Peptides as probes for food authentication
Abstract
The aim of this review is to provide a brief summary of the recent applications of peptide molecules in the food area. Since, in the last few years, there has been growing interest in food authenticity, the role of peptides in this area has assumed greater importance. A fundamental part, as discussed in the article, was played by the growth of innovative analytical techniques, based on mass spectrometry (MS), which allows identifying and quantifying protein fragments in complex mixtures of different components. These procedures have been successfully applied for the identification of peptide markers in food authenticity. Because food-derived proteins have high variability in chemical and physical properties, it is obvious that an exhaustive proteomic analysis cannot be applied by using a single technique for all objectives. The report shows some examples of various methodologies applied to different food matrices and some trivial aspects are also critically described.
Graphical Abstract
1 INTRODUCTION
Food authentication is a rapidly growing research field due to the development of the knowledge concerning food quality and safety. Authenticity refers to the adherence, during food production, to specific ingredients, specific origins of production, or specific technologies.
The claim of specific quality issues in high-value food products is particularly interesting, since these goods are often goals of fraudulent labeling. Therefore, for food safety, food quality, and consumer assurance, the traceability is an important topic, as well as the conformity with national and international regulations.1
Among food authenticity issues, the authenticity related to the animal or vegetal species is particularly relevant, with high economic relevance.2 Wines obtained from selected grape varieties, cheeses made from the milk of particular mammalian species (like goat or water buffalo), and olive oils obtained from specific olive varieties are just a few examples of specific quality parameters associated with the authenticity of species. The consumers also perceive “pure” species products as having superior quality, which often translates into increased market prices. Close to that, in some cases, the presence of other species, different than those declared on the label, hides more serious threats, such as in the case of hidden, not declared, allergenic ingredients. Indeed, as a matter of fact, also declaring the absence of allergenic ingredients is a case of species authenticity.
Therefore, the authentication of food labeling claims must be guaranteed and accurate, and reliable analytical methods should be developed for testing the food ingredients and checking their quality according to purchaser's demand and to the claims of the seller.3
2 TRADITIONAL ANALYTICAL TECHNIQUES USED TO ASSESS FOOD AUTHENTICITY
Typical methods used to assess species authenticity in foods are based on the screening of genetic differences among species and sometimes even among varieties of the same species, detected at the level of DNA or protein. DNA assays (such as RAPD, RFLP, or the most recent Barcode Reading, just to name a few)4, 5 are indeed very specific, providing a clearly identified DNA sequence, which gives enough inter-species variability and intra-species stability. The common problem of DNA assays is that DNA molecules are easily degraded or modified by the technological processing taking place in food production, yielding often unclear or inconsistent results. Close to that, quantification of different species present in mixtures is also difficult, since DNA has to be extracted and amplified by Polymerase Chain Reaction (PCR). As a result, the final DNA amount is only loosely related to the initial amount. For this reason, accurate DNA quantitation requires the use of specific techniques, such as Real-Time PCR. Even in this case, quantification of DNA does not immediately translate in the quantification of the undeclared ingredient, since DNA content of animal or vegetal tissues is variable.
Proteins carry the same level of information as DNA, since protein sequences are genetically determined. The advantage of addressing protein testing rather than DNA is that, in many foods, proteins are much more abundant than DNA, making the tests somehow simpler. Protein assays are typically carried out by immune enzymatic methods, where specific antibodies recognize specific protein sequences. Examples of such an approach are ELISA tests, which are also quantitative methods, or the use of lateral flow devices.6 These methods have the great advantage of being very user-friendly and much easier to carry out than DNA testing. Anyway, these approaches strongly rely on the robustness of the chosen antibodies in selectively recognizing a species-specific protein, a parameter often overlooked. Besides antibody performance, this recognition process is easily interfered by the food matrix and by the technological treatments applied to food, which might modify protein structure, thus altering the final results. False positives and false negatives are indeed very common with these methodologies, requiring continuous cross-checks and calibrations.
3 THE ADVENT OF PEPTIDE PROBES AS A VALUABLE TOOL IN ANALYTICAL TECHNIQUES
In the last years, a third alternative approach for species authentication in food started to be more and more applied. First in the scientific literature, and then rapidly expanded to the analytical laboratories. The approach relies on the analysis of specific short peptide sequences derived from the proteins contained in the food. These peptide sequences are analyzed by liquid chromatography coupled to mass spectrometry systems, which provide a very robust method for their separation and clear-cut identification at the level of the amino acidic sequence. Peptides are usually created by enzymatic digestion of the proteins extracted from food. Anyway, in some food matrices having very intense proteolysis related to the food production process (i.e., cheeses), peptides are naturally very abundant, thus the method can be further simplified by directly extracting the naturally occurring peptides (Figure 1).
