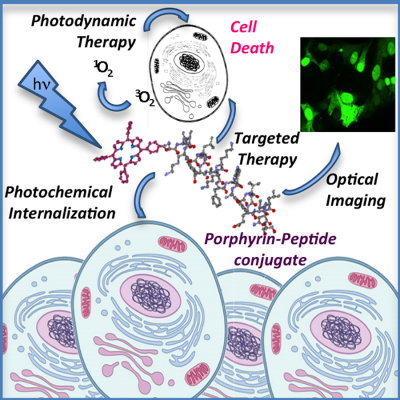
Porphyrin–peptide conjugates in biomedical applications
Abstract
The conjugation of peptides to porphyrins and related tetrapyrrole based macrocycles have extended their applications in biological systems, where the photophysical properties of these molecules and their ability to coordinate metals can be exploited for detecting and opposing to the onset of important diseases. This review presents a survey of the published literature over last years on biomedical applications of these hybrid compounds, mostly in the field of photodynamic therapy (PDT), but also in sensing key metabolites and peculiar structures of biomolecules in living systems. Peptides based strategies to improve the efficacy of the photosensitizer (PS) in PDT will be discussed, as well as solutions to overcome some limitations recently emerged in the use of peptides as delivery vehicles. An overview on possible application of porphyrin–peptide conjugates in detection of biomarkers of physiological and pathological states will be also presented.
Graphical Abstract
1 INTRODUCTION
Porphyrins and other tetrapyrrole macrocycles have been actively investigated due to their interesting photophysical and chemical properties. Since 1931 when Hans Fisher was awarded the Nobel Prize for his work on haeminin synthesis, the interest in synthesis and application of porphyrin derivatives in various areas of science has grown significantly.1-6 Particularly strong driving forces for the study of these macromolecules were the synthesis of catalysts and model systems for biology, as well as light-responsive sensitizers for photodynamic therapy where the photophysical properties of porphyrins proved particularly useful. In nature the porphyrinic macrocycle represents an ideal scaffold to firmly incorporate metal atoms in tetradentate fashion in proteins, constituting the prosthetic group of several metalloproteins involved in fundamental biological processes. The heme-proteins family represents a fascinating example in this respect, in which the heme cofactor promotes a variety of functions, such as dioxygen storage and transport, electron transfer, hydroxylation and oxidation of organic substrates, and hydrogen peroxide disproportion.7, 8 Structural and site-directed mutagenesis studies from an increasing number of heme-proteins have shown that the protein matrix, which surrounds the heme-active site, controls the intrinsic reactivity of the prosthetic group. Therefore, it is not surprising that over last 20 years a large number of porphyrin–peptide conjugates have been synthesized and studied as model of metalloenzymes and heme-proteins. Beside this important topic, on which a number of excellent reviews have been published,9-11 new applications for porphyrin–peptide conjugates have been emerged from the literature, spreading from medical to material sciences. Herein we intend to present a survey of the literature for the point of view of the biomedical applications of tetrapyrrole–peptide conjugates, beyond imaging techniques. In most of conjugates presented in this review the tetrapyrrole macrocycle has been linked to the peptide through an amide bond involving the amino group at the peptide N-terminus or a lysine side chain. Other conjugation strategies have exploited the cysteine side chain or the azide–alkyne cycloaddition of proper functionalized components. For an overview on the synthetic approaches for the conjugation of porphyrins and related macrocycles to peptides and proteins, readers are requested to refer to the excellent review of Giuntini et al.12
2 ANTIMICROBIAL PHOTODYNAMIC THERAPY
Porphyrins and their derivatives have widely been used in the past decades for application as sensitizers in photodynamic therapy (PDT).13 PDT is a localized therapy for the treatment of cancer and other non malignants conditions that involves the combination of light, oxygen and a visible light absorbing molecule (the photosensitizer, PS) to generate the cytotoxic reactive oxygen species (ROS) responsible for cell death.14 Tetrapyrrole compounds, such as porphyrins, phthalocyanines, and chlorins (Figure 1) possess favorable properties as PSs: (a) strong absorption bands in the red to near-infrared spectral region, 600–700 nm, where the light can deeply enter in tissues; (b) a substantial triplet quantum yield, leading to good production of ROS upon irradiation, and (c) minimal toxicity in the dark that offers a certain degree of intrinsic selectivity, because the generation of ROS occurs upon irradiation of the sensitizer. On the other hand, recognized drawbacks are the instabile nature of some molecules (f.i. bacteriochlorins15) and their poor bioavailability, being either too hydrophilic or conversely too hydrophobic and with limited water solubility. Moreover singlet oxygen, a key species in PDT cytotoxicity, has a short half-life (<0.04 µs) and limited diffusion distance (0.01-0.02 µm) in a biological environment16 which significantly inhibit its ability to impair central cellular counterparts.
