The association between sleep duration and detailed measures of obesity: A cross sectional analysis in the ADDITION-PRO study
Abstract
Background
Sleep duration is associated with BMI and waist circumference. However, less is known about whether sleep duration affects different measurements of obesity differently.
Objective
To investigate the association between sleep duration and different measures of obesity.
Methods
In this cross-sectional analysis 1309, Danish, older adults (55% men) completed at least 3 days of wearing a combined accelerometer and heart rate-monitor for assessing sleep duration (hours/night) within self-reported usual bedtime. Participants underwent anthropometry and ultrasonography to assess BMI, waist circumference, visceral fat, subcutaneous fat, and fat percentage. Linear regression analyses examined the associations between sleep duration and obesity-related outcomes.
Results
Sleep duration was inversely associated with all obesity-related outcomes, except visceral-/subcutaneous-fat-ratio. After multivariate adjustment the magnitude of associations became stronger and statistically significant for all outcomes except visceral-/subcutaneous-fat-ratio, and subcutaneous fat in women. The associations with BMI and waist circumference demonstrated the strongest associations, when comparing standardized regression coefficients.
Conclusions
Shorter sleep duration were associated with higher obesity across all outcomes except visceral-/subcutaneous-fat-ratio. No specifically salient associations with local or central obesity were observed. Results suggest that poor sleep duration and obesity correlate, however, further research is needed to conclude on beneficial effects of sleep duration regarding health and weight loss.
1 INTRODUCTION
Traditionally, strategies to prevent and reduce obesity have focused on diet and physical activity. Yet, insufficient sleep is increasingly recognized as an important risk factor for obesity and through this pathway for obesity-related disease and mortality.1-3 The current evidence on the association between sleep duration and obesity relies predominantly on Body Mass Index (BMI)2 and shows either negative4-7 or U-shaped associations8, 9 where short and in part long sleep duration have been associated with higher BMI. These associations have been shown cross-sectionally2, 10 and prospectively.2, 6, 8
Particularly abdominal obesity has been demonstrated to be strongly associated with health- and cardiometabolic risk and studies also show strong associations between sleep duration and waist circumference (WC).5, 11-13 However, WC is not able to distinguish between visceral- and subcutaneous fat, whereof visceral fat has been identified as one of the strongest determinants for cardiometabolic risk and related mortality.14, 15 On the other hand, subcutaneous fat is not found particularly negatively associated with health risk.15, 16 Further, aging is associated with increased visceral fat, whereas the amount of subcutaneous fat declines with age,16 suggesting that measurements as BMI and WC may be less reliable for risk-assessment in older populations.
Only a handful of studies have investigated the association between sleep duration and obesity, including assessment of visceral- and subcutaneous fat depots in adult populations.11, 17-20 Studies that have, generally show shorter sleep duration to be associated with higher or increased visceral- and/or subcutaneous fat, however with varying strengths of associations.11, 17-19 Limited evidence is available for how sleep duration affects obesity measures differently. Further, most of the current evidence relies purely on self-reported sleep duration, which has been found to overestimate true sleep duration by up to an hour, with error varying by for example, sex, race, and subjective health rating.21, 22 Thus, it can be argued that the mechanisms linking sleep duration, different operationalizations of obesity and cardiometabolic risk are not yet fully understood.
The aim of this study was therefore to investigate the cross-sectional associations between objectively measured sleep duration and BMI, WC, visceral fat, subcutaneous fat, fat percentage and visceral-subcutaneous fat ratio, and to compare the magnitude of associations across measurement type. The study hypothesized sleep duration to be negatively associated with all obesity-related outcomes.