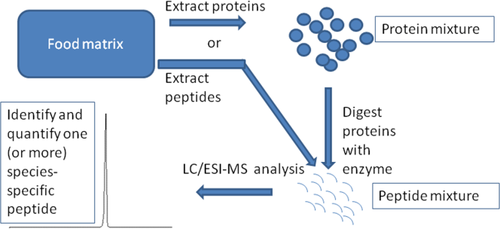
Species authentication in food through peptide analysis by LC/ESI-MS. Peptides might be generated by enzymatic digestion of the extracted proteins or, in peptide-rich foods, they can be directly extracted from the food matrix
This approach carries the same information as the previous one based on protein analysis, but with the advantage that peptides are much easier analyzed than proteins, and they can be identified and quantified in a very robust way by LC/ESI-MS. Moreover, allowing the analysis of single distinct peptide sequences, the analyst can focus only on the sequences that actually carry useful information. Indeed, when determining the authenticity in closely related species (such as in cheeses made from cow or water buffalo milk), homologous proteins from the two species carry only a few different amino acids among hundreds, and immune enzymatic tests might give erroneous results more easily. In peptide analysis, many peptides will actually be useless, having exactly the same sequences in the two species. But few of them will actually be very meaningful and much easier to discriminate, since the single different amino acid will not be anymore among hundreds of identical amino acids but only in a short stretch of few of them.
Even simple, relatively less expensive, single quadrupole instruments allow easy identification of the peptides by single ion monitoring (SIM) analysis (particularly if the target peptides are part of proteins that are particularly abundant in the food matrix, such as caseins in cheeses or muscular proteins in meat), but peptides can also be analyzed by means of multiple stage MS instruments (e.g., triple quadrupole, Q-ToF, and others), which provide a more robust identification of the target peptides if also present in trace amounts by increasing both sensitivity and specificity of the detection.
To generate the peptides to be analyzed, in the case enzymatic digestion is needed after the extraction, any enzyme in principle will work on the extracted proteins, but trypsin is by far the most used one for several reasons. Trypsin cleaves in a very specific way after positively charged residues (lysine and arginine), which are more or less equally distributed in many proteins, in this way generating peptides having similar dimensions. This avoids sensitivity distortion effects, in MS detection, related to the different mass and size of very diverse peptides. Moreover, it ensures that every peptide has one single positively charged amino acid, which also helps to maintain homogeneous ionization and the same sensitivity for all peptides during MS detection (which is usually in the positive mode).
In some food matrices such as cheese, as said above, peptides are spontaneously generated by the natural proteolytic process. The advantage is that peptides can be directly extracted but, being the enzymatic process not anymore under control of the analyst, peptides can have any charge and any length, thus differing greatly in the sensitivity of MS detection.
The analytical techniques described up to here fall in a research field named proteomics or more specifically peptidomics.
Peptidomic analyses are based on the use of highly efficient chromatographic techniques or polyacrylamide gel electrophoresis (2D-PAGE) combined with enzymatic digestion methods, followed by MS analysis in order to obtain data which will be processed by specific software to get peptide sequence identification.7
Given the high variability of the food-derived proteins and considering their differences in chemical and physical properties, it is evident that a comprehensive proteomic exploration does not consist in the application of a single technique for all purposes. Rather, proteomic studies use multiple technologies aimed to improve proteome resolution and coverage and to provide complementary results.
In the following sections, some examples of various methodologies applied to different food matrices, are shown and critically described.
4 DAIRY PRODUCTS
Cheeses are a particularly important food commodity as far as authenticity is concerned. Many kinds of cheese, particularly in Southern Europe, carry a Protected Designation of Origin (PDO) and many of them have to be made starting from the milk of specific species different from the cow: sheep, goat, and water buffalo.8 Being the milk of these species usually more expensive than cow's milk, frauds might take place partly substituting the more expensive milk with the cow's milk. During cheese production, milk proteins (mostly caseins) are degraded by the lactic acid bacteria and by the rennet used in the manufacturing, generating complicated mixtures of peptides. For detecting milk substitution, a simple qualitative approach is needed. Because the cow's milk does not have to be present, the identification of a peptide derived from cow's milk proteins is enough to spot the fraud. Several examples have been reported by our group in mixtures of peptides extracted from supposedly 100% sheep cheeses taken from the market.9, 10
Liquid chromatography/mass spectrometry (LC/MS) technique has been applied to detect two peptides produced during the proteolytic process (fragments 1–14 and 1–23 from αS1 casein), which show some amino acid differences in sheep and cows. This method could be successfully applied for detecting peptides deriving from cow's caseins in very low concentrations and to detect the presence of undeclared cow's milk.