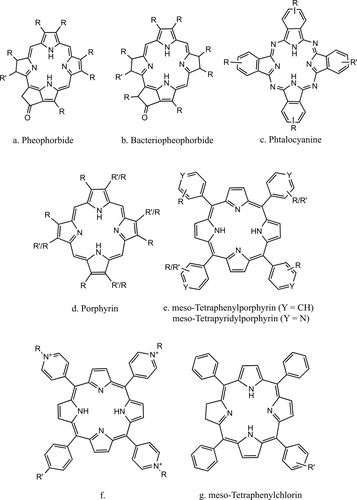
Basic structures of porphyrin-based PS. R means an alkyl or alkenyl substituent and R′ the functional group for the covalent linkage with the peptide chain
Conjugation strategies that modify the PS by linking to certain peptides or proteins have been used to improve the selectivity and solubility of the PS. In the field of antimicrobial PDT,17 poly-amino acids and cationic peptides have been conjugated to porphyrins to increase the affinity of the PS for the surface of Gram-negative bacteria. In comparison to Gram-positive bacteria the more complex cell envelope of Gram-negative bacteria forms an effective permeability barrier that tends to restrict the binding and penetration of many PS structures. Thus, strategies able to permeabilize this barrier in combination with non-cationic or cationic PS are effective against Gram-negative species.2 Cationic antimicrobial peptides are critical components of the innate immune systems of most living systems.18, 19 Their overall positive charge ensures accumulation at the polyanionic microbial cell surfaces that contain acidic polymers, such as lipopolysaccharides and wall-associated teichoic acids in Gram-negative and Gram-positive bacteria, respectively. Moreover, they act on different bacterial targets: most of them are membranolytic (f.i., magainin, cecropin) others (f.i., apidaecin, buforin) cross bacterial membranes, without apparent damages, to reach intracellular targets. Dosselli et al.20-22 prepared a number of conjugates between an antimicrobial peptide and a hydrophobic or cationic porphyrin (Table 1, entries 1–3). Besides improving the solubility of the hydrophobic PSs, the antimicrobial peptide was expected to address the photoactive drug against different bacterial targets. A carboxy functionalized meso-tetraphenylporphyrin (TPP) was covalently linked to the N-terminal end of apidaecin, magainin, or buforin and the conjugates exhibited high phototoxicity against both Gram-positive and Gram-negative bacteria under illumination. On Gram-negative bacteria the TPP-apidaecin and TPP-magainin resulted several orders of magnitude more phototoxic than the porphyrin alone but also loosely bound to bacteria and, depending from their amphiphilicity, more or less easily removed by washings. Thus, the apidaecin ability to penetrate Gram-negative bacteria was unfortunately lost after conjugation to the PS. TPP-buforin exhibited a huge phototoxic activity against Escherichia coli, comparable to the most active cationic PSs,17 that was fully retained also after extensive cell washing.22 Despite the selective toxicity toward bacterial cells exhibited by these cationic antimicrobial peptides and their porphyrin conjugates in the dark, under illumination all tested conjugates resulted more cytotoxic than the corresponding free porphyrins on human fetal fibroblasts.22 Very recently a conjugate between a cationic porphyrin and an analogue of the antimicrobial lipopeptide polymyxin B has been synthesized. Polymyxins are known as permeabilizer of the outer membrane of Gram-negative bacteria and when used in combination with a PS they are expected to improve the photodynamic effect. The conjugate showed a broad spectrum of antibacterial activity under light irradiation, and a better efficacy than the cationic porphyrin alone against Gram-negative bacteria. Correspondingly its cytoxicity against human dermal fibroblasts increased.23
| Entry | Reference peptide/receptor | Peptide sequencea, b | Type of PSc | Refs. |
|---|---|---|---|---|
| Antimicrobial peptides | ||||
| 1 | Apidaecin | *GNNRPVYIPQPRPPHPRL | e, f | 20-22, 43 |
| 2 | Magainin | *GIGKFLHSAKKFGKAFVGFILNS | e, f | 22, 43 |
| 3 | Buforin | *GTRSSRAGLQFPVGRVHRLLRK | e | 22, 43 |
| 4 | YVLWKRKRFC*FI | d | 25 | |
| Cell penetrating, Nuclear and Mitochondrial localizing peptides | ||||
| 5 | HIV-1 Tat(48–60) | *GRKKRRQRRRPPQ | e | 35, 39 |
| 6 | HIV-1 Tat(48–57) |
*GRKKRRQRRR GRKKRRQRRR* |
e, d | 24, 53, 95 |
| 7 | Bactenicin 7(15–24) | *PRPLPFPRPG | d | 94, 95 |
| 8 | pVEC | RRMKWK*G | e | 53 |
| 9 | LLIILRRRIRKQAHAHSK* | e | 53 | |
| 10 | CPP | *Rn | d, e, g | 35, 37, 41, 47, 95, 96, 98 |
| 11 | CPP/MMP-2 cleavable | *R9GPLGLAGE8 | d | 49 |
| 12 | NLS SV40 |
*PKKKRKV *MGLGLHLLVLAAALQGAWSQAPPKKKRKVG |
e | 33, 35, 39, 40 |
| 13 | Penetratin/NLS SV40 | *RQIKIWFQNRRMKWKK APPKKKRKVG | e | 39, 40 |
| 14 | NLS SV40/Tat(48–60) | *APPKKKRKVEDPRKKRRQRRRPPQG | e | 40 |
| 15 | Nucleoplasmin/Tat(48–60) | KRPAATKKAGQAKKKLEDPRKKRRQRRRPPQG | e | 39, 40 |
| 16 | MLS | *MSVLTPLLLRGLTGSARRLPVPRAKIHSL | e | 52 |
| 17 | *KLAKLAKKLAKLAK | d | 26, 57 | |
| 18 | MLS/CPP | MGRTVVVLGGGISGLAAGC*GRRRRRRRRR | d(Pt) | 96 |
| 19 | MLS/CTSB cleavable | *βCD-CGKRKGFLGEEHAIYPRH | g | 59 |
| Tumor homing peptides | ||||
| 20 | Neuropilin-1 | *ATWLPPR | g, d | 66-68 |
| 21 | EGF Receptor | *LARLLT | c,d | 69-71 |
| 22 | EGF Receptor | *YHWYGYTPQNVI | c, d | 69, 71 |
| 23 | Integrins αvβ3/αvβ5 | *RGD | d,e,g | 68, 75, 76 |
| 24 | Integrins αvβ3/αvβ5 | cyclo[RGDf*K] | g | 74, 76 |
| 25 | Integrins α6β1 | *TWYKIAFQRNRK | f(Ga) | 34 |
| 26 | GRP Receptor | *yQWAVAβH-Thi-Nle | d | 79 |
| 27 | µ-opioid Receptor | *YrFAβ | e | 83 |
| 28 | Bladder cancer αvβ3 Int. |
cycloSS[*cQDGRMGFc] cycloSS[*cGRLKEKKc] *RrRK cycloSS[cGRLKEKKc] |
f(Er) | 84 |
| 29 | Polo-like kinase 1 |
*PLHS(PO3H2) *PLHSD |
e | 86 |
| Other peptides | ||||
| 30 | *GDEVDGSGK-Phol | a | 63, 64 | |
| 31 | *GdevdGsGk-Phol | b | 15 | |
| 32 | MMP-7 | *GPLGLARK-Qu | a | 89 |
| 33 | FAP | *TSGPNQEQK | a | 90 |
| 34 | AK*AEAEKAAAEKAA*EEA | f | 101 | |
| 35 | Collagelin-a | *SGSGCPGRVMHGLHLGDDEGPC | e | 105 |
- a Single letter code for natural amino acids: upper case for L-amino acids or glycine, lower case for D-amino acids; other abbreviations: Aβ, β-alanine; βCD, β-cyclodextrin adamantine complex; Nle, L-norleucine; Phol, folic acid; Qu, black hole quencher (BHQ-3); Thi, L-thienylalanine.