2 METHODS
2.1 Study design and participants
This study was a cross-sectional analysis of the measurements from the ADDITION-PRO cohort study. Design and population of ADDITION-PRO have been described in detail elsewhere.23 In brief, ADDITION-PRO was a longitudinal cohort study of the cardiovascular experience of individuals at high diabetes risk, recruited from Danish primary care in 2001–2006, through the stepwise screening program that formed the first recruitment step for the Danish arm of the ADDITION-Europe trial. Participants, aged 40–69 years who participated in the screening program and had varying levels of diabetes risk but did not have diabetes, were invited to participate in the ADDITION-PRO cohort study in 2009–2011. 4188 individuals were invited to the health examination, and 2082 (50%) attended. The study was approved by the scientific ethics committee in the Central Denmark Region (no: 20000182) and was conducted in accordance with the 1996 Helsinki Declaration. All participants gave written informed consent before any measurements were performed.
Prior to the health examination, participants completed a health questionnaire reporting demographic, lifestyle, and clinical information. At the examination participants underwent anthropometry, ultrasonography and were instructed to wear a combined accelerometer and heart rate monitor (ActiHeart; CamNTech Ltd., Cambridge, U.K) for 7 days and nights and to maintain their usual physical activity pattern.24 To ensure individual calibration of the heart rate-energy expenditure relationship, participants completed a submaximal step test at the day of the examination as described in detail elsewhere.25, 26 Only participants with sufficient wearable sensor data and without any missing information on obesity-related outcomes and covariates were included in the present study (n = 1309).
2.2 Assessment of sleep duration and physical activity
Participants' average nocturnal sleep duration (hours/night) was based on a minimum of three and a maximum of seven nights of combined heart rate and accelerometry recordings, combined with self-reported sleep pattern information. Sleep was self-reported via the open-end question: “At what time do you normally get up?”, “At what time do you normally go to bed?” on weekdays and weekends. Times-series data of energy expenditure were derived from the wearable sensor data (see further details below). Sleep was then defined as time when the energy expenditure was <1.04 metabolic equivalent of task (MET), within the reported hours of bedtime, with 1 MET being defined as 3.5 ml O2/kg/min. Nights with <3 h of estimated sleep duration were excluded, due to a strong indication of measurement-errors in recordings. Participants with missing sleep records, resulting in less than three included nights of data were excluded from the analyses (n = 181).
Physical activity volume was summarized as physical activity energy expenditure (PAEE) kJ/kg/day, derived by combining heart rate and accelerometry measures using branched equation modeling,27 with the relationship between heart rate and PAEE calibrated using data from the individually performed step test. This approach has been shown to be valid against gold-standard stable isotope measures.28 For participants who did not complete the step test, group calibration was used, based on the average heart rate response to stepping from 1046 ADDITION-PRO participants26 (n = 300).
For sensitivity analyses, fraction of time spent with moderate-to-vigorous physical activity (>3 METs) was also included. Participants also completed a modified, Danish version of the Recent-Physical-Activity-Questionnaire to access recent self-reported physical activity. This information was only included in sensitivity analyses, to compare the impact of adjustment for objectively assessed PAEE versus self-reported physical activity on the associations between sleep duration and obesity-related outcomes.
2.3 Obesity-related outcomes
Fat percentage and weight was measured by bioelectrical impedance using a Tanita Body Composition Analyser. Height was measured to the nearest 0.1 cm with a stadiometer (Seca, Medical Scales and Measuring Systems) with the participant wearing light indoor clothing but no shoes. BMI was defined as weight (kg) divided by height (m) squared. WC was measured to the nearest 0.1 cm. Visceral fat was defined as the depth in cm from the peritoneum to the lumbar spine and was assessed by ultrasonography (Logiq 9 machine, GE Healthcare), with the participant lying down. Validation studies have demonstrated ultrasonography assessments to correlate strongly with CT-assessment of visceral fat depots,29, 30 supporting validity of this method. The measurement was made where the xyphoid line crosses the waistline and was measured using a 4C abdominal convex transducer placed longitudinally. Scan depth was individually set for each image. Information on subcutaneous fat was obtained likewise but defined as the depth in cm from the skin to the Linea Alba.