More recently, proteomics-based methods have also been developed for the qualitative and the quantitative determination of cow, sheep, and goat milk in raw products.
In particular, Chen et al.,11 have shown the development of a quantification method of goat/sheep milk (GSM) and cow milk (CM) in the major raw material of dairy products, milk powder and whey protein powder.
The UPLC-MS-TOF method set up together with tryptic digestion allowed identifying two peptides which could be used as markers for assessing the presence of GSM or CM. In particular, the two peptide sequences, derived from β-lactoglobulin, were LSFNPTQLEEQCHI in CM and LAFNPTQLEGQCHV in GSM, and they differ for three points of mutation. The corresponding isotope labeled sequences were synthesized and used for performing the quantification step. The proteomic method was compared with a commercial ELISA kit, which could only indicate the presence of CM in the sample and not prove the presence of GSM.
This method seems to be very robust and promising for future applications in the field of fraud, since the two peptides used as markers are very efficient in discriminating the two animal origins (Figure 2).
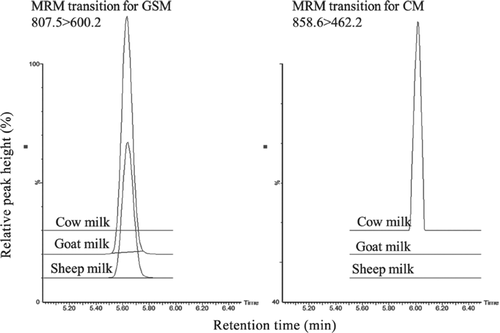
Specific selectivity test of the pure cow, goat, and sheep milk in 2 multiple reaction monitoring (MRM) channels. CM = cow milk; GSM = goat and sheep milk. (Reproduced from Chen et al. Journal of Dairy Science 2016 with permission from Elsevier, License Number: 4210720765300) (Ref. 11)
With the aim of fitting the request of the dairy producers and consumers for minimizing raw material (milk) adulterations, Arena et al.12 promoted a very efficient MALDI-TOF-MS method that tests milk quality through the identification of specific peptide markers. The analysis of fresh and frozen buffalo milk was performed for this purpose; a robust statistical processing of the obtained data allowed characterizing different milk samples.
Interestingly among the 28 peptide sequences identified, Glycosylation-dependent cell adhesion molecule 1 (GLYCAM1) phosphorylated peptide (1–53) was found and characterized here for the first time in milk, and it could be considered a marker of adulteration by frozen milk. In particular, its increasing formation in frozen samples was attributed to an unknown protease stable at low temperatures.
This work can be considered a good example of a MALDI-TOF-MS platform for a polypeptide profiling for a high-quality food characterization.
Recently a work of Ebener et al.13 showed how the peptide fingerprint of different milk samples can reflect milk processing conditions. Usually, in order to preserve microbiological safety and stability, raw milk undergoes heat treatment before consumption.
To achieve this task, some technological processes are currently used, which can be classified according to the specific thermal treatment applied-pasteurization techniques and high temperature short-time (HTST) pasteurization, which can be classified as mild, ultra-high temperature (UHT) treatment, which is more rigorous; and recently extended shelf life (ESL) milk has been brought into the market.
ESL milk should combine low heat treatment and the taste of pasteurized milk with prolonged shelf life. Because there is not a law regulating this milk type at the moment, various heating techniques are used for the production. Heating conditions can negatively alter nutritional properties of milk, by reducing the availability of vitamins and essential amino acids. Concerning amino acids moieties, they could be degraded after the Maillard reaction with lactose, which mostly happens on lysine side chains.14
A MALDI-TOF-MS method was used to monitor the trend of several native peptides and it was found that their relative quantities change as a function of the heat load.
In particular, MALDI-TOF peptide profiles of different commercial milk products showed 16 peptides that presented highly significant intensity differences (increase or decrease) between pasteurized milk and UHT milk. Microfiltered ESL milk behaved in the same way as the corresponding HTST pasteurized milk, while high-temperature ESL milk was different from both pasteurized and UHT milk. By performing different heating and storage experiments, it was demonstrated that the presence of these 16 peptides was influenced either by heating or storage. Interestingly, relevant proteolytic activity was observed during storage.