- b The asterisk* shows the position of the PS in the conjugate.
- c See Figure 1; for metallated PS, the ion coordinated by the tetrapyrrole macrocycle is shown in brackets.
Besides natural antimicrobial peptides, de-novo designed cationic peptides have been linked to a PS to improve its association with the bacterial outer membrane, thus increasing the photodynamic activity.24-27 A chlorin derivative (purpurin-18) covalently linked to a six residues oligoarginine peptide resulted high phototoxic to Gram-positive bacteria, thanks to a higher penetration of the PS into the thick and porous peptidoglycan layer. The conjugate exhibited a considerable photohemolytic activity also on mammalian cells, even though considerably reduced in the presence of bacteria.27 Another example has been reported by Liu et al. who linked one or two copies of a lipopolysaccharide binding cationic peptide (Table 1, entry 4) to a protoporphyrin IX (PpIX) derivative, for fluorescent imaging and photodynamic inactivation of Gram-negative bacterial strains (Figure 2).25 Both the monomeric and the dimeric PpIX-peptide derivatives displayed higher phototoxicity than the free PS against tested bacterial strains, including strains with drug resistance properties. In confirmation of the importance of a close association of the PS with the bacterial envelope, the dimeric conjugate resulted more phototoxic than the PpIX-peptide on bacterial cells and no cytotoxic toward mammalian cells both in the dark and under light irradiation. The contrasting results on the toxicity of PS-peptide conjugates on mammalian cells suggest that the amphiphilicity of these compounds plays an important role in determining their selectivity toward bacterial cells and highly lipophilic PS could compromise the targeting specificity of peptide moiety.
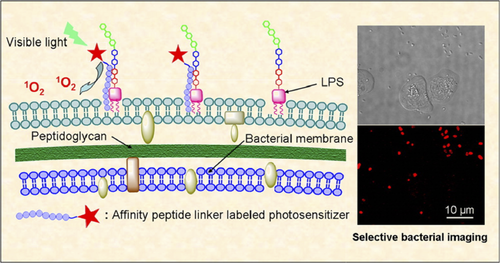
Fluorescent imaging and photodynamic inactivation of Gram-negative bacterial strains by a PpIX–antimicrobial peptide conjugate. Reproduced with permission from Ref. [ 25]
3 TUMOR PHOTODYNAMIC THERAPY
3.1 Peptide carriers for tetrapyrrole based photosensitizers
Though PDT was discovered in the field of microbiology, it has been mostly applied for cancer treatment, particularly of superficial tumors.28, 29 Since the end of the last century peptides were covalently linked to tetrapyrrole derivatives30 to increase their biological effectiveness as PSs and in particular to improve their tissue penetration and their accumulation in cancer cells. Cellular localization of the PS is important to the efficacy of PDT and a number of delivery systems have been suggested to enhance subcellular accumulation. Since the beginning nuclear localization signals (NLS)31 and cell penetrating peptides (CPPs)32 have been the most frequently used vectors of PSs to tumor cells. The group of Vicente synthesized a number of functionalized derivative of TPP bearing one, two, three or four copies of the eptapeptide PKKKRKV, corresponding to the minimum sequence of the NLS of the simian virus large T-antigen. The NLS was covalently linked through a short spacer to one, two, three, or four aromatic rings of the porphyrin macrocycle, or to a single position as three consecutive copies. The studies conducted on human carcinoma Hep2 cells showed that the tumor cell uptake and phototoxicity of the conjugates do not depend from the number of NLS units per molecule but by the amphiphilic character of the conjugate. NLS symmetrically substituted at the porphyrin periphery showed a reduced uptake and toxicity of the conjugate, whereas an amphiphilic conjugate containing a single peptide sequence, bearing multiple NLS, resulted the most phototoxic. Although the NLS was supposed to address the PS to the cell nucleus, all conjugates localized intracellularly within endosomal vesicles and lysosomes.33
PDT requires high nanomolar to micromolar quantities of PS34 and its functionalization by cell-penetrating peptides (CPP) has been shown to be effective in delivering a large amount of PS to the cell interior.35 Natural penetrating peptides and short oligoarginine sequences, inspired to protein transduction domains, have been conjugated to hydrophobic porphyrins and chlorins to enhance their uptake in cancer cells.24, 35-39 TPP linked through a hydrophilic spacer to the basic domain sequence (48–60) of the HIV-I transcriptional activator (Tat-peptide) (Table 1, entry 5) showed low dark toxicity and high phototoxicity on PC-3M human prostate cancer cells. The biodistribution of this conjugate in vivo was compared with that of the FDA-approved purified hematoporphyrin and the TPP-Tat conjugate resulted more selective and accumulated to a significantly greater extent in the tumor.38 Contrary to expectation, TPP conjugates with hybrid peptide sequences, containing both CPP and NLS (Table 1, entries 13 and 14), resulted less phototoxic than the TPP-Tat conjugate.39, 40 Li et al. have recently proposed natural cell-based carriers as “Trojan Horse” for delivering PSs to tumors. The porphyrin was anchored to the carrier cell by an arginine-rich sequence (KRRRR), ending with a palmitic acid residue for membrane insertion. The tumoritropic migratory properties of the carrier cells allowed accumulation of the porphyrin at the tumor site and under illumination only tumor cells in close contact with carrier cells were destroyed by PDT.41
Like CPPs, some antimicrobial peptides have the ability to translocate across biological membranes in a non-disruptive mode and they have been used to introduce fluorescent probes in mammalian cells.42 Three antimicrobial peptides were tested as porphyrin carriers in cancer cells. The corresponding TPP conjugates (Table 1, entries 1–3) were effectively taken up in A549 carcinoma cells and after irradiation with blue light they proved to be phototoxic at nanomolar concentration, whereas micromolar concentrations of the free PS were required to obtain the same effect.43 The dominant mechanism of cell internalization for all of porphyrin–CPP conjugates has been shown to be endocytosis but their intracellular localization has been reported in different subcellular compartments (lysosomes, endoplasmatic reticulum, Golgi apparatus, etc), depending on the peptide sequence and the overall charge of the conjugate.40, 43, 44
In spite the presence of several charged amino acids in CPP, their conjugates with hydrophobic PS can exhibit considerable self-aggregation in aqueous medium,36, 45 that has been usually hindered by inserting a hydrophilic spacer between the peptide and the porphyrin moieties.35, 36, 39, 40 As alternative approach to overcome solubility and aggregation problems, the encapsulation of the hydrophobic PS in β-cyclodextrin derivatives has been proposed46 and the cellular uptake of a supramolecular inclusion complex between a β-cyclodextrin and a TPP-octarginine has been recently reported.47 In a serum-free medium the host–guest complex was efficiently taken up by HeLa cells and dispersed in the cytosol, as a result of a direct transmembrane pathway. Nevertheless, in serum-containing medium the complex quickly dissociated, the conjugate was solubilized by serum proteins and accumulated in the endosome of cell. Thus, in spite of the ability of β-cyclodextrin derivatives in carrying large amount of porphyrin–CPP conjugate into the cytosol, the stability of the host-guest complex in serum containing media needs to be improved.
