Peptides with regularly alternating enantiomeric sequence: From ion channel models to bioinspired nanotechnological applications
Dedicated to Prof. Pasquale De Santis who first envisioned the potentiality of l,d-peptides.
Abstract
Peptides are versatile building blocks that have been extensively used as peptide-based organizers to generate bioinspired hybrid materials. Among them, those peptides characterized by regularly alternating enantiomeric sequences (l,d-peptides) have attracted much interest. Their structures, which are not accessible to the corresponding homochiral peptides, have been exploited to achieve hybrid conjugates, the chemical and structural properties of which can be predetermined by correct design. Molecules that self-assemble into hollow tubular architectures with side chains on the outer surface can form, allowing the introduction of new functionalities on amino acid residues containing reactive side chains. This provides an effective strategy to develop engineered nanotubes with the surface suitably tuned for applications in different fields, spanning from electronics to medicine.
Graphical Abstract
1 INTRODUCTION
Peptide self-assembly is a very common process in the biological world and a source of inspiration to generate novel materials using a hierarchical bottom-up approach. The wide use of peptides as starting building blocks exploits the chemical versatility of the amino acid side chains and several conformations accessible to the peptide backbone. Such chemical and structural properties govern the self-assembly of peptides toward predictable ordered architectures and allow the introduction of new functions that could result in stimuli responsive materials.
Many attempts have been made to produce nanotubular materials due to their versatile use in different fields of nanotechnology. In this respect, peptides characterized by regularly alternating enantiomeric sequences (l,d-peptides) are appealing for their propensity to self-organize in rod-like aggregates.
Cyclic and linear l,d-peptides were theoretically and experimentally investigated during the 1970s and 1980s as models of ion-channels and carriers. They were bioinspired by the prototype of ion-channel gramicidin A, a linear l,d-pentadecapeptide with antibiotic activity, whose ion-conducting structure in bilayer membranes was still unknown at that time. Among several models proposed in the effort to identify the gramicidin active conducting form, Urry1 postulated a head-to-head single helix dimer in β-like conformation with a topology very similar to the structure that was later demonstrated to be the main conducting form.2-4
The first part of this review focuses on the theoretical model proposed by De Santis et al.5 who first stated the rules that govern the conformations allowed for l,d-peptides, and the experimental efforts carried out to identify l,d-sequences as models of ion channels in membranes. In the second and third part, the nanotechnological applications developed by exploiting the self-assembling properties of linear and cyclic l,d-peptides will be described with focus on hybrid materials based on the l,d-α-peptide building blocks.
2 THEORETICAL MODEL
Interesting for their structures that are similar to the naturally occurring antibiotic peptides and depsipeptides involved in selective ion transport across bilayer membranes, l,d-sequences were theoretically and experimentally investigated in 1970s and 1980s. Although previous molecular mechanic calculations had been proposed to identify possible conformations of l,d copolypeptides,6 a relevant advance in the field came with the theoretical paper by De Santis et al.5 By an elegant and effective approach, the general rules that govern the conformational stability of any l,d- or ll,dd sequence were established and their propensity to assemble in tubular structures was predicted.5
Under the general conditions that associate stereo-chemical to chemical equivalence, l,d-sequences generate cyclic structures that contain a rotoreflexion axis Sn. An even number of monomeric units is required to ensure the conformational equivalence and the perfect closure and planarity of the rings. Slight perturbations of the conformational equivalence transform a cyclic structure into a low-pitch helical turn that can produce either right-handed or left-handed helical conformations when repeated along a polymer chain. In such case, the symmetry Sn is lost and no restraint on the monomer number is required.
The preferred structures for linear and cyclic l,d-peptide are β-like conformations where CO and NH bonds, almost parallel to the helical axis, are oppositely oriented owing to the trans-conformation of the peptide backbone. This structural feature favors assembling l,d-peptides in tubular architectures ruled by hydrogen bonds between the donor CO and the acceptor NH groups. As examples, the structural models of the dimeric assemblies of the hexapeptide cyclo[-(d-Phe-l-Lys)3-] (Figure 1A) and linear octapeptide H-(d-Lys-l-Lys)4-OH (Figure 1B) are shown in axial and radial projections. Owing to the large conformational space allowed for β-like structures, linear l,d-peptides can adopt right-handed and left-handed single helical structures and parallel and antiparallel double helices. Accordingly, the oligomer H-(d-Ala-l-Ala)n-OH was modeled in a left-handed single helix with β6.2 conformation (Figure 1C), and antiparallel double stranded helix with β8.2 conformation (Figure 1D). The structural models reported in Figure 1 are achieved as reported in Ref.7
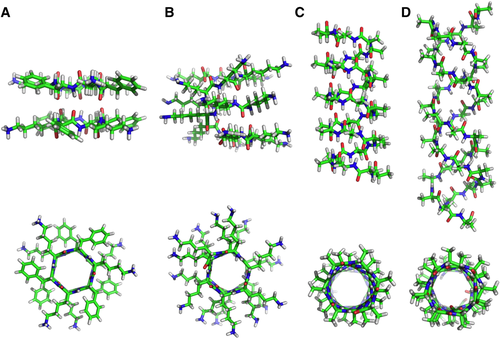
Structural models of antiparallel dimeric assembly of A, the cyclic hexapeptide (d-Phe-l-Lys)3; B, the linear octapeptide H-(d-Lys-l-Lys)4-OH in right-handed β-helix conformation with 6.2 monomer per turn; the polymer H-(d-Ala-l-Ala)n-OH in single left-handed β6.2-helix conformation (C) and antiparallel double helix with β8.2-conformation (D). The structures are reported in axial and radial projections. The images of the structures were realized with the PyMOL Molecular Graphics System.8 (Color code: oxygen: red, nitrogen: blue, hydrogen: white)
All the structures have the optimal geometry to establish a wide network of intramolecular and intermolecular hydrogen bonds that stabilize helices or tubular assemblies. They are characterized by hydrophilic inner channels that can bind ions or small hydrophilic molecules, activating their passage in membranes. The residue side chains lie on the outer surface of the tubular structures. As an important consequence, the chemical and structural properties of such architectures can be tuned by introducing new functionalities if the amino acids with reactive side chains are inserted in the peptide sequence. In this respect, the presence of lysine residues, as in the models shown in Figure 1A and B, makes the tubular assemblies versatile templates to exploit in the design of new materials, as discussed below.