The work shows a combination of C18-stage tip extraction and MALDI-TOF-MS analysis as a possible method for high sample throughput by the fast generation of peptide fingerprints of different samples.
In this work, as in the previous one, MALDI-TOF-MS is used for a quantitative analysis. The technical limitations of MALDI measurements are well known.
However, Arena et al., to overcome these limitations, shows a comparison with quantitative LC results, which were in perfect agreement with deduced MALDI-TOF-MS polypeptide profiling.
5 MEAT
The high commercial value of meat and its derived products makes them very susceptible to food fraud. In fact, the Food Fraud Network (FFN) activity report15 found that meat was the most counterfeit product in 2014, followed by fish and honey (other high added value products). Most of the violations were ascribable to labeling noncompliance (25%), false documentation (22%), and substitution (17%). In addition to food security issues, a truthful label is necessary for the consumer, for a conscious choice (e.g., Halal or Kosher food products). Given the increasing awareness of the risk of food fraud in the meat sector, especially after the horse meat scandal of 2013, huge efforts were made in the research for robust, reliable, and accurate analytical methods for meat authenticity assessment.
After the European scandal of 2013, it has been somehow natural that most of the researchers went in the direction of horse marker peptides identification. A range of heat stable marker peptides for horses (mainly from myosin and myoglobin) were identified in processed food. Moreover, a database of potential peptide markers for nine animal species of food interest was constructed by comparing the amino acid sequence of the most heat-resistant proteins, allowing a semi-targeted approach.16 The limit of detection (LOD) of the method was determined for horse meat, and it was equal to 0.5% (w/w) in processed foodstuff. Specific peptides for horse, beef, and pork meat (troponin and myosin, mainly) were identified to detect fraudulent addition of beef and pork meat in a wide variety of raw and processed products, with very low limits of detection (0.13-0.24% w/w).17, 18 Peptide fingerprint coupled with statistical analysis (such as OPLS-DA) has been successfully used to differentiate beef, horse, pork, chicken, and turkey both in raw and cooked meat mixtures.19-21 In processed products, protein aggregation can lead to a limited extraction and digestion efficiency, thus, microwave treatment can be used to assist the tryptic digestion, enhancing sample solubilization.22 However, some reproducibility issues make it inadequate for quantitative purposes. Heat stable peptide markers were also identified for chicken, duck, and goose, allowing their label-free relative quantification with the LC-QTOF-MS/MS technique, down to 1% (w/w).23 Relative quantification of meat species in binary mixture is achievable by means of “corresponding protein, corresponding peptide”24- homologues proteins of different species (e.g., myoglobin) will share almost identical sequences, with the exception of some amino acid mutations that are typical for a certain species. After tryptic digestion, these amino acid mutations will result in a number of different peptides, which can be used as markers. Moreover, the sequence similarity allows analogue extraction, proteolysis, and detection behavior. It follows that the relative abundance of these peptide markers reflects the relative abundance of the two species. Myoglobin was exploited for the identification and the relative quantification of beef, horse, pork, and lamb, down to 1% w/w.25 In absence of a clear regulation, the threshold of 1% is conventionally taken as the watershed between an involuntary contamination and a fraudulent activity. Pork meat consumption is forbidden in several religions (e.g., Judaism or Islam), so particular attention was also paid to detect the voluntary or involuntary addition or contamination of foodstuffs with pork meat. Porcine specific peptide markers (from serum albumin and lactate dehydrogenase) were identified to detect pork contamination in processed meat samples of beef, chevron, and chicken.26
With the banning of processed animal proteins after the bovine spongiform encephalopathy outbreak, the identification of peptide markers has become an important tool for the assessment of their fraudulent addition to animal feed. For this purpose, several species-specific peptides were identified for bovine, porcine and ovine proteins, when added in feed at concentrations higher than 5% (w/w).27
Collagen is the most abundant protein in bones, skin, and connective tissues, and it is ubiquitous in the animal kingdom. Its compact helical structure, conferred by the numerous proline and hydroxyl proline residues, makes it very stable over time and resistant to high temperature and/or pressure treatments. Thus, collagen tryptic peptides have been widely studied as potential biomarkers in archaeological remains, feed, and food using both peptide mass fingerprint (PMF) or LC-based methods.28 In food products, collagen is present as a natural constituent of meat (connective tissue) or for the addition of its main derived product, gelatin. Gelatin is a thickener agent produced from the by-products of the bovine, porcine, and fish industry through severe heat treatment followed by acid or alkaline extraction. These conditions are deleterious to DNA and proteins. However, collagen's structure makes it quite resistant to degradation and intact marker peptides can be identified. More specifically, 14 marker peptides for bovine collagen and five peptides for porcine collagen were identified, allowing also the identification of post-translational modifications such as hydroxyl-proline.29 The developed method allowed screening several gelatins present in the Western Europe market, and two of them were found to contain additional species to those declared on the label. Peptide markers from collagen were also used to develop a quantitative LC-MS method for the determination of beef and pork meat in Bolognese sauce, giving a good linearity and accuracy of the method.30 The particular innovation of this study is that the method here developed allows to quantify beef and pork meat in a complex and highly processed food matrix.