2.4 Covariates
Information on age (years) and sex was derived from the unique Danish Civil Registration number. Ethnicity (Caucasian/other) was based on birthplace. Smoking status (current/not current) and alcohol consumption (units per week) were self-reported. Information on depressive symptoms was self-reported via the EQ-5D question: “I am… [not, moderately, extremely] …anxious or depressed” and were defined as present in participants who reported feeling moderately or extremely anxious or depressed.
2.5 Statistical analyses
Descriptive characteristics of the final study population were reported stratified by sex in Table 1. Categorical variables were expressed as number/proportion and continuous variables were expressed in mean/standard deviation. The main analyses investigated the association between sleep duration and all obesity-related outcomes, using multiple linear regression models, all including a sleep-by-sex interaction. Sleep duration and obesity-outcomes were treated as continuous variables. The interaction term was not statistically significant in any of the models (p > 0.05) but was kept based on biological differences in body composition tendencies across sex.31 Based on previous literature demonstrating a U-shaped association between sleep duration and obesity, inclusion of a quadratic term was tested. This term was statistically significant in five of 12 models, but as predicted values for the linear- and polynomial models showed minor differences, only linear models were reported here, whereas the polynomial models were provided as Supporting Information S1. Model 1 was adjusted for age and sex and model 2 was additionally adjusted for ethnicity, physical activity (PAEE), depressive symptoms, smoking and alcohol consumption. The models were performed for each obesity outcome separately. Subsequently, regression-coefficients were standardized to facilitate comparison of effect estimates. The standardization of regression coefficients for models with women as reference group were based on only women's outcome means and standard deviations and vice versa when having men as reference group.
| Men | Women | |
|---|---|---|
| N | 722 (55%) | 587 (45%) |
| Sleep duration (h) | 6.6 (0.8) | 6.7 (0.6) |
| BMI (kg/m2) | 27.6 (4.1) | 26.7 (5.0) |
| WC (cm) | 100.7 (11.4) | 90.1 (13.4) |
| Visceral fat (cm) | 9.0 (2.8) | 7.0 (2.4) |
| Subcutaneous fat (cm) | 2.3 (1.0) | 2.9 (1.2) |
| Fat percentage (%) | 27.1 (6.3) | 37.6 (6.8) |
| Visceral-/subcutaneous fat ratio (cm) | 5.0 (3.3) | 2.8 (1.6) |
| Age (years) | 66.4 (6.5) | 65.3 (7.3) |
| Ethnicity | ||
| Caucasian | 686 (95%) | 558 (95%) |
| PAEE (kJ/kg/day) | 34.1 (15.4) | 31.0 (13.8) |
| Depressive symptoms present | 73 (10%) | 107 (18%) |
| Smoker | ||
| Current | 145 (20%) | 76 (13%) |
| Alcohol consumption (units/week) | 12.6 (10.6) | 6.4 (6.2) |
- Note: Values are expressed in mean (sd) or n (%).
To examine the associations' robustness to different ways of adjusting for physical activity, sensitivity analyses included additional adjustment for proportion of time spent in moderate-to-vigorous intensity activities (>3 MET) and replacement of objective measurements of PAEE with self-reported physical activity.
The difference between the linear- and polynomial models was explored by predicting and comparing obesity-related outcomes for men and women at the fifth, 25th, 50th, 75th, and 95th percentiles of sleep duration.
All analyses were performed in RStudio version 4.1.1 (The R Foundation for Statistical Computing, www.R-project.org) at a 0.05 significance level.
3 RESULTS
3.1 Characteristics of the study population
The study population consisted of 55% men with a mean age of 66.4 ± 6.5 years (Table 1). Women were slightly younger (65.3 ± 7.3) and more likely to report depressive symptoms (n = 107, 18%) than men (n = 73, 10%). Men were more likely to be current smokers (n = 145, 20%) than women (n = 76, 13%) and had a higher mean of weekly consumed alcohol (12.6 ± 10.6 units) than women (6.4 ± 6.2 units). Men had a mean sleep duration of 6.6 ± 0.8 h/night, which was 6.7 ± 0.6 h/night in women.
