect some of its functional characteristics as an A-form peptide (i.e., with respect to E. coli), it does not result in a complete switch to an F-form type of function.
3.6 Bacterial membrane permeabilization by LL-37 analogs
S. aureus cells were exposed to increasing peptide concentrations in the presence of propidium iodide (PI), to determine the capacity of these to permeabilize the bacterial membrane. The concentration dependent effects of RL-37, LL-37, and CTD are shown in Figure 5A, and RL-37 is more potent at permeabilizing the membrane than LL-37, with 3-fold lower PI50 (concentration at which 50% of cells are PI+). CTD turns out to be the most potent in permeabilizing the bacterial membrane, with a PI50 < 0.5 mM, apparently in contrast with its low antimicrobial potency. From this, one may deduce that it can cause membrane permeabilization at quite low concentrations, but that it is not sufficient to inactivate the bacterium, or that it is somehow able to recover, until it is overwhelmed at the significantly higher peptide concentrations required to overcome the sequestering effect of the peptidoglycan layer.
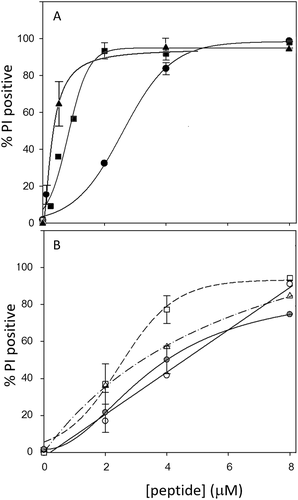
Permeabilization of S. aureus cells to propidium iodide. S. aureus ATCC 25923 cells were treated for 30 min with increasing concentrations of peptides in the presence of 10 μg/mL propidium iodide (PI) before analysis on the flow cytometer. A) LL-37 (—•—), RL-37 (—▪—), CTD (—▴—); B) LL-37(E10K11) (—○—), LL-37(K16E18) (-- --), LL-37(E10K11 K16E18) (—· -Δ-·—), and LL-37(G15) (—- -□- —). %PI positive is the number of PI+ cells with respect to total (10,000 events)
--), LL-37(E10K11 K16E18) (—· -Δ-·—), and LL-37(G15) (—- -□- —). %PI positive is the number of PI+ cells with respect to total (10,000 events)
Permeabilization of the S. aureus membrane to PI by the LL-37 analogs is shown in Figure 5B, and while LL-37(G15) behaves similarly to LL-37, the concentration dependence of permeabilization for the three inversion analogs is different to both that of LL-37 or RL-37.
3.7 Analysis of bacterial membrane lesions using fluorescent probes
It has previously been possible to distinguish the type of lesion caused by LL-37 and RL-37 on model membranes using atomic force microscopy (AFM), which suggested that LL-37 formed discrete pores while RL-37 a more heterogeneous membrane disruption.10 In later studies, a flow cytometric method showed that LL-37 permeabilized the membrane less efficiently to PI than RL-37, but allowed passage of fluorescently labeled dextran particles of up to 40 kDA (9 nm) at higher concentrations, while RL-37 did not, irrespective of its concentration.12, 13 This is consistent with the formation of relatively large, discrete pores by LL-37, and more widespread but smaller heterogeneous lesions for RL-37, in accordance with a carpet-like detergent effect, and in agreement with the AFM experiments.
A similar experiment was performed with the LL-37 analogs, in comparison to RL-37, LL-37, and CTD, using all the peptides at the same concentrations (2–8 μm) and fluorescent probes of several different sizes, ranging from 1.6 nm diameter (PI) to 9 nm diameter (FITC-dextrans) (see Figure 6). The experiment confirmed that RL-37 only forms small lesions, allowing efficient entry of PI (populated LRQ, see Figure 6B) but not of larger probes (≥ 2.8 nm). This was observed not only at 2 μM peptide (not shown), but also at the relatively high peptide concentration of 8 μM (see Figure 6B). LL-37, on the other hand, permeabilized significantly less to PI at 2 μM (not shown), but at higher concentrations allowed efficient entry of both PI and FD40 dextran probe (populated URQ, see Figure 6C). With respect to the LL-37 analogs, the effect appears different to that of either LL-37 or RL-37. LL-37(G15) and (E10K11) showed a behavior resembling that of LL-37 (Figure 6E,F), although they are less efficient in permeabilising to the larger FD40 probe. LL-37(K16E18) and (E10K11K16E18) behave more like of RL-37 (Figure 6G,H).