A brief scheme of the methodology applied is shown in Figure 3. Determining the amount of each meat species allows the discrimination between simple contamination and voluntary fraud and enables us to monitor the label compliance of mixed meat products. A schematic summary of available literature about meat authentication through species-specific marker peptides is provided in Table 1. Meat is by far the food matrix for which the greatest efforts have been made in terms of LC-MS methods development. The limit of detection of these peptidomic methods greatly depend on the type of meat and, even more, on the harshness of the conditions encountered during food processing. Limits of detection of LC-MS methods are comprised in the range 0.1–5%, fully comparable with those achievable using PCR (0.1–5%)31 and a bit higher than those of ELISA (0.05-0.5%)6 However, the different nature of the sample tested (and in particular the different degree of processing and thermal treatment) prevents direct comparisons among the different techniques. For what concerns time and costs, liquid chromatography coupled to mass spectrometry is slightly more expensive than PCR and ELISA, costing on average about 8$ for a sample,32 against the 2–5$ of PCR33 and the 4–6$ of ELISA.34 These higher costs probably depend on the absence of “ready to use” kits, as commercially available for genetic and immune enzymatic assays. The time required for the analysis can vary on the basis of the food matrix, being mostly ascribable to the extraction (and eventually purification) of DNA or proteins, but normally falls within the range of 2–3 hr for ELISA34 and 3–4.5 hr for RT-PCR.33 Longer times are usually required for LC-MS, with analysis lasting for at least 5 hr.32 However, despite these drawbacks, LC-MS usually shows its strengths when working on heavily processed food products, where DNA is often degraded and antibody recognition could be hampered.
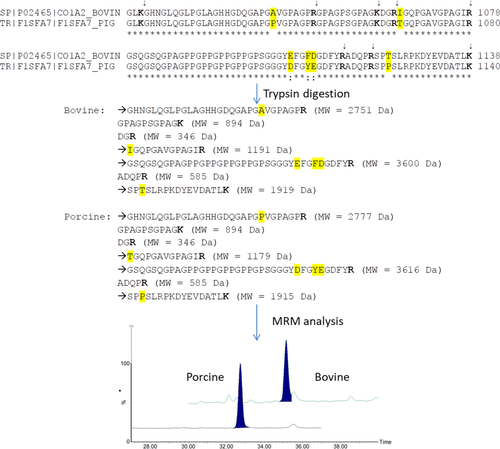
Brief scheme of the quantitative LC-MS method set up for the determination of beef and pork meat in Bolognese sauce (Ref. 30)
| Species | Food matrix | Protein | Examples of peptide markers | LOD (w/w) | Reference |
|---|---|---|---|---|---|
| Equine | Corned meat | Myosin, myoglobin | Equine: TLALLFSGPASADAEAGGK; Bovine: TLALLFSGPASGEAEGGPK | 0.5% | 16 |
| Equine, bovine, porcine | Raw meat | Troponin, myosin, myoglobin, pyruvate kinase, haemoglobin | Equine, porcine: YDIINLR; [Bovine: YDITNLR] | 0.13% | 17 |
| Equine, bovine, porcine | Meatballs, meatloaf, corned meat, salami, Bolognese sauce, hamburger, sausages | Troponin, myosin | Porcine: TLAFLFAER; [Bovine: TLAFLFAGG]; [Equine: TLALLFSGP] | 0.24% | 18 |
| Equine, bovine, porcine, chicken, turkey | Raw meat | Myosin, actin, myoglobin, troponin | – | ND | 19 |