Men had higher mean BMI (27.6 ± 4.1 kg/m2), WC (100.7 ± 11.4 cm), and visceral fat (9.0 ± 2.8 cm), but lower mean subcutaneous fat (2.3 ± 1.0 cm), fat percentage (27.1 ± 6.3%) and visceral-/subcutaneous fat ratio (5.0 ± 3.3 cm) than women (BMI: 26.7 ± 5.0 kg/m2, WC: 90.1 ± 13.4 cm, visceral fat: 7.0 ± 2.4 cm, subcutaneous fat: 2.9 ± 1.0 cm, fat percentage: 37.6 ± 6.8 cm, visceral-/subcutaneous fat ratio: 2.8 ± 1.6 cm).
Men had on average 166.4 h (6.9 days) of consolidated record time during the period of wearing the sensor, whereas women had on average 160.6 h (6.6 days) (data not shown).
3.2 Associations between sleep duration and obesity-related outcomes
Sleep duration was inversely associated with all obesity-related outcomes after multivariate adjustment, except for visceral-/subcutaneous fat ratio in men. In the analyses adjusted only for age and sex (Model 1), 1 hour longer sleep duration was associated with −0.3 kg/m2 BMI (95% CI: −0.8 to 0.2, p = 0.18) in men (Table 2) and −1.0 kg/m2 BMI (95% CI: −1.6 to −0.4, p < 0.01) in women (Table 3). For WC, 1 hour longer sleep duration was associated with −1.3 cm WC (95% CI: −2.6 to 0.04, p = 0.06) in men and −2.4 cm WC (95% CI: −4.1 to −0.8, p < 0.01) in women. Only estimates in women were statistically significant in Model 1, but when additionally adjusting for potential confounders in Model 2, the magnitude of associations became stronger and also associations in men turned statistically significant: BMI (men −0.7 kg/m2 [95% CI: −1.2 to −0.3, p < 0.01], women: −1.2 kg/m2 [95% CI: −1.8 to −0.6, p < 0.001]), WC (men: −2.5 cm [95% CI: −3.8 to −1.2, p < 0.001], women: −3.1 cm [95% CI: −4.7 to −0.6, p < 0.001]).
| Outcome | Linear regression | |||
|---|---|---|---|---|
| Model 1; β (95% CI) | p | Model 2; β (95% CI) | p | |
| BMI (kg/m2) | −0.3 (−0.8 to 0.2) | 0.18 | −0.7 (−1.2 to −0.3) | <0.01** |
| WC (cm) | −1.3 (−2.6 to 0.04) | 0.06 | −2.5 (−3.8 to −1.2) | <0.001*** |
| Visceral fat (cm) | −0.2 (−0.4 to 0.1) | 0.32 | −0.4 (−0.6 to −0.1) | 0.01* |
| Fat percentage (%-point) | −0.1 (−0.8 to 0.6) | 0.82 | −0.7 (−1.4 to < −0.001) | 0.05* |
| Subcutaneous fat (cm) | −0.1 (−0.2 to 0.04) | 0.18 | −0.1 (−0.3 to −0.02) | 0.02* |
| Visceral/subcutaneous fat ratio | 0.2 (−0.1 to 0.4) | 0.32 | 0.1 (−0.2 to 0.4) | 0.40 |
- Note: Reference group: Men. Model 1: Adjusted for age, sex. Model 2: Model 1 + ethnicity, physical activity (PAEE), depressive symptoms, smoking and alcohol consumption.