3 SYNTHETIC L,D-PEPTIDES AS MODELS OF ION CHANNELS AND CARRIERS
The l,d-oligo and polypeptides are characterized by an average inner diameter capable of accommodating alkali ions. Based on these features, l,d-peptides were considered adequate models of ion-conducting channels. The roles that various structural factors can have in the conformations of l,d-peptides, such as the length of the chain, the nature of the side chains, and the specific configuration motif (dld or ldl), were investigated. To this end, l,d-α-peptides with different sequences and lengths were synthesized and their conformational properties were investigated.9-19
Interesting examples are Cbz-(l-Ala-d-Val)4-OCH3 (Cbzbenzyloxycarbonyl) and Boc-(l-Val-d-Val)n-OCH3 (Boctert-butyloxycarbonyl, n = 4, 6, 8).
The linear l,d-octapeptide Cbz-(l-Ala-d-Val)4-OCH3 was proposed as an ion channel model under the hypothesis that the expansion of the pattern l-Ala-d-Val could reproduce the main features of gramicidin A.9 Experimental findings demonstrated that Cbz-(l-Ala-d-Val)4-OCH3 adopts single-strand β-like conformations. Further, it self-organizes in rod-like structure and acts as an ion channel when artificial bilayer membranes are doped with octapeptide.19
Some years later, the linear sequence Boc-(l-Val-d-Val)4-OCH310, 16 was identified to model the channel-type ionophores. Single crystal X-ray diffraction analysis and spectroscopic results in solution established that the octamer Boc-(l-Val-d-Val)4-OCH3 was prevalently in antiparallel double-stranded β-helix conformation closely related to the X-ray crystal structure of gramicidin A.20, 21 However, structural investigations on Boc-(l-Val-d-Val)6-OCH312 and Boc-(l-Val-d-Val)8-OCH314 revealed that single-stranded β-helices are stable conformations for longer sequences.
Finally, cyclo[-(l-Val-d-Val)3-] and cyclo[-(l-Phe-d-Phe)3] were proposed as models of alkali ion carriers.18 Both assumed β-like conformation with an inner cavity able to bind alkali ions as X-ray diffraction analysis displayed. Further, the backbone amide groups were almost perpendicular to the peptide average plane, although they were strongly hydrogen bonded to the solvent molecules that bridged adjacent layers of cyclic peptides.
These early results proved the ability of linear and cyclic l,d-α-peptides to adopt β-like conformations capable of self-assembling in tubular architectures. Such investigations attempted to elucidate the mechanism involved in the transport of ions or small molecules through membranes. Although not conclusive in all respects, the large number of theoretical and experimental findings was predictive of the potential use that such peptides would have for future nanotechnological applications.
4 EXPLOITING SELF-ASSEMBLING L,D-PEPTIDES FOR NANOTECHNOLOGICAL APPLICATIONS
Tubular structures obtained by hierarchical self-assembling processes can be attractive molecular-sized building blocks to produce new materials with tailored properties. In the 1990s, Ghadiri and collaborators first proposed a new class of bioinspired nanotubes, which exploited the structural features of cyclic l,d-α-peptides with an even number of residues and envisioned their applications in various fields ranging from pharmaceutics to electronics.22-26 A very effective synthetic strategy was described to control the self-association process by proper modification of size and outer surface chemical properties of tubular aggregates. In this way, peptide nanotubes provide versatile templates that, while taking advantage of amino acids with reactive side chains, can be conceived with properties specific to any applications introducing unprecedented functionalities.22-26
A variety of cyclic l,d-α-octapeptides were designed and their structural properties were investigated. As theoretically predicted,5 they adopt β-like conformation and self-organize in rod-like structures with antiparallel arrangement due to the amide groups that are almost perpendicular to the average plane of the ring and stabilize the stacked cyclic peptides by a wide network of hydrogen bonds. The side chains face outwards and drive possible aggregation of single nanotubes in bundles by van der Waals interactions.22 Very recently, the X-ray crystal structures of two cyclic l,d-α-octapeptide nanotubes with parallel and antiparallel association arrangements were first reported by Silk et al.27 The structures of the tubular aggregates obtained at atomic resolution substantiated the previously proposed structural models.5, 22 The high resolution, however, revealed a less favored parallel stacking of one of the cyclic peptides, probably due to the side-chain interactions and distortions of the rings toward an oval shape, which could compensate for the lower stability of this arrangement.