Although CPP can be considered excellent PS vectors, the presence of several positively charged amino acids in their sequence can cause strong nonspecific interactions, making them inapplicable for many in vivo applications. To avoid a premature capture of the conjugate, Li et al.48 linked PpIX to an “activable” CPP (Table 1, entry 11), formed connecting the positively charged CPP to a neutralizing polyanionic peptide sequence, through a specific proteolytically cleavable linker.49 After accumulation of the PS-neutral peptide conjugate at the tumor site, the anionic segment can be selectively cleaved off by the matrix-metalloproteinases present in the tumor extracellular matrix, thus releasing the PS-CPP. In vivo studies on animal model showed that the activable conjugate was quite stable in the blood-stream and extensively accumulated in tumor for in vivo imaging. Moreover, repeated treatments with the conjugate under illumination reduced size and weight of the solid tumor with a low systemic toxicity for the animal.
In spite of the enhanced cellular uptake in tumor cells achieved with PS-peptide conjugates, most of them localize in cytoplasmic membranes rather than in sensitive intracellular sites (such as mitochondria, the endoplasmatic reticulum, and the nucleus) where ROS produced by the photodynamic effect can be more effective. Considering that mitochondria are widely distributed in the cytoplasma and, as the energy center of cells, they manipulate many metabolic activities, they have become an important target in PDT.50 Several mitochondrial targeting sequences51 have been identified and used for the specific delivery of drugs. Sibrian-Vazquez et al. synthesized the conjugate between TPP and a 14 residue mitochondrial localization sequence (Table 1, entry 16), inserting a hydrophilic spacer between two moieties to increase the water solubility and hindering aggregation phenomena.52 The uptake of the conjugate in human carcinoma Hep2 cells was very efficient but it localized mainly within the cell lysosomes, resulting less phototoxic than a porphyrin bearing only a guanidinium group.
A notable improvement to the availability of the PS, and of drugs in general, into subcellular compartments is expected from the photochemical internalization (PCI) technology,53 the light-induced release of therapeutic agents sequestered into vesicles after uptake by endocytic mechanisms. In contrast to PDT, PCI is a site-specific drug delivery technique in which the therapeutic action is exerted by the drug itself rather than the PS. In PCI a sub-lethal light dose is applied to a PS, that localizes in endo/lysosomal membranes, which is sufficient to cause partial rupture of these intracellular organelles (mediated by singlet oxygen production), releasing the entrapped drug.54 TPP or chlorin-e6 linked to different CPP by a variety of bioconjugation techniques has been recently proposed as active component in PCI. PCI efficacy was tested in combination with a protein toxin, saporin, which was shown to co-localize with the PS-peptide conjugate into lysosomes. The release of the toxin was trigger by a short time irradiation and a significant reduction in cell viability was achieved in comparison to saporin or PS treatment alone, also when sub-lethal doses of PS and drug were combined together.44, 55, 56
Han et al.57 applied a dual-stage light irradiation strategy to reduce the sequestration in endosomes of the conjugate between PpIX and the pro-apotoptic peptide (KLAKLAK)258 (Figure 3). This strategy integrates PCI and PDT, using a short light irradiation to increase the cellular internalization of the conjugate by membrane permeabilization and a long time light irradiation to generate ROS in the mitochondria, leading to significantly improved PDT. The efficacy of this dual strategy was also tested in vivo on a murine model confirming minimal systemic cytotoxicity and efficacious long-term tumor inhibition.57 A sophisticated approach to deliver efficiently the PS to mitochondria was proposed by Tong et al.59 These authors synthesized a “smart nanocarrier” composed of three functional peptidic units: (a) a heptapeptide with specific binding ability to the transferrin receptor, overexpressed in highly proliferative cancer cell lines; (b) a cathepsin B cleavable sequence (GFLG); and (c) a mitochondria targeting peptide sequence (CGKRK) bearing at N-terminus an adamantane residue (Table 1, entry 19). Through this residue the peptide was non-covalently linked to a β-cyclodextrin functionalized with the PS (chlorine e6), forming a host–guest complex60 (Figure 4). This nanocarrier allowed a programmed mitochondria delivering of the PS through three stages: a receptor mediated intracellular accumulation of the complex in cells overexpressing the transferrin receptor (MF7); after internalization, the enzymatic cleavage by cathepsin B of the GFLG sequence caused exposure of the mitochondria targeting sequence; accumulation of the PS in mitochondria, where generation of ROS upon light irradiation triggered cell apoptosis. The progression of each step in MF7 cells was easily monitored by fluorescence microscopy and, upon light irradiation, a dose-dependent photodynamic cytotoxicity was achieved.59
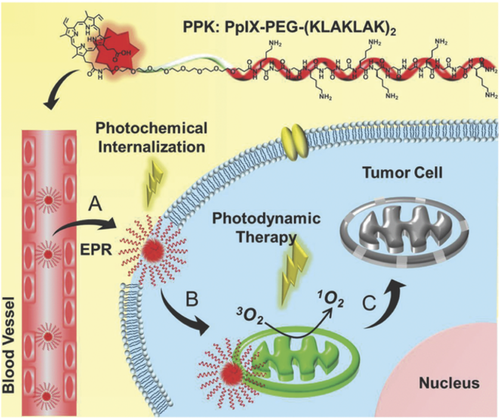
