Intranasal glial heterotopia in an infant boy
ABSTRACT
Introduction
Nasal glial heterotopia is a rare congenital developmental disorder characterized by meningeal epithelium and/or glial components.
Case presentation
A 2-month-old boy presented for treatment of a congenital mass in the right nasal cavity near the pharynx. The preoperative diagnosis was congenital intranasal neoplasm. Nasal endoscopic resection of the nasopharyngeal mass was performed under general anesthesia. Histological findings in the resected tissue supported a diagnosis of intranasal glial heterotopia. The surgical outcome was good and no surgical site infection occurred. During 1 year of follow-up, the patient did not exhibit recurrence of heterotopia or related symptoms.
Conclusion
Transnasal endoscopic surgery is recommended for patients with intranasal glial heterotopia. Thorough preoperative imaging should be performed before glioma resection. The mass should be differentiated from encephalocele to prevent cerebrospinal fluid leakage and meningitis.
1 INTRODUCTION
Glial heterotopia is a rare congenital developmental disorder in which neuroglial tissue forms in extracranial sites, typically in the midline. Because glial heterotopia commonly occurs in or around the nose, it is often regarded as a nasal glial heterotopia (NGH).1 The reported incidence of congenital nasal masses ranges from 1 in 20 000 to 1 in 40 000 live births.2, 3 Herein, we describe a 2-month-old boy who presented with NGH; we also review similar cases reported in the literature.
2 CASE REPORT
A 2-month-old boy presented for treatment of a nasal obstruction that had been present since birth. The obstruction was obvious on the right side; it was persistent and had gradually worsened, such that it caused choking during bottle feeding. Fiberoptic bronchoscopy showed a 2.0 cm × 1.5 cm polypoid substance in the right nasal cavity near the pharynx (Figure 1). Computed tomography (CT) of the nasopharynx showed that a small area of high-density shadow in soft tissue of the right nasal cavity. The radiodensity of the mass was approximately 26–35 Hounsfield units and it did not show obvious enhancement; furthermore, there was no obvious bone defect in the skull base and the tumor did not exhibit communication with the brain. Magnetic resonance imaging (MRI) showed high-density shadows in the right nasal canal and vestibular soft tissue (Figures 2 and 3). The size of the soft tissue mass was approximately 22.1 mm × 17.3 mm × 10.9 mm; it exhibited heterogeneous enhancement. Furthermore, the mass extended to the back of the right nasal bone, and was adhered to both middle nasal septum and nasal bone (the right inferior turbinate was attached to the lateral edge of the mass). The middle nasal septum was slightly convex and curved on the left, while the right nasal canal was widened; the right nasal bone was slightly collapsed and deformed, while the right maxillary sinus cavity was slightly narrowed. The base of the mass extended from the lateral wall of the nasal cavity; the front boundary of the mass was at the level of the anterior end of the inferior turbinate, while the rear boundary of the mass was at the level of the posterior end of the middle turbinate. The upper boundary of the mass was approximately 2–3 mm from the top of the nasopharynx. The overall appearance of the mass was polypoid. The preoperative diagnosis was congenital intranasal neoplasm.