Potential applications in the material science of tubular nanostructures require their uniform and specific size. Tuning of the pore diameter can be achieved by self-assembling cyclic l,d-α-peptides with different numbers of the amino acid residues.24 However, this strategy fails for cyclic peptide units larger than twelve due to an increased flexibility that produces more disordered assemblies, while the large strain of cyclic units containing less than six residues hindered the needed geometry for stacking.26
The conformational properties of cyclic l,d-α-octapeptides and their propensity to self-assemble in nanotubes suggest their use as artificial ion channels, drug delivery systems and cytotoxic agents.23 Hydrophobic cyclic l,d-α-peptides were designed to favor their partitioning into the lipidic environment of membranes. Their ability to form trans-membrane channels mediating the transport of ions (Figure 2),23, 28 small molecules, such as glucose29 and hydrophilic drugs, such as the antitumor 5-fluoroacil30 was demonstrated.
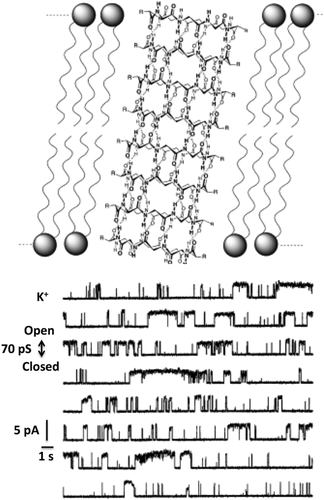
Structural model of the trans-membrane ion channel formed by self-assembling cyclo[-(l-Trp-d-Leu)3-l-Gln-d-Leu)-] (top); K+ single channel conductance recorded in bilayer membranes doped with cyclo[-(l-Trp-d-Leu)3-l-Gln-d-Leu)-] (down) [Ref. 28]. Reprinted with permission from Organic Nanotubes. D. T. Bong, T. D. Clark, J. R. Granja, M. R. Ghadiri, Angew. Chemie Int. Ed. 2001, 40, 988. Copyright (2001) WILEY-VCH
5 APPLICATIONS OF CYCLIC L,D-PEPTIDES TO BIOMEDICINE
The cyclic l,d-peptide property to enhance membrane permeability by β-sheet rod-like structures and the increased resistance to proteolysis due to the l,d-motif have paved the way to a variety of pharmaceutical applications.
Properly tailored hexameric and octameric cyclic l,d-α-peptides showed strong antibacterial activity,31, 32 while the antiviral properties of different families of amphiphilic cyclic l,d-α-octapeptides were successfully screened providing a broad spectrum of antiviral agents.33, 34 More recently, the great potentiality of histidine- and tryptophan-rich cyclic l,d-α-octapeptides as novel antidiabetic drugs has been demonstrated in vitro.35
Cyclic l,d-α-peptide self-assemblies were proposed to treat degenerative pathologies, such as Parkinson's and Alzheimer's diseases, that seem related to the deposition of materials accumulated in toxic amyloid fibrils.36 Based on the possible interaction between the rod-like organization of fibrils and the tubular architecture of self-assembled cyclic l,d-α-peptides due to the structural similarities, the anti-amyloidogenic activity of a family of cyclic l,d-α-hexapeptides was established in vitro,37, 38 suggesting a rational pharmacological approach for preventing neurodegenerative diseases.
Very recently, the ability of cyclic l,d-α-peptide scaffolds to remodel the high-density lipoprotein complexes, which are postulated to remove cholesterol from the bloodstream, reducing the formation of sclerotic plaques, was exploited to develop new prospective therapeutics to treat heart diseases.39 The efficacy of a wide class of cyclic l,d-α-peptides of tuning shape and the function of high-density lipoproteins was investigated, and sequence patterns that make such peptides effective in vitro were identified. Interestingly, modifications of peptide sequences, which inhibited tubular aggregations, reduced their remodeling function. This result suggests that only the supramolecular assemblies act as therapeutic agents.
Recently, the antitumor activity of valinomycin was reported. Among natural ionophores, the valinomycin, a 36-membered macrocycle that consists of the regular repetition of a ll,dd motif, has a hydrophilic cavity suitable to accommodate potassium ions that can be delivered across membranes.40 Due to the synthetic difficulties to functionalize the valinomycin, its pharmacological potential was little explored, except for the well-established antibiotic activity. These drawbacks were overcome by Iacobazzi et al., who proposed an effective synthetic strategy to obtain several valinomycin-derived conjugates whose antiproliferative action on cancer cells was demonstrated in vitro.41
Finally, self-assembled cyclic l,d-α-peptides were also used to develop new systems for drug delivery and gene transfection, taking advantage of the ability of tubular architectures to interact with membranes forming transmembrane channels.
Li et al.42 first prepared cationic rod-like aggregates by self-assembling the cyclo[-(l-Lys-d-Ala)4-] functionalized with a single arginine analog that clamps the stacked cyclic peptides, stabilizing the tubes despite the electrostatic repulsions among the charged lysine residues. The association complexes obtained in physiological conditions between such positively charged nanorods and DNA demonstrated surprising gene transfection effectiveness.
Chen et al.43 explored the viability of self-organizing cyclic l,d-α-peptides to carry small molecular anticancer drugs through the artificial channels they form across the cell membranes. A hydrophobic cyclic l,d-α-decapeptide was designed with an inner pore suitable to complex selectively hydrophilic molecules with a smaller size than 1 nm, such as the anticancer drug 5-fluoracyl. The association complexes between 5-fluoracyl and the nanotubes revealed a strong inhibition of tumor growth either in vitro or in vivo.