- *p < 0.05, **p < 0.01, ***p < 0.001.
| Outcome | Linear regression | |||
|---|---|---|---|---|
| Model 1; β (95% CI) | p | Model 2; β (95% CI) | p | |
| BMI (kg/m2) | −1.0 (−1.6 to −0.4) | <0.01** | −1.2 (−1.8 to −0.6) | <0.001*** |
| WC (cm) | −2.4 (−4.1 to −0.8) | <0.01** | −3.1 (−4.7 to −0.6) | <0.001*** |
| Visceral fat (cm) | −0.3 (−0.7 to 0.04) | 0.08 | −0.4 (−0.8 to −0.1) | 0.02* |
| Fat percentage (%-point) | −0.8 (−1.7 to 0.1) | 0.06 | −1.2 (−2.0 to −0.3) | <0.01** |
| Subcutaneous fat (cm) | −0.1 (−0.3 to 0.03) | 0.13 | −0.1 (−0.3 to 0.001) | 0.05 |
| Visceral/subcutaneous fat ratio | −0.2 (−0.5 to 0.2) | 0.42 | −0.1 (−0.5 to 0.2) | 0.43 |
- Note: Reference group: women. Model 1: Adjusted for age, sex. Model 2: Model 1 + ethnicity, physical activity (PAEE), depressive symptoms, smoking and alcohol consumption.
- *p < 0.05, **p < 0.01, ***p < 0.001.
The same tendency was observed for remaining outcomes, except for the visceral/subcutaneous fat ratio: In model 1, 1 hour longer sleep duration was non-significantly associated with lower fat percentage (men: −0.1% [95% CI: −0.8 to 0.6, p = 0.82], women: −0.8% [95% CI: −1.7 to −0.1, p = 0.06]), less visceral fat (men: −0.2 cm [95% CI: −0.4 to 0.1, p = 0.32], women: −0.3 cm [95% CI: −0.7 to 0.04, p = 0.08]) and less subcutaneous fat (men: −0.1 cm [95% CI: −0.2 to 0.04, p = 0.18], women: −0.1 cm [95% CI: −0.3 to 0.03, p = 0.13]), and after multivariate adjustment associations became stronger and turned statistically significant: Fat percentage: men: −0.7% (95% CI: −0.1 to < −0.001, p = 0.05), women: −1.2% (95% CI: −2.0 to 0.3, p = 0.006), visceral fat: men: −0.4 cm (95% CI: 0.6 to 0.1, p = 0.01), women: −0.4 cm (95% CI: −0.8 to −0.1, p = 0.02), subcutaneous fat: men: −0.1 cm (95% CI: −0.3 to −0.02, p = 0.02), except for subcutaneous fat in women, which remained insignificant (p = 0.052). For the visceral/subcutaneous fat ratio, estimates were non-significant in both Model 1 (men: 0.2 cm [95% CI: −0.1 to 0.4, p = 0.32], women: −0.2 cm [95% CI: −0.5 to 0.2, p = 0.42]) and Model 2 (men: 0.1 cm [95% CI: −0.2 to 0.4, p = 0.4], women: −0.1 cm [−0.5 to 0.2, p = 0.43]) and contrary to remaining outcomes, longer sleep duration was non-significantly associated with higher visceral to subcutaneous fat ratio in men.
When converting estimates to standardized regression coefficients, largest effects were found for BMI and WC in both men and women, whereas visceral/subcutaneous fat ratio showed the smallest effect compared to remaining obesity-related outcomes (Figure 1).
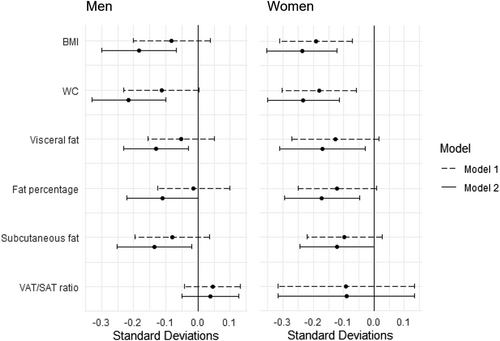
Forest plot: sleep duration (1 h) and standardized regression coefficients of obesity measures in men and women. Standardization performed by sex. VAT/SAT ratio: Visceral-/subcutaneous fat ratio
The observation that associations were stronger and statistically significant only after multivariate adjustment suggested that estimates in Model 1 were affected by reverse confounding, causing underestimation of the associations in Model 1. Physical activity (PAEE) had the strongest impact of the magnitude of associations, which may be explained by participants with shorter sleep durations having higher PAEE-levels (data not shown).