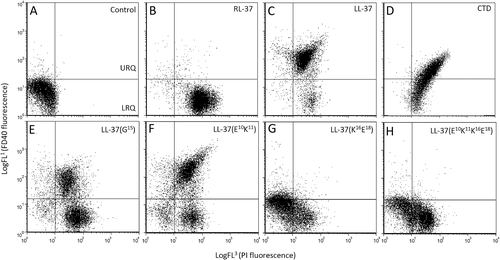
Biparametric dot plots for S. aureus exposed to RL-37, LL-37 and its analogs. Permeabilization was measured using two fluorescent probes of different size (propidium iodide and FITC-dextran 40 kD). S. aureus cells were treated with 8 μM peptides for 30 min in presence of PI (1.6 nm, FL3) and FD40 (40 kD, 9 nm, FL1). All control cells (A) are in the lower left quadrant [LLQ, double negative PI(-)/FD40(-)]. After RL-37 treatment (B), cells populate only the LRQ (PI+ only). For LL-37 and CTD treated cells, they populate mostly the URQ, so are double positive [PI(+)/FD40(+)]. LL-37 analogs (E-H) behave differently to both LL-37 and RL-37
Permeabilization to probes of different sizes, at the intermediate peptide concentration of 4 μM, is summarized in Table 3. RL-37 only permeabilizes to the smaller PI probe. LL-37 permeabilizes with decreasing efficiency up to a probe size of 9 nm, while the obligate dimer CTD permeabilizes efficiently to all probe sizes. All variants are less efficient than either RL-37 or LL-37 in permeabilizing to PI, but some are more efficient than RL-37 in permeabilising to the 2.8 nm probe.
| % positive cellsa | |||||
|---|---|---|---|---|---|
| Probesb | PI 1.6 nm Ø |
FD-4 2.8 nm Ø |
FD-10 4.6 nm Ø |
FD-20 6.6 nm Ø |
FD-40 9 nm Ø |
| RL-37 | 90 | 3 | <1 | <1 | <1 |
| LL-37 | 83 | 60 | 31 | 20 | 35 |
| CTD | 93 | 93 | 91 | 82 | 79 |
| LL-37(K16E18) | 58 | 9 | <1 | <1 | <1 |
| LL-37(E10K11) | 42 | 21 | 5 | 3 | 6 |
| LL-37 (E10K11 K16E18) | 57 | <1 | <1 | <1 | <1 |
| LL-37(G15) | 77 | 9 | <1 | <1 | <1 |
- a Flow cytometric analysis of S. aureus cells treated with 4 μM peptide for 30 min in 20% MHB; values represent the % cells positive for fluorescent probes.
- b Propidium iodide or FITC-labeled dextran of 4, 10, 20, and 40 kDa (FD-4 to FD-40, respectively), with the indicated diameters. Results are the mean from at least two experiments with SEM of < ± 4.
4 DISCUSSION
We have previously reported that LL-37 and its macaque orthologue have different propensities for helix formation and oligomerization in aqueous media, which lead to different types of interaction within the bacterial membrane, and different modes of damage induction.8, 10-13 This has led to paradigms for the mode of action of A-form and F-form peptides on the bacterial membrane; A-form peptides act via toroidal pore formation, F-form peptides by promoting smaller and more heterogeneous lesions. Other types of helical AMPs have also been reported to interact in different manners with bacterial membranes, resulting in different types of lesions. In particular, magainin 2 forms toroidal pores with 2–3 nm diameter,35 while melittin acts via a carpet mechanism leading to a less well defined, detergent-like disruption of the membrane.36
We have designed analogs of LL-37 (Table 1) to probe the role played by intramolecular salt-bridges in stabilizing the helical conformation of this A-form peptide and in determining its peculiar mode of pore formation. These were designed so that minimal sequence alterations might disrupt salt-bridging to different extents, without altering other functionally important parameters. Modeling of the structures indicated that most of the residues chosen for these alterations were likely to be involved in salt bridging. The effect on conformational stability was then assessed using CD spectroscopy in different environments, and results were quite revealing. Despite disruption of at least one salt-bridge in three analogs, these were still capable of adopting a helical structure, to some extent, in the presence of salt ions. This may indicate that helical structuring of LL-37 depends also on other types of interactions (e.g., H-bonding), and/or that it can somehow compensate for the loss of salt-bridges by forming new ones, with its dynamic structure. Furthermore, intermolecular electrostatic, H-bonding and hydrophobic interactions may be acting concurrently to favor both helix formation and oligomerization, and these may not have been affected by sequence modifications. While it was apparent that the central E16-R19 salt-bridge has a more relevant role in stabilizing the helix, we can conclude that that is not possible to switch the behavior of LL-37 from that of an A-form to an F-form peptide by limited disruption of the intramolecular salt-bridging pattern.