6 APPLICATIONS OF L,D-PEPTIDES TO ELECTRONICS
Several other strategies have been developed to produce noncovalently rod-like nanostructures. Homo-configurational short peptides,44, 45 peptide-based amphiphiles,46, 47 and surfactant-like oligopeptides48 provide self-organized nanotubes, nanotapes, nanofibrils that can be exploited as templates to drive hierarchical association up to the micro- or macroscale. However, the self-assembly of cyclic l,d-peptides is one of the most successful approaches for the ease to control the nanotube diameter, which only depends on the number of the amino acids forming the cycle. Furthermore, the self-assembly process and the nanotube length can be controlled if proper stimulus-responsive or covalently modified residues are inserted in the ring.
Along with the possibility to modulate size and the stability of the cyclic peptide assemblies, the chemical profile of the amino acid residues with reactive side chains can be substantially modified, introducing unnatural ligands and a large variety of new functions. This approach and the intrinsic propensity of cyclic peptides to provide highly ordered self-assemblies with nanometric size have paved the way to transform the original insulating materials into new building blocks with potential applications in electronics and opto-electronics.
A rational strategy to generate electronic nanodevices with tunable photoluminescence properties was proposed by Horne et al.49 Cyclic l,d-α-peptides functionalized with various fluorescent probes provided excimers and promoted tunable charge transfer in the tubular assemblies.
An elegant and effective approach aimed at investigating the mechanisms of charge transfer in the wires obtained by cyclic peptide self-assembly was recently proposed.50 By a layer-by-layer deposition of three self-assembling cyclic d,l-α-octapeptides, cyclo[-(d-Tyr-l-Lys-d-Tyr-l-Cys)3-], cyclo[-(d-Trp-l-Glu)4-] and cyclo[-(d-Tyr-l-Lys)4-], vertically oriented nanotubes of tunable length were produced exploiting the ability of the cysteine-containing peptide to anchor to gold surfaces and the attractive electrostatic interactions between lysine-rich and glutamic-rich peptides. An efficient charge transfer was achieved on the molecular junctions fabricated utilizing the oriented nanotubes anchored to gold substrates and a conductive atomic force microscopy tip as a second electrode, demonstrating that the cyclic d,l-α-peptide units can be engineered to properly modify the electronic properties of their self-assemblies in view of nanodevice fabrication.
The proton conduction of self-organized cyclic d,l-α-octapeptides was also investigated revealing that the presence of aromatic amino acid residues (tryptophan and tyrosine) induced the conductivity with different mechanisms. The ion conduction was strongly dependent on the long-range order of the assemblies in dehydrated conditions while the insertion of the proton donor amino acids (glutamic acid) in the sequence greatly promotes conduction in a wet environment even though a loss of order occurs.51 These findings demonstrated that properly functionalized peptide sequences provide versatile building blocks for a rational design of highly proton conducting materials for electrochemical applications.
Linear d,l-α-peptides were also proposed for the design of nanotools with potential applications in electronics.52 Three linear lysine-containing d,l-α-octapeptides, For-(d-Leu-l-Lys)4-OH, For-(d-Phe-l-Lys)4-OH and H-(d-Lys-l-Lys)4-OH, were synthesized, and their hydrogen-bonded tubular structures were used as scaffolds to bridge the lysine side chains by forming the corresponding metal chelates via Schiff base derivatives as demonstrated by absorption and circular dichroism spectroscopies and molecular mechanic calculations. When metals with high electron spin states are used, their coupling could produce magnetic materials useful to shape patterns of nanometric lattices driven by their orientation under magnetic fields.
7 NANOTECHNOLOGICAL APPLICATIONS OF α,γ-CYCLIC PEPTIDES WITH REGULARLY ALTERNATING ENANTIOMERIC SEQUENCES
The potential to reshape the chemical features of the outer surface of cyclic peptide nanotubes has been widely exploited to fabricate novel materials. However, peptides with a regularly alternating enantiomeric sequence offer the opportunity to modulate the polarity of the inner pore, as proposed by Granja and colleagues. Hybrids were designed where d-α-amino acids alternate with cyclic γ-residues with the suitable chirality that, properly functionalized, allow tuning the inner core properties.53, 54 The insertion of cycloalkane γ-amino acids preserves the flat conformation of cyclic α,γ-peptides and the perpendicularity of the peptide backbone to the ring, ensuring the optimal conditions to produce stacked nanostructures. The contemporary functionalization of the inner core and outer surface enables the preparation of amphiphilic nanotubular architectures with more selective cavities, which can entrap guests with different chemical properties.55 In particular, hybrid heterodimeric assemblies were achieved by self-complementary cyclic α,γ-peptides.56 The conjugation with hybrid functionalities allowed the design of novel materials to build biosensors,58 artificial photosystems,57, 58 delivery and release nanomedicine systems,59, 60 transmembrane channel transporters,61, 62 nanotools for electronics,63 and responsive molecular machines containing properly designed reversible covalent bonds that are sensitive to environmental stimuli.64
8 D,L-α-PEPTIDE-BASED BUILDING BLOCKS TO GENERATE HYBRID MATERIALS
In recent years attention has been paid to cyclic d,l-α-peptide-polymer conjugates to introduce a broader range of functionalities that are not accessible to small molecules. Compared with other tubular polymeric architectures, such hybrid conjugates can provide the unique ability to form tubes with rigid rod architectures and subnanometer control over the inner diameter. Cyclic d,l-α-peptide-polymer nanotubes can be synthesized either by divergent growth of a polymer from the peptide scaffold used as a template or by convergent coupling of polymers to the cyclic d,l-α-peptide before the peptide assembles.65-67
The first example of a cyclic d,l-α-peptide-polymer conjugate was reported by Couet et al. who prepared the octapeptide cyclo[l-Asp-(d-Ala-l-Lys)3-d-Ala-] grafted with the thermo-sensitive poly(N-isopropylacrylamide) (PNIPAAM).68 Cyclic peptide initiators were self-assembled in nanotubes in aqueous solution and used as templates. Then, surface-induced atom transfer radical polymerization (ATRP) reaction of NIPAAM was carried out, as sketched in Figure 3A. The nanotubes observed after grafting were present as distinct single-rod structures, as proved by atomic force microscopy images (Figure 3B), showing that the conjugation with PNIPAAM avoided the aggregation of the single nanotubes in bundles otherwise observed in the precursor cyclic peptides. However, the strategy of carrying out living radical polymerizations on templates after self-assembly could not ensure high density polymer chains, an important feature to account for any potential applications of these systems since it confers stability to the peptide core and aggregates.