Chemical structure of the PPK conjugate and schematic diagram of the mitochondria-targeted self-delivering process. Reproduced with permission from Ref. [ 57]
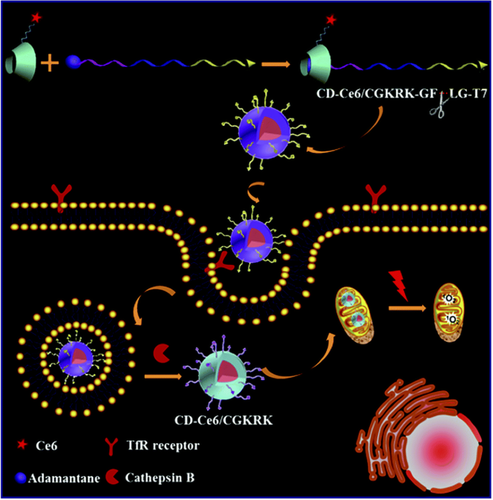
Schematic illustration of the programmed PS nanocarriers with dual targeting ability for enhanced PDT. Reproduced with permission from Ref. [ 59]
3.2 Tumor homing peptides
Although some PS used in PDT are found to be preferentially retained by the tumor cells compared with the surrounding healthy tissue, the exact mechanisms that drive this process are not fully understood. Tumor selectivity could be improved by targeted delivery agents that can transport the PS from the site of administration to the tumor tissue, leading to the selective accumulation of the PS in the tumor and limiting the damages to surrounding healthy tissues.61 Tumor cells express many molecules on their surface that distinguish them from normal cells. Traditionally, such molecules were detected with antibodies, but small targeting ligands such as folic acid, peptides, and aptamers have greatly expanded the number of tools available for selective binding to tumor cells.62 Zheng's group exploited folic acid to address a number of PS–peptide conjugates to tumor cells and tissues overexpressing the folate receptor (Figure 5).15, 63, 64 In these PS–peptide–folate adducts (Table 1, entry 30) the peptide improves the water solubility of the conjugate, makes the targeting agent more accessible to its receptor and acts as pharmacomodulator in vivo, decreasing the retention of the PS in excretion organs in favor of higher accumulation in tumor.63 Using bacteriochlorophyll as PS, a three component conjugate (Table 1, entry 31) was used as theranostic agent in vivo for near infrared fluorescence imaging and in PDT.15 Bacteriochlorophyll is very attractive for fluorescence imaging because it has a strong absorption between 600 and 900 nm, providing deeper tissue penetration due to the low tissue absorption and low autofluorescence. With pyropheophorbide-α as PS, on the same peptide, the conjugate could be converted in a positron emission tomography (PET) probe, by stabile chelation of the porphyrin macrocycle with the 64Cu radionuclide, thus providing a stable probe for prediction and quantitative measurements of the PS accumulation in tumors.64
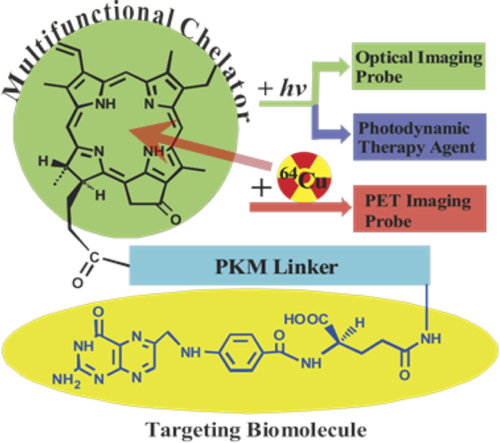
Structure of the PpIX–peptide folate conjugate for targeted PET imaging and PDT. Reproduced with permission from Ref. [ 64]
Peptides as tumor targeting agents have been discovered since the 90s, supported by the development of peptide library approaches.65 In PDT most of the efforts in the development of tumor targeting-PSs have been focused on the targeting of markers over-expressed on the tumor vasculature. Targeting the vasculature allows physiological barriers that prevent penetration of the PS into the tumor to be overcome, receptors at the surface of tumor vessels are readily accessible by circulating compounds and tumor vasculature is not specific for the type of cancer.61 Growth factors are key mediators of angiogenesis in cancer, a crucial step in tumor translation to metastatic form, capable of spreading it to other parts of the body. Thus, receptors for growth factors are excellent targets for specific PS delivery systems. Tirand et al. linked tetraphenylchlorine through a short spacer to the heptapeptide ATWLPRP, specific for the vascular endothelial growth factor co-receptor neuropilin-1 (Table 1, entry 20). In vitro the cell uptake and the photodynamic activity of the conjugate resulted significantly enhanced in comparison to the free PS66 and in vivo the conjugate exhibited good pharmacokinetic parameters and accumulated rapidly in the tumor. However, the peptide component was rapidly degraded in organs of the reticuloendothelial system, mainly liver, leading to loss of selectivity of the peptide moiety.67 The same peptide conjugated to a PpIX via the propionic side chains exhibited low dark- and photo-toxicity on cancer cell lines.68
In recent years another receptor to whom considerable attention has been paid is the epidermal growth factor receptor (EGFR), overexpressed in various human tumors of epithelial origin. Among the EGFR-targeting biomolecules reported for selective delivery of cytotoxic drugs to tumor sites, two small peptides (Table 1, entries 21 and 22) were used in PDT and fluorescence imaging for delivering the PS.69-71 In particular a phthalocyanine, bearing one or more hydrophilic modifications on the macrocycle to improve its water solubility, was covalently linked to the LARLLT peptide. The conjugate efficiently targeted EGFR over-expressing cells and was internalized in tumor cells according to the degree of EGFR expression. All of these PS-peptide conjugates were essentially nontoxic in the dark but, upon illumination, they became low to high phototoxic on cancer cells, depending on the light dose.69, 70 In vivo the fluorescence signals from the PS were detectable in tumor after 24 hours and remained consistent until 48–96 hours.68, 69 Overall these studies show that a high tumor selectivity can be achieved only by a careful balance between the hydrophilicity and the hydrophobicity of the conjugates.