3.3 Sensitivity analyses
Additional adjustment for time spent specifically in moderate-to-vigorous physical activity (>3 MET) in Model 2 did not change results. However, if objectively assessed PAEE was replaced with self-reported physical activity in Model 2, results changed, so that only associations for BMI and WC in women remained statistically significant.
Analysis of deviation from linearity showed a statistically significant quadratic term in five out of 12 models. In all these cases the quadratic term was positive, indicating that the shape of the association was concave. When examining the differences between linear and polynomial models percentual differences in predicted values were on average 0.14% in men and 0.21% in women and did not exceed 2.84% (Supporting Information S1). Largest percentual deviations between values predicted by the polynomial- and linear models were seen for the outermost (fifth, 95th) percentiles, so that the predicted obesity levels at either end of the sleep duration distribution were slightly higher than estimated by the linear models.
4 DISCUSSION
This cross-sectional study investigated the associations between sleep duration and a range of adiposity outcomes in an older, adult population. Shorter sleep duration was associated with higher degree of obesity across all outcomes, except for visceral-/subcutaneous fat ratio. The strongest associations were observed for BMI and WC, whereas the weakest associations were observed for visceral-/subcutaneous fat ratio in both men and women. Results suggested that sleep duration was related to general obesity rather than specifically located obesity. Adjustment for physical activity (PAEE) strengthened the observed associations, and most associations were only statistically significant after multivariate adjustment. This suggested that estimates in the unadjusted analyses were affected by reverse confounding, causing underestimation of the associations if objectively assessed physical activity was not taken into account.
Sleep duration was assessed by a combination of self-reported sleep habits to identify the main timing of sleep and wearable senor data to reclassify time segments as not asleep when energy expenditure was above resting. Participants' average estimated sleep duration of 6.6 h/night in men and 6.7 h/night in women was consistent with previous literature assessing sleep duration objectively.4, 5, 32, 33 Some studies have observed a U-shaped association between sleep duration and obesity.8, 9 This study also found indication that the association between sleep duration and obesity was not fully linear after testing inclusion of a quadratic term in all models. This supported the suggestion that not only short sleep duration, but also long sleep duration might be associated with higher degree of obesity.8, 9 Exploration and comparison of the polynomial- and linear models showed that differences were only minor and restricted to the extremes of the distribution, indicating that for a large majority of the population the linear models provided an adequate description of the association between sleep and obesity.
Results of this study confirmed findings of a vast number of studies demonstrating that short sleep duration was associated with higher or increased BMI, WC, and fat percentage in adult populations, including both cross-sectional and prospective studies, with most of these assessing sleep duration by self-report.2, 6, 8, 13 Fewer epidemiological studies in adults have included visceral- and subcutaneous fat but results of those who have, point in the same direction as this study, where shorter sleep duration was associated with higher or increased visceral- and/or subcutaneous fat, however with varying statistical significance.11, 18, 19, 34 Dekker et al. suggested that although sleep duration was associated with different measures of overall adiposity (BMI, WC, and fat percentage), short sleep duration was not specifically associated with visceral fat in a Dutch study population.11 They demonstrated statistically significant associations between short sleep duration and higher BMI, WC, fat percentage and visceral fat, but when additionally adjusting for total body fat, the statistical significance of models with visceral fat disappeared. These results were in line with findings of this study, suggesting that sleep duration was associated with general rather than specifically located obesity in older adults.