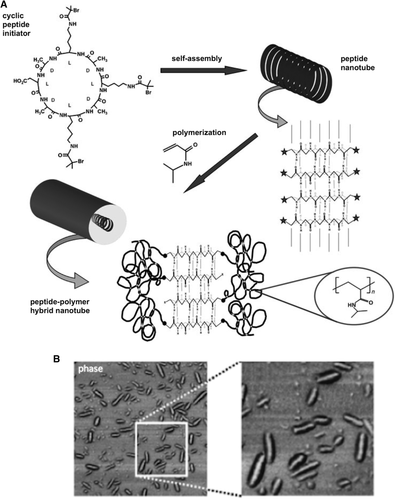
A, Scheme of the synthesis of cyclopeptide-PNIPAAM conjugate: the self-assembled cyclopeptides with polymerization initiator groups on the outer surface of the nanotube were employed as templates for the surface-initiated polymerization in the presence of the monomer NIPAAM generating a polymer shell. B, Atomic force microscopy phase image of cyclopeptide-PNIPAAM nanotubes on a silicon wafer (scale: 2 μm) and an enlarged area (scale: 0.8 μm) [Ref. 68]. Reprinted with permission from Peptide-Polymer Hybrid Nanotubes. J. Couet, J.D. Jeyaprakash, S. Samuel, A. Kopyshev, S. Santer, M. Biesalski, Angew. Chem. Int. Ed. 2005, 44, 3297 (Adapted Figures 1 and 3). Copyright (2005) WILEY-VCH
An alternative synthetic approach was adopted by conjugating the same octapeptide, used as initiator in disaggregating conditions, to poly(butylacrylate) (PBA).69 The two synthetic methodologies provided conjugates with analogous structural behavior. In fact, the new hybrid showed a solvent induced self-assembly with nanostructures very similar to those formed by the “grafted-from” PNIPAAM conjugate.68
The octapeptide cyclo[-(l-Gln-d-Ala-l-Lys-d-Ala)2-] was selected by ten Cate et al. to induce a tubular aggregation and synthesize a conjugate with two well-defined PBA chains. The polymer chains were prepared by ATRP and coupled to the opposite lysines of the cycles (Figure 4A).70 The controlled self-assembly of the conjugate led to uniform tube structures consisting of a core where stacked β-like cyclic peptides stabilized by an inter-ring hydrogen bonding network adopt a structure similar to the corresponding unsubstituted octapeptide, as schematically illustrated in Figure 4B and C and experimentally demonstrated by atomic force microscopy measurements (Figure 4D).
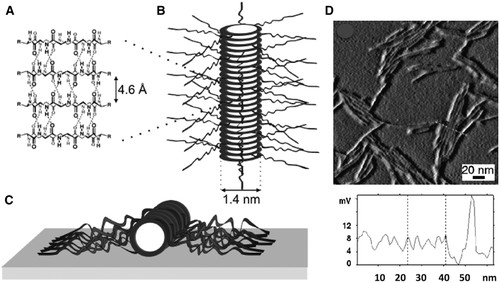
Structural model of the nanotubes self-assembled from polymer-cyclopeptide-polymer conjugates from poly(n-butyl acrylates) and cyclo[-(l-Gln-d-Ala-l-Lys-d-Ala)2-] (A), in solution (B), and on a mica surface (C). High resolution atomic force microscopy image of the nanotubes formed by deposition on to mica substrate after treatment with trifluoroacetic acid and reassembly in N,N-dimethylformamide/tetrahydrofuran [Ref. 70]. Reprinted with permission from self-assembling peptide-polymer conjugates comprising (d-alt-l)-cyclopeptides as aggregator domains. M. G. J. ten Cate, N. Severin, H. G. Börner, Macromolecules 2006, 39, 3297 (Adapted Figures 4 and 6). Copyright (2006) American Chemical Society
More recently, Perrier's group adopted a click chemistry reaction to couple a cyclic l,d-α-peptide pattern to various responsive polymers. Hybrids with different degrees of functionalization and sensitivity to the environmental stimuli were produced.71-78
A copper-catalyzed azide-alkyne click reaction was used to graft an azide functionalized l,d-α-octapeptide, cyclo[-(l-Lys(N3)-d-Leu)4-], with poly(hydroxy ethyl acrylate) (PHEA) synthesized via reversible addition fragmentation chain transfer polymerization and end-functionalized with an alkyne group.71 A little efficiency of the click chemistry reaction and low functionalization of cyclic peptides were achieved with high molecular weight polymers, due to the steric hindrance of the chains. However, evidence of the self-assembly of the conjugates into nanotubes was found.