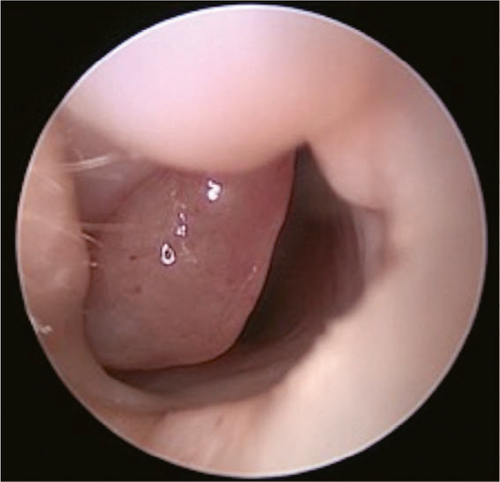
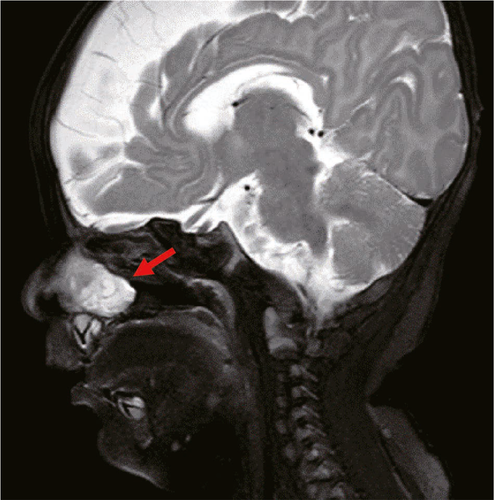
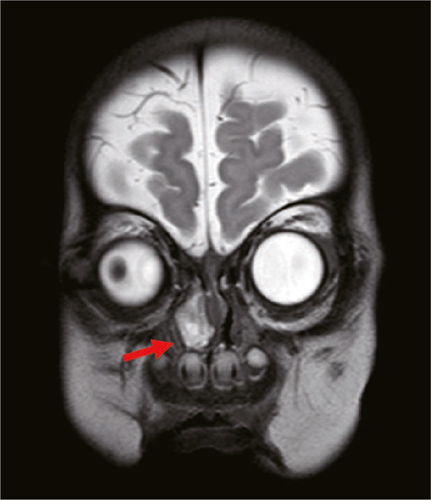
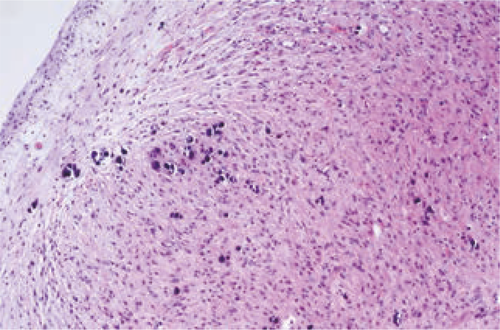
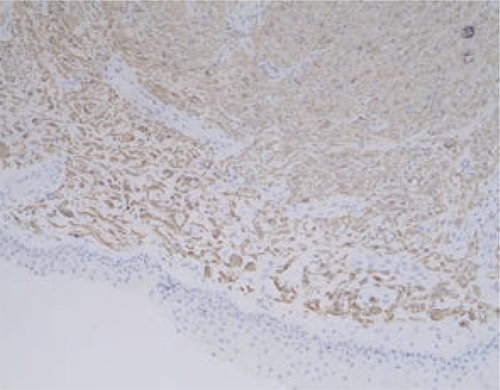
A nasal endoscope was used to excise the nasopharyngeal mass under general anesthesia. The base of the mass was extensive and located in the middle nasal canal of the lateral wall of the nasal cavity. The front and rear boundaries of the mass were consistent with those observed by MRI; notably, the rear boundary did not exceed the posterior nostril, nor did it reach the level of the nasopharynx and oropharynx. The upper boundary of the mass was also consistent with that observed by MRI. Histopathological analysis revealed that the mass was a glial heterotpia (Figure 4). Immunohistochemical analysis revealed the presence of glial fibrillary acidic protein and microtubule-associated protein 2, as well as the absence of Neuronal nuclei (NeuN) (Figure 5). The patient’s nasal obstruction was alleviated after surgery. At 3 months postoperatively, MRI confirmed that the right nasal cavity remained free of obstruction and that no abnormal density shadow was present. The patient did not exhibit recurrence of heterotopia during 1 year of follow-up.
3 DISCUSSION
A congenital midline mass in the nasal cavity is a rare developmental abnormality, with a reported incidence of 20 000 to 40 000 live births.2-5 NGH is presumed to constitute an encephalocele that has lost its intracranial connections. Most reports of NGH involve unilateral nasal or extranasal orbital, nasopharyngeal, middle ear, or scalp swellings.1 NGHs mainly occur in or near the nasal cavity; they occur outside of the nose in 60% of patients, inside the nose in 30%, and both outside and inside of the nose in 10%.6, 7 Notably, only 15%–20% of NGHs show intracranial communication.4, 8 In our patient, the NGH was located intranasally. Although glial heterotopias are benign lesions, they can cause clinical problems associated with their locations.9 Intranasal lesions may produce nasal obstruction as described in this report, epistaxis, or nasal deformity.