| Equine, bovine, porcine, chicken, turkey | Cooked meat | Myosin, troponin, myoglobin | Equine: ALGTNPTNAEIK; Turkey: ALGQNPTNAEMNK | 5–10% | 20 |
| Equine, bovine, porcine, chicken, turkey | Ham, spreads, corned meat, sausages, frankfurters | Myosin, myoglobin, GAPDH, enolase | Porcine: TLAFLFSGAQTGEAEAGGTK; Bovine: TLAFLFSGTPTGDSEASGGTK | ND | 21 |
| Equine, bovine, porcine, chicken, turkey | Cooked meat, sausages | Myosin, myoglobin | Bovine: HPSDFGADAQAAMSK; [Porcine: HPGDFGADAQGAMSK]; [Equine: HPGDFGADAQGAMTK]; [Chicken: HAADFGADSQGAMKK]; [Turkey: HAADFGADSQAAMKK] | ND | 22 |
| Chicken, duck, goose | Frankfurters, sausages, smoked and cooked meat | Pyruvate kinase, ATPase, Myosin binding protein, troponin, enolase, Haemoglobin, apolipoprotein | Chicken: LDVPISGEPAPTVTWK; Turkey: LEIPITGEPTPKVMWSK; Duck: LEIPITGEPTPKVMWSK; Bovine: MDVSITGEPRPVATWMK; Porcine: LDVSITGEPPPVATWMK | 0.8-1.0% | 23 |
| Bovine, equine | ND | Myoglobin | Equine: HPGDFGADAQGAMTK; [Bovine: HPSDFGADAQAAMSK] | 1% | 24 |
| Bovine, porcine, equine, ovine | Raw meat | Myoglobin | Horse: VEADIAGHGQEVLIR; Bovine, porcine, ovine: VEADVAGHGQEVLIR | 1% | 25 |
| Porcine | Cooked, sterilized and raw meat | Lactate dehydrogenase, serum albumin, creatine kinase | Porcine: LVVITAGAR; Bovine, ovine: LVIITAGAR; Chicken: LVIVTAGAR | ND | 26 |
| Bovine, porcine, ovine | Feed | Haemoglobin, heat shock protein, desmin, carbonic anhydrase, globin | Bovine: ALPAAAIEGPAYNR; [Porcine: IEGPAAVAAPAYSR] | 5% | 27 |
| Bovine, porcine, ovine | ND | Collagen | Bovine: PGEVGPPGPPGPAGEK; Ovine: AGEVGPPGPPGPAGEK; Porcine: PGEAGPPGPPGPAGEK | ND | 28 |
| Bovine, porcine | Gelatine, pharmaceutical capsules, confectionery, stock cubes | Collagen | Bovine: GETGPAGPAGPIGPVGAR; Porcine: GETGPAGPAGPVGPVGAR | 0.4-1.0% | 29 |
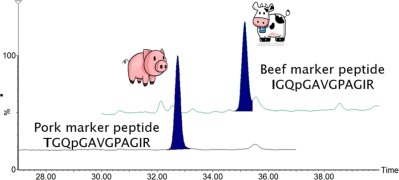
| Bovine, porcine | Bolognese sauce | Collagen | Porcine: TGQPGAVGPAGIR; Bovine: IGQPGAVGPAGIR | 1.5% | 30 |
- ND: not determined.
- The protein source of the peptide marker(s) is also reported, together with the limit of detection (LOD). Sequences in brackets were not reported in the papers, but determined with BLAST (Basic Local Alignement Sequence Tool) as a comparison with the other species mentioned in the papers.
6 FISH
Fish is an important dietary component since it is a source of animal proteins, ω-3 polyunsaturated fatty acids, vitamins, and minerals. In the last few years, also for this food chain, the need to guarantee the traceability, authenticity and health benefits of such products has increased.35
As it has been described in the previous paragraphs, proteomic methodologies have also been developed and successfully used for the assessment of fishery products authenticity.