Adopting the same synthetic approach but reducing the degree of functionalization, Perrier's group prepared functional nanotubes from a range of polymers including PBA, poly(dimethylamino ethyl acrylate) (PDMAEA), poly(acrylic acid) (PAA), poly(styrene) (PS), and poly(hydroxyl ethyl acrylate) (PHEA). Tubular architectures with structural properties different from the starting cyclic peptides can be achieved by conjugation with responsive polymers. Further, self-aggregates with dual chemical properties can be generated by modular self-assembly of a mixture of differently functionalized cyclic peptides.72 This strategy has proved flexible and powerful and has allowed producing nanotubes with variable sizes and functions.
As an example, a thermo-sensitive cyclopeptide-polymer hybrid with a lower critical solution temperature was prepared by coupling of cyclo[-(l-Trp-d-Leu-l-Lys-d-Leu)2-] with two chains of the thermosensitive poly(2-ethyl-2-oxazoline).73 The resulting conjugate self-organized in nanotubular structures in an aqueous solution at room temperature; reversible transformation of soft nanotubes into hard polymeric microparticles occurred by increasing the temperature. These results showed that the coupling with proper polymers could dramatically affect the behavior of cyclic peptides, which were essentially insensitive to the temperature, and introduce unprecedented properties in the conjugates.
The character of copolymers consisting of hydrophilic-hydrophobic blocks and cross-linking of suitable monomeric units under aggregative conditions were also exploited. Based on the l,d-α-octapeptide cyclo[-(l-Trp-d-Leu-l-Lys-d-Leu)2-], a conjugate with two chains of a block-copolymer of PAA and poly(isoprene) (pI), [p(AA-b-I)]2-cyclopeptide, was prepared.75 Multishell tubular aggregates consisting of a hydrophobic internal channel formed by the cyclic peptide and the hydrophobic (pI) and hydrophilic (pAA) shells of the copolymer were found, as shown by transmission electron microscopy micrographs (Figure 5A). Better-defined nanotubular structures were obtained after crosslinking the acrylic acid shell (Figure 5B). An internal peptide channel was highlighted after the ozonolysis was conducted to remove the polyisoprene shell (Figure 5C). Interestingly, the strong inter-ring hydrogen bonds, which trigger aggregation in nanotubes, were preserved despite possible competitive effects arising from the amphipathic nature of the copolymer.
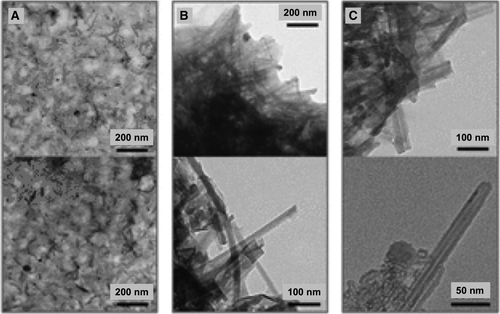
Transmission electron microscopy images of (p(AA-b-I))2-cyclopeptide nanotubes assembled A, before crosslinking; B, after crosslinking; C, after ozonolysis. [Reprinted with permission from Multishell Soft Nanotubes from Cyclic Peptide Templates [(Ref. 75]. R. Chapman, K. A. Jolliffe, S. Perrier Adv. Mater. 2013, 25, 1170 (Figure 2). Copyright (2013) WILEY-VCH]
Taking advantage of polymers with different hydrophobicity, Danial et al. first achieved Janus (the mythological Roman deity with two faces) cyclopeptide-polymer nanotubes by linking the same octapeptide cyclo[-(l-Trp-d-Leu-l-Lys(N3)-d-Leu)2-] to two different nonmiscible polymers, such as PS and PBA, which can undergo phase separation (Figure 6).76 For this purpose, an innovative and efficient two-step convergent synthetic route using well-defined polymer and cyclic peptide building blocks was proposed to obtain aggregates with a surface characterized by dual chemical properties (Figure 6A). Although linear15, 19 and cyclic23 l,d-α-peptides were shown to be active as ion channels in bilayer membranes, Janus rods could open a new vision in the design of macropores capable of mimicking transmembrane protein channels. The partition process and self-assembly into transmembrane channels could be better modulated by conjugation of hydrophobic and hydrophilic polymers.77 Moreover, this approach along with the conjugation of cyclic peptides with thermosensitive polymers allowed generating transbilayer channels whose structure, interaction mechanism with the membrane, and transmembrane transport were under temperature control.
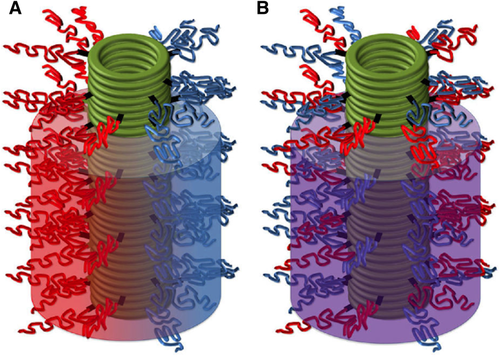
Scheme of Janus tubular assembly with ‘demixed’ corona of cyclopeptide-polymer nanotubes (A) and hybrid assembly with mixed corona (B) [Ref. 76]. [Reprinted with permission from Janus cyclic peptide-polymer nanotubes. M. Danial, C. M.-N. Tran, P. G. Young, S. Perrier, K. A. Jolliffe, Nature Commun. 2013, 4, 2780 (from Figure 1). Copyright (2013) WILEY-VCH]
The pH-dependent tubular architectures can also be fabricated by grafting the pH-sensitive charged polymer poly(dimethylamino ethyl methacrylate) (PDMAEMA) to cyclo[-(l-Trp-d-Leu-l-Lys-d-Leu)2-].78 The self-assembling properties of the cyclopeptide dramatically change as a function of pH. Variations of pH involve a different protonation of the polymer chains in the corona and permit the difficult task to govern the aggregation number and the length of the supramolecular aggregates by protonation/deprotonation of the amine groups on the PDMAEMA chains.