The integrins are a family of membrane spanning receptors that mediate the attachment of a cell to the surrounding extracellular matrix proteins. αVβ3- and αVβ5-Integrins are highly expressed in tumor endothelium and have been exploited as target in tumor imaging and therapy.72 The tripeptide sequence RGD, found in many extracellular matrix proteins, is a promiscuous binder of integrins and has been widely used to address drugs and probes to tumors.73 Linear and cyclic-RGD peptides have been conjugated to different types of PS68, 74-76 (Table 1, entries 23 and 24) to promote selectivity and PDT in tumor cells. In αVβ3-over-expressing cells the uptake of the PS as RGD conjugate was considerably increased with respect to the free PS. Conversely not much more difference in the intracellular concentration of the PS was detected between the conjugate with the linear or cyclic, conformationally constrained, RGD moiety. Unfortunately, as a consequence of the increased cellular uptake, the PS-RGD conjugates resulted phototoxic on healthy HUVEC cells over-expressing αVβ3-integrins.76 Li et al. synthesized a theranostic probe using the RGD sequence and PpIX for tumor targeting PDT. Moreover, the theranostic probe comprised a caspase-3 responsive peptide linker connecting a Förster resonance energy transfer (FRET) couple (Figure 6) for the ratiometric imaging of the therapeutic effect.77 The authors showed that the conjugate was able to accumulate selectively in αVβ3-integrin overexpressing tumor cells, inducing under light irradiation cell-death. The therapeutic effect of PDT could be monitored in real time following the fluorescence induced by activation of caspase-3, a biomarker of cellular apoptosis, which interrupted the FRET process.77
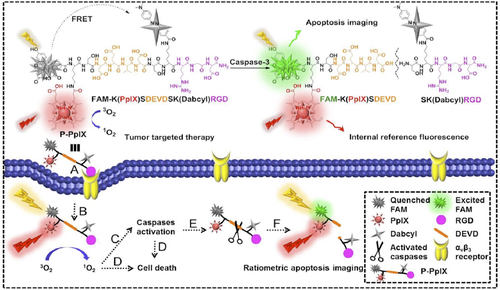
Chemical structure and schematic diagram of the theranostic probe (P-PpIX) for tumor targeted photodynamic therapy and ratiometric apoptosis imaging. Reproduced with permission from Ref. [ 77]
A dodecapeptide (Table 1, entry 25), with good affinity for a different family of integrins (α6β1) upregulated in multiple cancers,78 was recently used as targeting agent of a gallium(III) porphyrin, in pursuit of a cancer theranostic probe suitable as PET radiotracer and PS in PDT. The resulting conjugate proved to be selective and upon light irradiation cytotoxic on integrin over-expressing cells, showing the possibility of using the same conjugate as both PDT and PET agent.34 This conjugate represents an improvement of the strategy previously reported by Mukai et al.79 in which the conjugate between a tumor targeting peptide (a bombesin analogue,80 Table 1, entry 26) and a 64Cu radiolabelled porphyrin was prepared as PET agent to evaluate in vivo the pharmacokinetics of the PS prior of the PDT treatment. Porphyrin chelation with paramagnetic isotopes, such 64Cu, quenches the therapeutic action of the PS, requiring use of the unmetallated porphyrin as the treatment agent. Thus for theranostics, diagnostic and therapeutic agents have to be administered in separate doses. Unfortunately, PET-based imaging of tumor bearing mice treated with the radiolabelled PS-conjugate or with the radiolabelled porphyrin alone did not show significant differences in the biodistribution of the radio tracer, and its accumulation in the tumor site was not sufficient for in vivo targeting.
The blood–brain barrier largely restricts the movement of hydrophilic, high molecular weight molecules between the blood and the brain interstitial fluid, and this constitutes a major challenge in tumor therapy.
Recently a dermorphin analog81 (Table 1, entry 27), with high affinity for µ-opioid receptor and translocation ability through the blood–brain barrier, was linked via a hydrophilic spacer to a carboranyl porphyrin to achieve a conjugate for application in boron neutron capture therapy (BNCT),82 a binary treatment methodology for brain tumors and other cancers, that involves the irradiation of 10B-containing tumors with low-energy thermal or epithermal neutrons. The conjugate resulted non-toxic to human glioma T98G cells, both in the dark and upon exposure to low light irradiation, an important feature of boron-delivery agents because of the high amount of boron needed in this therapy.83 Moreover on in vitro cell model, it exhibited high permeability coefficient for the human blood–brain barrier, as required for a promising boron delivery vehicle for BNCT of brain tumors.
Zhou et al.84 exploited the multimodal properties of porphyrin–lanthanide complexes to kill bladder cancer by the singlet oxygen produced by the porphyrin moiety, simultaneously affording fluorescence imaging by the near infrared emission of the lanthanide. Three bladder cancer-specific peptide sequences (Table 1, entry 28) were proved to target this bi-functional tumor-imaging and PDT agent to cancer cells. Selective uptake of all of conjugates in bladder cancer cells was confirmed by in vitro imaging and high photo-cytotoxicity was achieved under illumination in the near-infrared region.