A possible mechanism linking short sleep duration and general rather than specific obesity has been suggested as increased calorie intake.7, 8, 35 Short sleep duration affects levels of leptin and ghrelin, causing changes toward increased feeling of hunger.1 Higher concentrations of ghrelin have also been demonstrated to affect food preferences toward higher consumption of carbohydrates and sweets.8, 35 With short sleep duration leaving more awake hours available for eating, contemporary with increased feeling of hunger and high-calorie food preferences, this may result in increased calorie intake and thereby overall weight gain, rather than specifically located fat accumulation.
Unlike most other studies, this study observed reverse confounding of physical activity, since participants with shorter sleep durations had higher PAEE-levels, which is further associated with lower degree of obesity. Cooper et al. demonstrated a similar tendency for associations between sleep duration and BMI and WC, showing stronger and statistically significant associations only after multivariate adjustment.32 The common denominator of this study and Cooper et al. was the capability of assessing sleep duration and physical activity by objective measurements, whereas other studies either accessed sleep duration and/or physical activity by purely self-report11, 18, 19, 34 or refrained from including physical activity.33 Thus, variation in findings and different influence of confounding among studies might have been related to different assessments of sleep duration and physical activity. Poor health conditions were not included as confounding variables, since obesity and short sleep duration increases the risk of poor health,3, 36 making underlying health conditions likely colliders.
The current study had a number of strengths. The assessment of sleep duration using a combination of self-reported usual sleep habits and objectively assessed data reduced the risk of systematic errors and has been recommended to overcome challenges of using one in isolation.21 The definition of sleep based on a MET-threshold of <1.04 has previously been shown to be consistent with being in a posture indicating sleep.32, 37 Also, the use of small, wearable combined heart rate and movement sensors made it possible to track participants' sleep over several days in their natural sleep environments, which reduced potential “first-night-effects” and increased the probability that the recorded sleep duration was representative of the participants' usual sleep duration exposure.
On the other hand, the assessment of sleep duration was constrained to participants' self-reported usual bedtime. Thus, only sleep within this window of time was recorded. Any naps or extra hours of sleep outside the reported bedtime was not captured, which may have led to underestimation of sleep duration. However, there is currently not strong enough evidence to judge whether sleep outside of normal bedtimes hours has a qualitatively different impact on obesity and metabolic outcomes than sleep during usual bedtime hours. The tendency to nap or to have unstable sleep habits has itself been suggested to be associated with higher degree of obesity,38, 39 and simply adding daytime sleeping hours to participants' sleep duration would potentially have introduced an information problem which may could have created a false association. Further, inclusion of naps would most likely have added only minor sleep time to participants' sleep duration.
Unlike most other studies, this study was able to adjust for objectively assessed physical activity. However, these measurements may indirectly have been dependent on the participant's sleep duration, since shorter sleep duration by definition means having more time awake, which may have led to higher total physical activity energy expenditure. However, sensitivity analyses demonstrated that inclusion of time spent in moderate-to-vigorous intensity activities in Model 2 did not change results. However, if replacing objectively assessed-with self-reported physical activity in sensitivity analyses results changed so that only associations between sleep duration and BMI and WC in women remained statistically significant. Thus, the objective assessment of physical activity improved the ability to adjust appropriately for physical activity and limited its (reverse) confounding effect on associations under study.
This study also had a number of limitations. The cross-sectional design implied that no firm conclusions on causality of the association between sleep duration and obesity-related measurements could be drawn. A bidirectional association between sleep duration and BMI has been demonstrated,5 and reverse causation could not be rejected. The total balance of evidence ranging from observational cohort studies to laboratory- and smaller intervention studies however points toward a causal role, where short sleep duration affects obesity and metabolism,2, 6-8, 40-42 even if some degree of reverse causation was also present.