Different phases of the proteomics schemes of action, as shown in Figure 4 and Figure 5 (discovery and target-driven phases),36 allow the identification and characterization of several peptide biomarkers that can be monitored by MS, enabling the unequivocal and fast authentication of fish species and allergen detection in any seafood product in <2 hr.37
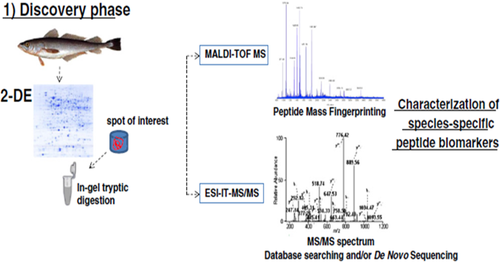
Proteomics pipeline used for fish authentication. In the discovery phase, 2-DE and mass spectrometric analyses of sarcoplasmic proteins are applied to identify and characterize species-specific peptide biomarkers. (Reproduced from Carrera et al. Food Res. Int. 2013 with permission from Elsevier, License number: 4210720238395) (Ref. 36)
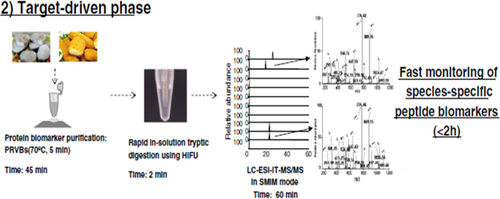
Proteomics pipeline used for fish authentication. In the target-driven phase, SMIM experiments are designed for monitoring species-specific peptide biomarkers. (Reproduced from Carrera et al. Food Res. Int. 2013 with permission from Elsevier, License number: 4210720238395) (Ref. 36)
In particular, the two MS techniques applied can be used for giving an initial quick identification of the protein of interest from the peptide mixture produced (MALDI-TOF-MS), then the complete characterization of the peptide fragments until a deep quantification process can be performed by ESI-MS/MS analysis.
Recently the same experimental approach was applied to establish a database containing protein patterns of common food fish prone to substitution. The database currently comprises 54 fish species. Aspects such as the sensitivity of identification on the species level and the impact of bacterial contamination of fish filets are assessed.38
7 CEREALS
Among vegetal-derived foods, cereals play an important role for authentication issues. A recent study had the aim to check the authenticity of wheat, spelt, and rye, regarding their addition in bread products,39 identifying the three different grain species as well as determining their composition in processed and nonprocessed cereal products. The described analytical biomarker peptide strategy was successfully applied to protein extracts from bread submitted to chymotryptic digestion in order to identify peptide mixtures from three cereal species of soft wheat, spelt, and rye by LC-MS/MS. The specific use of MRM transitions of selected peptides to identify closely related species such as T. spelta and T. aestivum presents a further elegant tool in protein analysis and, consequently, in ingredient classification/differentiation in complex food matrices. The basis of the biomarker validation was achieved by using pure cereal flours and own baked products via matrix calibration to normalize peptides and to test the authenticity of the cereals in bread. However, the sensitivity of the reported method should be improved by the use of synthetic peptide markers and their isotope labeled analogs.
Some examples of studies of authentication of wheat have been also reported by our group. In the first one, the presence of common wheat in durum wheat flour samples was detected by performing the identification of two marker peptides.40 One peptide was identified as present only in common wheat; the second was present in all wheat samples (both common and durum), so it was used as a marker of the total wheat content. The method used for the analysis and the identification was sample digestion by pepsin and chymotrypsin, followed by LC/ESI-MS of the obtained peptide mixture.
Another approach was used for the characterization of the protein CM3 which is one of the major allergens of wheat, by using LTQ-OrbiTrap analysis on peptides obtained from the enzymatically digested protein separated by gel electrophoresis.41 In this case, one of the identified peptides was used as a marker for quantification on UPLC/ESI-MS by using its isotopically labeled analog as an internal standard, allowing to determine the protein amount in the different samples.
8 METHODOLOGY OVERVIEW: TRICKS AND TIPS
In most of the cases that have been described for each food product, the major issue is to find, among many different peptides of unknown origin, the discriminating markers. On the other side, if postextraction protein digestion is needed, in principle, it is possible to predict at first glance which peptides are going to be generated from the known proteins and the known enzyme and pre-select the most suitable markers. However, the not complete predictability of proteolytic enzyme behavior (missed cleavages, selectivity among different cleavage sites,42 impurities in the enzyme batches, and unexpected side reactions during enzymatic reactions43) and the fact that some peptides might be modified by the technological processes make this theoretical approach quite unfeasible. Thus, in general, the most convenient procedure for finding marker molecules is to always carefully analyze the mixture of peptides (either already present or generated after extraction) and to identify among them the potential markers.
As already shown in the previous paragraphs, food processing might chemically modify proteins, or denature them, changing the way they are digested by the enzymes, and, thus, affecting the peptide mixture composition after digestion. This means that marker peptides found in an unprocessed food ingredient might not be present anymore after processing. A typical example was shown by our group when studying beef and pork marker peptides in Bolognese sauce,30 which is a heavily processed meat preparation. Because of matrix interference, it was not possible to find a univocal method for extracting proteins and peptides, and marker peptides were also identified in raw meat that were completely absent in the Bolognese sauce. These results show how the marker peptides chosen for species identification must be accurately matrix-matched, in order to make sure that their detectability is not hampered by the technological treatment.