Polo-like kinase 1, a critical cyclin-independent serine/threonine protein kinase involved in many central cell cycle events and over expressed in tumors, has been proposed as dual target for cancer therapy.85 Short size peptide inhibitors of this kinase suffer from poor cellular uptake and their conjugation to the porphyrin macrocycle has been recently proposed for improving their entrance in cancer cells and monitoring the cell cycle.86 Two porphyrin–peptide conjugates (Table 1, entry 29) were shown to bind with high affinity to this target and one of these exhibited a responsive emission enhancement upon binding, that allowed monitoring the cell division. In vitro the conjugates were able to inhibit the growth of cancer cells with a good selectivity respect to normal cells (IC50 in cancer cells was 10-fold less than in normal cells) and, under light irradiation, about 40% of cancer cell death was achieved at PS concentration 10-fold less than that used in the dark.
3.3 Activable photosensitizers
An alternative strategy to tumor homing peptides to enhance the specificity of PDT is using activable PSs,87 that is PSs that can be turn on by a molecular trigger characteristic of the tumor environment. As an extension of the approach of molecular beacons,88 a photodynamic molecular beacon consists of a PS, a quencher and a disease-specific linker connecting two moieties. The PS's photoactivity is silenced by the quencher until the disease-specific linker is cleaved in the presence of the target molecule, thus releasing the PS that can be activated under illumination (Figure 7). A number of enzymes are overexpressed in some types of cancer and, due to well-characterized catalytic activity, they are excellent targets for these type of PSs. Zheng et al.89 synthesized a photodynamic molecular beacon connecting a pyropheophorbide to a quencher (BHQ-3) through a short peptide sequence (Table 1, entry 32), which is recognized and cleaved by matrix metalloproteinase-7, an enzyme overexpressed in pancreatic, colon, breast, and non-small cell lung cancer. The enzyme triggered photodynamic cytotoxicy of the construct was demonstrated in vitro on cancer cells and in vivo in mice bearing the same type of tumor. The activation of the photodynamic molecular beacon could be monitored by fluorescence imaging, because the enzymatic release of the porphyrin restored its fluorescence besides its photodynamic activity upon illumination. The same approach was later used for imaging and PDT of epithelial cancers overexpressing the fibroblast activation protein (FAP), a serine protease highly expressed in over 90% of human epithelial neoplasms and that plays a critical role in tumor growth and metastasis. PpIX and the quencher were linked through a specific nanopeptide sequence derived from α2-antiplasmin (Table 1, entry 33). The authors could prove the enzyme specificity in cleaving the molecular beacon and its ability to release the PS both in vitro and in vivo, offering a potentially useful tool for epithelial cancer detection and treatment.90
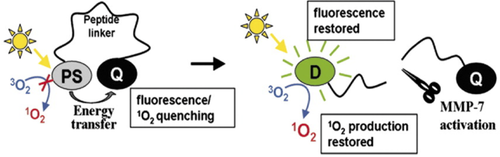
Schematic illustration of the concept of photodynamic molecular beacon. (A) PS's photoactivity is silenced by the quencher until the conjugate arrives at the tumor site; (B) here, the tumor associated protease recognizes and cleaves the specific peptide linker, releasing the PS which can generate the photodynamic effect. Reproduced with permission from Ref. [ 89]; copyright 2007 National Academy of Sciences
Using a similar architecture, Ke et al.91 developed a chemo-photodynamic prodrug, that can be selectively activated in the tumor microenvironment. They linked together the PS (a zinc phthalocyanine) and the drug (doxorubicin) through a tetrapeptide sequence (TSGP) sensitive to the FAP protease expressed in tumor environments. The singlet oxygen production of the prodrug was partially quenched by the presence of doxorubicin and the drug cytotoxicity was inhibited by the presence of the PS, that presumably hindered the binding of doxorubicin to DNA. In the presence of FAP (in solution or in HepG2 cells) the conjugate released the free PS and drug leading to significant enhancement of fluorescence emission and photo-cytotoxicity, suggesting a synergistic anticancer efficacy against HepG2 cells. The activation of the prodrug in vivo was confirmed by the fluorescence imaging of mice bearing a H22 murine hepatocellular tumor.91
4 BIOMARKERS DETECTION AND EMERGING THERAPIES
Unique spectroscopic properties of porphyrins allow the detection of small changes upon interaction with other molecules by using a plethora of spectroscopies, such as UV–visible, circular dichroism, nuclear magnetic resonance (NMR), fluorescence, and Raman. Not surprisingly porphyrin–peptide conjugates have been used to exploit these properties in sensors design.