Although linear l,d-peptides display structural and self-assembling features similar to the homologous cycles and simpler synthesis, a few examples of engineered linear l,d-peptides were reported. The tubular association of such peptides could be less stable, owing to a slightly distorted orientation of the peptide backbone with respect to the helical axis, resulting in weaker hydrogen bonds. However, the engineering of linear l,d-α-peptides could provide hybrids that enable an easier control of the size of the self-assemblies. In this regard, two hybrid conjugates, formulated as biocompatible carriers of hydrophobic drugs, were obtained by coupling of poly(ethylene glycol) (PEG, Mn = 2,000) to the C-terminus of a hexa- and octapeptide based on the pattern l-Ala-d-Val.79, 80 The two conjugates, Cbz-(l-Ala-d-Val)4-PEG79 and Cbz-(l-Ala-d-Val)3-PEG,80 self-organized in core-shell nanoparticles with very different morphologies, indicating that the length of the peptide moiety determines the shape of the nanostructures. In Figure 7A and C, scanning electron microscopy of Cbz-(l-Ala-d-Val)3-PEG and transmission electron microscopy micrographs of Cbz-(l-Ala-d-Val)4-PEG are shown. The PEGylated hexapeptides self-assembled in vesicles where a head-to-head association of the unimers in the layer can be speculated (Figure 7C). The conjugated octapeptide, instead, exhibited a rod-like structure (Figure 7B). Further, the micrograph in Figure 7B highlighted a gray tubular structure in the nanorod core. Interestingly, very similar results were reported by Chapman et al. for cyclopeptide-polymer conjugates.72, 75 The thicknesses of the internal channel and corona as obtained by transmission electron microscopy images are compatible with a tubular association of linear octapeptides in the most stable β6.2 helical conformation5 embedded in a dense polymer shell, as sketched in the proposed structural model of the aggregate (Figure 7D).79 However, Cbz-(l-Ala-d-Val)4-PEG and Cbz-(l-Ala-d-Val)3-PEG were revealed to be inefficient drug carrier systems since a low drug loading was measured when curcumin, a polyphenolic compound from Curcuma longa with manifold therapeutic activities,81 was used as a hydrophobic drug model.80 In fact, the uptake of hydrophobic drugs is very sensitive to the length and chemical properties of the hydrophobic core of nanoaggregates. Very recently, the synthesis and characterization of a new PEGylated d,l-octapeptide, For-(d-Phe-l-Cys(Acm))4-PEG (For, formyl) were reported.82 Designed to enhance the upload of hydrophobic and aromatic drugs, such as curcumin, by π-π stacking interactions with the phenylalanine residues, a high drug loading of curcumin in rod-like For-(d-Phe-l-Cys(Acm))4-PEG self-assemblies was obtained.
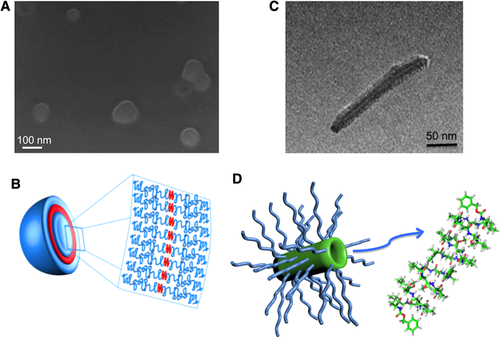
A, A representative scanning electron microscopy image of the self-assembled Cbz-(l-Ala-d-Val)3-PEG; B, transmission electron microscopy image of self-assembled Cbz-(l-Ala-d-Val)4-PEG; C, sketched structure of vesicles formed by assembled head-to-head Cbz-(l-Ala-d-Val)3-PEG; D, rod-like model of Cbz-(l-Ala-d-Val)4-PEG aggregates where the peptide core is modeled as antiparallel assembling of the octapeptides in β6.2 helical conformation. The image in D were realized with the PyMOL Molecular Graphics System.8 (Color code: oxygen: red, nitrogen: blue, hydrogen: white)
These results show that nanotubular architectures can also be formed with linear l,d-α-octapeptides-polymer conjugates, as proved by Cbz-(l-Ala-d-Val)4-PEG79 and For-(d-Phe-l-Cys(Acm))4-PEG82 self-assemblies. This occurs provided that the critical number of eight amino acid residues is reached. The β6.2 helical conformation is preferentially adopted by l,d-octapeptides or longer sequences.5 The latter, however, are further stabilized by a wider network of hydrogen bonds.