If qualitative identification is somehow easy by the methodology here described, quantification is instead a quite arduous issue. As it is shown in the examples reported in this survey, quantification of a peptide marker can be easily performed by using an external standard (identical peptide) or even internal (stable isotopically labeled identical peptide), but this quantification only provides the amount of the peptide in the mixture. Whereas when quantifying different food ingredients from different species, the amount of the ingredient is the information needed. To match the amount of the peptide with the amount of the food ingredient, many controls have to be performed. First, in order to have a 1:1 ratio between the protein of origin and the peptide, the enzymatic digestion must be complete, the sequence must be unique, and there should not be modifications in the peptide structure induced by the processing. If these parameters are fulfilled (and properly checked), the measurement of the amount of the original protein in the food can be considered accurate.40
It is important to also consider that often the ingredient is present in the mixture in small quantities, therefore, an accurate calibration obtained with standards of food preparations closely matching the real ones and containing variable amounts of all the species to be quantified, needs to be prepared and tested. As an example, this method was applied by our group for measuring the percentage of common wheat in durum wheat semolina. A marker peptide for common wheat was identified and semi-quantified with a peptide common to both common and durum wheat. The method was calibrated with several common wheats- durum wheat mixtures at various percentages and also using different wheat varieties to average the natural variability.41
9 CONCLUSIONS
The data presented in this report cover most of the scientific literature of the last few years on the use of peptide molecules in Food Science. According to the most relevant topics in this particular scientific area, we selected one main application field-food authenticity.
The approach of measuring proteolytic peptides (spontaneously generated or enzymatically generated) is a very robust method for species identification in food, which is being used more and more in analytical laboratories for assessing food authenticity. Albeit conceptually quite simple, the method needs a very careful set up and calibration, particularly when quantitative data have to be given, but there is no doubt that this approach (also with the expanding knowledge about proteolytic enzymes behavior in the next years) has the full potential to deliver high-quality robust data about the composition of a given food.
Some food products have been more deeply studied since they are naturally enriched in proteins or their authentication has an importance for economic issues. In the case of meat, after the European scandal of 2013, it has been somehow natural that most of the researchers went in the direction of the identification of horse marker peptides identification. In this field, there has been a large increase in the setting up of analytical techniques and robust methods for fraud identification.
However, in future studies, efforts should be addressed to some issues that are lacking in most of the studies. In particular, deeper implementation on reporting technical protocols details, on the number of representative samples and replicates, the use of powerful and adequate statistical processing analysis, and the development of more powerful MS techniques and bioinformatics tools.
With regard to availability of food samples, the major problem covers the protein extraction task from complex matrices and the low concentration of target proteins; therefore, further efforts have to be performed at the extraction and enrichment steps in order to increase sensitivity and reproducibility of the assays.
Another limiting factor is the restricted availability of reference materials, considering the wide range of possible analytes and matrix combinations of the real samples to be analyzed. Concerning this issue, the implementation of the use of chemical peptide synthesis protocols, as it has already been shown in few studies, could be very feasible for the introduction of isotopically labeled amino acid residues or chemical modifications induced by technological treatments.
Biographies

Tullia Tedeschi is currently an assistant professor in the Department of Food and Drug, University of Parma (Italy). She obtained a Ph.D. in chemistry here, and followed it with a fellowship at the Center of Molecular Biorecognition at the University of Copenhagen (Denmark). Her prime interest is the chemistry of peptides and proteins, including the chemical synthesis of amino acid and peptide derivatives, and enzymatic reactions. She has now published 58 papers in peer-reviewed journals and authored 9 book chapters.

Barbara Prandi is a fixed-term researcher at the Telematic University San Raffaele Roma (Italy), where she teaches Food Chemistry. She graduated in food science and technology at the University of Parma (Italy) in 2010, and in 2014 she successfully defended her Ph.D. thesis, entitled Wheat allergies: A peptidomic approach. Her research activities deal with the study of food allergens, the use of peptide markers for food authenticity purposes, and the characterization of food waste and by-products.

Stefano Sforza is full professor of organic chemistry in the Department of Food and Drug of the University of Parma (Italy), and visiting professor at the University of Wageningen (The Netherlands). His area of expertise is the chemistry, analysis and properties of peptides and proteins in food and feed biomasses. At present, he has published around 140 papers in peer-reviewed journals, authored 22 book chapters and is the holder of one patent.