Monitoring intracellular molecular oxygen (O2) offers important information on the viability, metabolic status and physiological behavior of living systems, because it is one of the key metabolites and functional parameters. Cellular O2 levels regulate metabolism, gene expression, formation of free radicals and many other processes and abnormal supply of O2 to the tissue is associated with many common pathological states and diseases.92
Among several methods for direct and indirect assessment of O2 in cells, minimal invasive strategy based on phosphorescent Pt(II)– or Pd(II)–porphyrins complexes have been proposed.93 For this application targeted delivery of the porphyrin into the cell, tissue or particular subcellular compartments is an essential requirement. To improve the intracellular delivery and distribution of metallated porphyrins, Papkovksy et al. functionalized the macrocycle periphery with cell-penetrating peptides of different length and charge (Table 1, entry 10) and compared their cellular distribution and O2 sensitivity in live mammalian cells.94-97 From these studies it emerges that, rather than the overall positive charge of the conjugate, its amphiphilicity and the spatial distribution of positive and negative charges on the molecule play an important role in determining the cell-penetrating ability. Peptides composed of eight-nine arginine residues ensure optimal cell penetration of coproporphyrin conjugates and linear or tetra-substituted derivatives produced high loading signals in all tested cell lines.96 For all these conjugates the main mechanism of cell entry was reported to be endocytosis, with different cell distribution for linear or branched derivatives. However, mitochondria could not be reached even inserting mitochondria targeting sequences in the peptide vector. In intracellular O2 sensing experiments several conjugates exhibited good analytical performance, representing a valuable tool for the measurement of the intracellular oxygen level at resting conditions and on metabolic stimulation.95, 96
Porphyrins are also one of the most studied DNA binding agents. The binding of cationic porphyrin derivatives to nucleic acids has been the subject of extensive research98 and recently some bioactive moieties were introduced onto the periphery of cationic porphyrins in order to utilize the tetrapyrrole macrocycle as DNA targeting agent.99 Bis-intercalating compounds show higher DNA binding affinities and slower dissociation rates than the corresponding monomers and a great number of dimeric forms of DNA intercalators has been developed as potential anticancer drugs.100 To achieve e selective recognition of DNA sequences, Byron and Voyer101 linked two cationic porphyrins on different positions of a α-helical peptide (Table 1, entry 34), to orient properly two DNA intercalating moieties on this peptide framework. All of the bis-porphyrin peptide conjugates were able to intercalate to double-stranded DNA, exhibiting a tremendous preference for guanine–cytosine over adenine–thymine sequences. Contrary to expectations, the length and structural properties of the peptide framework did not play an important role in the mode of interaction of porphyrins with various DNA. An alternative approach to improve the cellular uptake and the binding of cationic porphyrins to DNA was the introduction at the periphery of the macrocycle of one or two copies of a branched tetrapeptide.99 A dual mode of interaction of the conjugates to DNA was detected by spectroscopic techniques (intercalation and external binding) and intercalative binding was more feasible with the tricationic conjugate, especially on nucleoprotein complexes.99, 102 In vitro the uptake of porphyrin–tetrapepeptide conjugate was higher for the dicationic species and their cellular localization was proved in cytoplasmic organelles but not in the nuclear region.103
The research of biomarkers for early disease diagnosis has constantly grown in recent years and designing of imaging probes for targeting biomarkers in living tissues is a great challenge. Quantitative and qualitative changes in type I collagen, which is a major component in the extracellular matrix of skin and other vital organs, are often associated with the pathophysiology of these organs. Collagelin-a,104 a specific collagen binding peptide (Table 1, entry 35), was covalently linked to TPP as a fluorescent molecular probe for diagnostic imaging of early liver fibrosis. Chronic liver disease is characterized by the accumulation of extracellular matrix proteins, especially collagen, that destroys the hepatic architecture by forming fibrous scar. Chilakamarthi et al.105 showed that the TPP-collagelin specifically binds to collagen in vitro, with a marginal reduction of the binding respect to the unconjugated peptide. In ex vivo and in vivo conditions the probe could differentiate fibrotic and normal livers fibrosis, giving good opportunities in the management of chronic liver disease.
Even though PDT has been mostly applied in the treatment of tumors, interesting results have been recently reported in degradation of amyloid β-monomer and oligomers associated with Alzheimer's disease.106 Under photoirradiation the conjugate between a TPP derivative and the pentapeptide KLVFF, a key element in aggregation of 40- and 42-residue amyloid β peptides,107 was more efficient to degrade oligomers than the porphyrin alone. In vitro, the toxic effect of amyloid β peptides on neuron-like cells (PC12) was greatly inhibited by the conjugate in a dose dependent manner, without effect on the cell viability. A possible mechanism of inhibition of amyloid β peptides formation by a zinc–porphyrin–KLVFF conjugate has been recently proposed, consisting in the generation of off-pathway aggregated forms of amyloid β peptides not evolving into oligomeric or proto-fibrillary toxic forms.108
5 CONCLUSIONS
Peptides have contributed to extend the applications of porphyrins and related tetrapyrrole macrocycles in biological systems in different ways. By improving the solubility in aqueous environment of hydrophobic porphyrins, promoting their accumulation into cells by exploiting the cell penetrating properties of specific amino acid sequences and, thanks to the peptide specificity in recognition phenomena, addressing tetrapyrrole based PSs selectively to cells expressing specific markers. Most of porphyrin–peptide conjugates have been used in PDT, achieving significant results on treatment of tumor cells both in vitro and in vivo. Further research is needed to solve problems related to entrapment of porphyrin–peptide conjugates in endosomal vesicles and, for in vivo studies, to the instability in blood of conjugates with cationic peptides, but interesting solutions have been proposed such as PCI and the use of cleavable sequences. The possibility to use porphyrin–peptide conjugates as single probe in combined therapies (f.i., PDT/PET, PDT/BNC) is an important goal to overcome some limitation of PDT. Besides PDT, the ability of porphyrin–peptide conjugates in “sensing” physiological or pathological conditions in living systems is a very promising research area that can contribute to detection of biomarkers for early disease diagnosis.
The research on porphyrin–peptide conjugates has been constantly growing and it is expanding also in the nanomedicine field where nanostructures derived by the self-assembling properties of porphyrin–peptide conjugates can accumulate in the tumor tissue through the enhanced permeability and retention effect, overcoming some limitations of conventional PS formulations.109-112 Moreover new biomedical applications are emerging for these nanostructures, such as contrast agents in photoacoustic imaging and photothermal agents in thermal ablation of cancer cells and tumors.113-115
Biographies

Francesca Biscaglia graduated in Chemical Sciences at the University of Padova in 2014 with a dissertation focused on peptide-porphyrin conjugates for photodynamic therapy. She is currently pursuing her PhD at the University of Padova in the group of Professor Marina Gobbo. Her research interest is currently focused on the synthesis of peptides conjugates and preparation of plasmonic nanostructures as biosensors for cancer diagnostics and therapeutics.

Marina Gobbo received her PhD in Chemical Sciences in 1987 from the University of Padova. After a postdoctoral training in Prof Wünsch's group at Max-Planck Institute of Biochemistry in Martinsried (Germany), she was appointed as CNR researcher in the Biopolymer Research Centre in Padova. Since 2004 she has been associate professor in organic chemistry at the University of Padova. Her research interests are focused on the synthesis of conjugates of biologically active peptides for biomedical applications.