9 SUMMARY AND FUTURE APPLICATIONS
Cyclic and linear l,d-α-peptides have had a large variety of applications from their first use as models of ion channels and carriers up to the design of novel peptide-based materials. In this respect, the use of cyclic d,l-α-peptides for nanotechnological applications spanning from electronics to medicine was an extraordinary intuition of Ghadiri et al.22, 23
From a theoretical point of view, the approach proposed by De Santis et al.5 was exhaustive in predicting all the possible structures of l,d-peptides and their self-assembling propensity in tubular architectures. Further, the theoretical model is general and applies to any regularly alternating enantiomeric sequence. Accordingly, the α,γ-cyclic peptides, first proposed by Granja and collaborators, widen the significance of l,d-sequences. Such cyclic peptides are structurally similar to the homologous l,d-α-peptides. However, contrast to the cyclic l,d-α-peptides, the insertion of γ-units allows the functionalization of the inner pore of the cycle whose polarity and size can be tuned, generating more selective and specific systems.53-64
The use of cyclic l,d-α-peptides to design new pharmaceutical classes to fight serious pathologies, such as diabetes, neuro-degenerative diseases and cancer, was proposed. Results achieved prevalently in vitro are encouraging, although most applications suggest that the pharmacological activity of cyclic l,d-α-peptides is poorly specific and mainly due to their self-assembled structure.34-39
In contrast, the development of novel systems for drug delivery43 and gene transfection42 appears more sound since it is based on the well-proved propensity of cyclic l,d-α-peptides to interact with cells by forming trans-membrane channels. Applications of linear l,d-α-peptides in this fields could also be envisioned since the ability of these peptides to self-assemble in long rod-like aggregates79 and to act as ion channels in bilayer membranes were shown.15, 19
Compared with the homologous cycles, linear l,d-α-peptides enable a better tuning of the hydrophobicity/hydrophilicity ratio in amphipathic sequences. This feature is of crucial importance in peptide-polymer conjugates, which self-assemble due to hydrophobic collapse, since the conjugation with longer hydrophobic peptide sequences provides more stable assemblies.
A linear l,d-α-peptide can be functionalized through the N- and C-termini with less perturbation of the peptide backbone. Adopting a proper synthetic strategy, it would be possible to introduce novel functionalities with different chemical and structural features on the N- and C-termini. Hybrid three-block lipid-hydrophobic l,d-peptide-hydrophilic polymer could be designed with potential therapeutic applications. The lipidic tail should favor the partitioning into the hydrophobic environment of the membranes and cell uptake,83 while the hydrophilic polymer component should ensure the solubility of the system in water and protection of the peptide core from the proteolitic degradation.
Lipidated l,d-α-peptides could also be used as a platform for the development of new nanostructures with potential therapeutic applications. The hydrophilic peptide component can be conjugated with one or more lipid tails, combining the structural properties of the amphipathic surfactants with those of the l,d-α-peptide moiety. Charged amino acid residues, such as lysines, glutamic, and aspartic acid, could be inserted in the peptide sequence to generate pH sensitive self-assemblies, while the conjugation with lipid chains would introduce temperature sensitivity. In suitable conditions of concentration, pH, and temperature, these hybrids could self-assemble in nanofibers and form hydrogels that could find applications in tissue engineering, as an example.
In the rational design of new nanosystems, attention is focused on the conjugation of l,d-α-peptides with molecules that introduce multiresponsiveness that is impossible to obtain with the peptides alone. Such self-assemblies are usually the result of complex noncovalent interactions that produce a dynamic behavior in the system. In the presence of reactive groups, covalent bonds could be added by cross-linking reactions stabilizing the supramolecular aggregate. To this end, the insertion of cysteine residues in the l,d-α-peptide sequences could allow the covalent stabilization of the assemblies through oxidative coupling.
Advances in the field could occur in hybrid self-assemblies formed by weak covalent bonds, which break and re-form in response to environmental stimuli, causing reversible assembling and disassembling of the aggregates. As an example, l,d-α-peptide sequences could be functionalized with proper aromatic amines and crosslinked by reaction with dialdehydes according to a synthetic strategy proposed by Yu et al.84 The formation of imine bonds with aromatic amines could provide l,d-α-peptide polymers that are stable at acidic pH. Disassembling occurs at basic pH when the imine bonds-break.
In summary, this review has covered the theoretical and experimental aspects of linear and cyclic l,d-peptides with a particular focus on l,d-α-peptide-based materials and their nanotechnological applications. The limited number of studies on the potential use of the hybrid conjugates based on linear l,d-peptides has been highlighted. Some future perspectives are suggested. However, the potential of such peptides could also benefit from other areas of chemistry. Inorganics, nucleic acids, and polysaccharides could be effectively integrated into l,d-peptide nanostructures to develop novel hybrid materials.
ACKNOWLEDGMENTS
We thank CNIS Laboratory of Sapienza Università di Roma and Dr. Francesco Mura for providing the SEM micrographs. This work was supported by Fondi Ateneo Sapienza 2015 and 2016.
Biographies

Giancarlo Masci has a PhD in Chemistry (Sapienza University of Rome, 1993). The goal of his group is the synthesis of smart polymers (pH and thermosensitive) by atom transfer radical polymerization (ATRP). He has expertise in the conjugation of these polymers with polysaccharides, peptides, and other biomolecules and in the study of their self-assembled soft nanoparticles in aqueous media.

Anita Scipioni obtained the PhD in Chemical Sciences in 1989 from Sapienza University of Rome. Her scientific activity concerns theoretical and experimental investigations on structural properties of peptides, DNA and DNA-protein association complexes with the main purpose to unravel the superstructural elements that rule the genic expression. In the last decade, the goal of her group is focused on the design of self-assembling peptide-based conjugates to obtain bioinspired soft materials for applications in nanomedicine.