In our patient, the lesion was present at birth and did not exhibit a fibrous stalk connecting it to the intracranial space. The presence of glial tissue can be confirmed by assessing the immunohistochemical staining response to glial fibrillary acidic protein or S100 protein. Neurons are rare or absent in NGH, consistent with the findings in our patient. Although ependymal tissue is not always found in encephaloceles, its presence is more likely to lead to a diagnosis of encephalocele.4 On histological examination, NGH typically cannot be distinguished from encephalocele, as both types of lesions can contain various proportions of neurons and glia. And the two lesions have similar embryological origins and can both manifest as intranasal masses.
Nasal encephaloceles may be frontoethmoidal or basal.10 In frontoethmoidal encephalocele, brain tissue herniates through a defect of the frontal or ethmoidal bone into soft tissues of the forehead, nose, and orbit; these types of encephalocele are designated as nasofrontal, nasoethmoidal, or nasoorbital, respectively.11 Basal encephaloceles occur in the nasal cavity, rather than as an external mass; their developmental herniation is located posterior to the cribriform plate. The incidences of related developmental abnormalities in patients with encephalocele vary from 0% to 40%.12 The most common site of encephalocele is the occipital lobe (75%), followed by the frontal lobe (25%). NGH and nasal encephalocele are very rare diseases. It is difficult to distinguish them by histology. The diagnosis depends more on imaging, tumor location and intraoperative exploration, which requires multidisciplinary evaluation and management.13
The clinical manifestations of NGH are similar to those of congenital hemangioma, and it is difficult to distinguish between congenital hemangioma and NGH by using prenatal ultrasonography. The blood flow velocity in Doppler examination is rapid for congenital hemangioma, whereas it is slow for NGH. On T2-weighted MRI, both lesions show high signal intensity, but the intensity of NGH is lower than that of congenital hemangioma.14 Although it may be difficult to distinguish between NGH and congenital hemangioma, this does not directly influence treatment; both lesions require surgical treatment.14
CT and MRI are important tools in the diagnosis of NGH. CT aids in assessment of bone defects.15 Bone defects in patients with developmental abnormalities may be associated with NGH, but not with intracranial tissue.15 MRI is superior to CT for acquiring detailed information about soft tissue; it is also more useful for identification of an intracranial connection. In our patient, MRI showed no intracranial connection. Preoperative biopsy and resection are contraindicated without preoperative imaging to determine the extent and location of the mass, as well as to exclude any connection with the central nervous system; the avoidance of biopsy and resection prevents complications such as cerebrospinal fluid leakage, meningitis, or encephalocele. Thus, our patient was not biopsied preoperatively.
The preferred treatment for NGH is complete surgical excision. The potential for an intracranial connection must always be kept in mind when considering how to surgically treat a congenital midline mass to prevent the risk of cerebrospinal fluid leakage.16 NGH grows in a slow and benign manner, without the possibility of malignant transformation.17 However, early surgical treatment is advocated because continued gliosis may lead to deformity and facial bone erosion.17 NGH requires multidisciplinary management but has a good prognosis.18 Transnasal endoscopic surgery is recommended for patients with intranasal glial heterotopia. Due to advances in endoscopic equipment and technology, intranasal glial heterotopias can now be properly exposed and completely removed.4 For most patients with intranasal glial heterotopia or mixed NGH, endoscopic sinus surgery is feasible and does not involve increased operation time, nor does it involve increased rates of residual disease or complications.13 Our patient did not exhibit recurrence during 1 year of followup. The key clinical practice point illustrated by this report is that thorough preoperative imaging should be performed prior to glioma resection.
4 CONSENT FOR PUBLICATION
Consent was obtained from the patient’s parents.
CONFLICT OF INTEREST
None.