This was confirmed by SPR in an oligomerization experiment. Free flowing LL-37 formed high order oligomers with monomeric, chip-anchored peptide, and also aggregated strongly on the surface of immobilized DOPC LUVs. Free-flowing LL-37(K16E18) had a significantly decreased capacity to oligomerize with monomeric anchored peptide, but it was not completely abrogated. With respect to membrane binding, LL-37 analogs showed a behavior intermediate to RL-37 and LL-37 at lower concentrations, but unlike these, disrupted LUVs at higher concentrations. This suggests they switch to a lysis mechanism that is different to that of either the A-form or F-form peptides. Interestingly, the fact that RL-37 does not cause material loss in SPR experiments with LUVs indicates that its particular mode of membrane interaction does not involve micellization. The LL-37 analogs instead may switch to a mechanism causing this type of damage, at higher concentrations.
These conclusions are supported by studies using fluorescent probes of different sizes. At lower LL-37 concentrations (< 2 μM), only a minor percentage of bacterial cells are permeabilized to PI, but increasing the concentration (> 4 μM) cells become completely permeable to PI and also substantially to fluorescent probes up to 9 nm. RL-37 is very efficient at permeabilizing bacterial cells to PI but entry of larger probes was never observed, irrespective of concentration or exposure time. In agreement with SPR, this precludes micellization as it would result in the formation of broad lesions and allow entry of larger probe molecules. Results are more consistent with small and profuse lesions for RL-37, observed also in supported model membranes by AFM10. LL-37 also does not cause material loss in SPR experiments, so that entry of the larger probe particles are not due to micellization. Rather, results are consistent with formation of larger pores or lesions, consistent with the discreet lesions observed by AFM10.
With respect to the LL-37 analogs, only the G15 point mutant and E10K11 inversion analog result in permeabilization to both PI and the larger FITC-Dextran probes, at the highest tested concentration. This is consistent with a more relevant role of the E16-R19 salt-bridge in determining the structure/function characteristics of LL-37, as reflected also in the antimicrobial activity (see Table 2). The concentration dependence of permeabilization is however different for all the analogs to either LL-37 or RL-37 (see Table 3 and Figure 5). In general, the analogs would appear to have lost the pore-forming capacity of LL-37, without having gained the carpet forming capacity of RL-37, suggesting they switch to a mode of action that is dissimilar to either.
5 CONCLUSIONS
Our studies with minimal sequence variations in the key human host defense peptide LL-37 suggest that its primary structure has been tuned by evolution to provide it with the precise structural characteristics it needs to carry out its pleiotropic roles immunity and healing, which are different to those of the primate orthologue RL-37. It does not appear to be facile to engineer the switch from one type of behavior to the other, as they likely depend on the complex interplay of a network of intra- and intermolecular interactions. We have shown that it is possible to combine a simple method for identifying potential electrostatic interactions with a method for predicting their presence in a model structure, to guide minimal sequence modifications that can selectively knock out specific salt bridges. This permits to begin assessing their role, independently of other functionally important parameters. We have started to do this at three positions and have identified another two (bridges centered on Asp4 or on the C-terminal loop) that can be probed in future studies. These salt-bridges can be knocked out either sequentially or cumulatively, and it will be interesting to determine if it is possible to eventually hit on a combination that will allow for a switch from A-form to F-form behavior. The use of such rational, minimal sequence variations will in any case provide useful information on key structural and functional aspects of an important human innate defense effector, and may help in the design of therapeutically useful variants in the future.
ORCID
Alessandro Tossi http://orcid.org/0000-0001-9087-5764