This study was not able to adjust for obstructive sleep apnea (OSA), which has an increasing prevalence with both age and obesity42 and it could not be rejected that OSA to some extent may have confounded the results, especially since the associations were examined in older adults. Further, analyses were not adjusted for menopausal status, which is associated with both disrupted sleep and increased fat mass relative to body weight, particularly in the visceral area.43 However, female participants were on average > 10 years older than the average menopausal age.43 Even though the increase in fat continues after menopause, the menopausal effect on sleep would no longer have been present. Further, studies investigating the association between sleep and obesity observed no change of results when adjusting for menopause.11, 17
The population of this study were older adults with elevated diabetes risk and results are considered generalizable to similar population segments. This is most likely population segments who are more frequently consulted in general practice, which further makes the results particularly interesting in terms of prevention of obesity-related diseases among older adults in primary health care. The generalizability to younger and non-Caucasian populations should be considered carefully.18, 42
In conclusion, this study provided further insight to the association between sleep duration and obesity by including different obesity-related outcomes, comprising assessment of the visceral- and subcutaneous fat depots. Results demonstrated statistically significant, inverse associations, where shorter sleep duration was associated with higher BMI, WC, visceral- and subcutaneous fat and fat percentage, but not with the visceral/subcutaneous fat ratio after multivariate adjustment in a population of older adults. Associations with BMI and WC were particularly strong, and results suggested that shorter sleep duration was associated with higher overall rather than specific obesity. Future studies should strive to assess physical activity and sleep duration objectively as well as to adjust for OSA. To provide further insight, future studies might seek to examine how other aspects of sleep is associated with different obesity-related outcomes, preferably using a prospective design in order to get closer to a more solid judgment of which aspect of sleep is causally related to obesity, since results of this study cannot conclude on the clinical- and interventional potential of sleep duration.
AUTHOR CONTRIBUTIONS
Mie M. Andersen contributed to the development of the research question and discussion, cleaned and analyzed the data, and wrote and edited the manuscript. Anne-Louise Bjerregaard collected and researched data, contributed to the discussion and reviewed and edited the manuscript. Tinne Laurberg, Dorte Vistisen, Jonas S. Quist, and Jens M. Bruun contributed to discussion and reviewed and edited the manuscript. Annelli Sandbæk and Daniel R. Witte designed the study, obtained funding, contributed to discussions, and reviewed and edited the manuscript. Søren Brage contributed to PA data analysis and discussion and reviewed and edited the manuscript. All authors approved the final version of the manuscript. Mie M. Andersen is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ACKNOWLEDGMENTS
The authors acknowledge the ADDITION-PRO study centers and are most grateful to the support staff and the participants for their contribution in the study. Data from the ADDITION-PRO study are not publicly available. Data can be made accessible upon reasonable request, with approval of the ADDITION-Denmark Steering committee and under a collaboration agreement. The study protocol is publicly available. The ADDITION-Denmark study was supported by the National Health Services in the former counties of Copenhagen, Aarhus, Ringkøbing, Ribe, and Southern Jutland in Denmark; the Danish Council for Strategic Research; the Danish Research Foundation for General Practice; Novo Nordisk Foundation; the Danish Centre for Evaluation and Health Technology Assessment; the Diabetes Fund of the National Board of Health; the Danish Medical Research Council; and the Aarhus University Research Foundation. Additionally, the ADDITION-PRO study was funded by an unrestricted grant from the European Foundation for the Study of Diabetes/Pfizer for Research into Cardiovascular Disease Risk Reduction in Patients with Diabetes (74550801), the Danish Council for Strategic Research, and internal research and equipment funds from Steno Diabetes Center Copenhagen. Anne-Louise Bjerregaard additionally received a scholarship funding from the Capital Region of Denmark. The Steno Diabetes Centers in Aarhus and Copenhagen receive part of their core funding from unrestricted grants from the Novo Nordisk Foundation.
CONFLICT OF INTEREST
Dorte Vistisen and Daniel R. Witte own shares in Novo Nordisk A/S. Daniel R. Witte has received funding from Novo Nordisk A/S. No other potential conflicts of interest relevant to this article were reported. The ADDITION-Denmark Steering Committee consists of Dorte Vistisen, Daniel R. Witte and Annelli Sandbæk.