Biotin Mitigates Alcohol Withdrawal-Induced Anxiety and Depression by Regulating Serotonin Metabolism, BDNF, Inflammation, and Oxidative Stress in Rats
Funding: The authors received no specific funding for this work.
ABSTRACT
Introduction
Substance use disorders, particularly alcohol use disorders, represent a significant public health problem, with adolescents particularly vulnerable to their adverse effects. This study examined the possible anxiolytic and antidepressant effects of biotin, a crucial vitamin for brain function, in attenuating the behavioral and neurobiological changes associated with alcohol withdrawal in adolescent rats.
Materials and Methods
Sixty male Sprague–Dawley rats were exposed to a 20% ethanol solution for 21 days, followed by a 21-day drug-free period to assess long-term behavioral and physiological changes. Behavioral assessments included the Open Field Test, Elevated Plus Maze, and Forced Swimming Test, administered post-withdrawal to evaluate anxiety and depression behaviors. Additionally, biochemical analyses were performed to measure serotonin levels, monoamine oxidase-A (MAO-A) activity, and BDNF concentrations.
Results
The results indicate that ethanol withdrawal significantly induced anxiety- and depression-like behavior in the rats. However, treatment with biotin, particularly at higher doses, effectively attenuated these withdrawal-related behavioral changes. Mechanistically, biotin administration was found to regulate serotonin levels, monoamine oxidase activity, brain-derived neurotrophic factor, and glial fibrillary acidic protein, and alleviate oxidative stress markers in cortical tissue.
Discussion
The results of this study suggest that biotin may have therapeutic potential for alleviating the negative effects of alcohol withdrawal, particularly those related to anxiety and depression. Further research is needed to elucidate the underlying mechanisms and examine the clinical effects of biotin supplementation for individuals undergoing alcohol withdrawal.
1 Introduction
Substance use disorders in adolescents have emerged as a critical global public health challenge [1], requiring a critical reassessment of research priorities that have traditionally focused on substance use patterns in adults. This shift is supported by a growing body of evidence highlighting distinct physiological changes in adolescent neurobiology [2]. During this period of development, the central nervous system undergoes significant structural and functional changes, which include axonal maturation [3], pruning of synapses [4], a reduction in cortical gray matter volume, and especially changes in the dynamics of the neurotransmitter system related to reward processing [5]. These neurodevelopmental changes are closely related to the development of adolescent-specific characteristics, such as an increased tendency to search for new things, risk-taking behavior [6]; and the beginning of experimenting with and consuming substances, especially alcohol [7]. Alcohol consumption during adolescence increases the risk of developing alcohol use disorder in adulthood by altering the brain's cortical and subcortical reward circuits, which undergo maturation during this developmental period [8]. Both acute and chronic ethanol (EtOH) consumption have been shown to induce structural, physiological, and functional changes in the central nervous system (CNS), potentially leading to neurodegenerative processes that result in neuronal death and subsequent neurobehavioral changes such as anxiety and depression [9-14]. Furthermore, alcohol exposure in adolescents may not only directly contribute to the development of these negative states but also indirectly promote their development by altering the brain's response to stress [15]. Recent studies have uncovered several key cellular mechanisms that could explain these relationships, particularly oxidative damage [16, 17], which impairs the body's antioxidant defenses and leads to the accumulation of free radicals and lipid peroxidation within cellular structures. This process appears to play a significant role in the negative effects of alcohol consumption [18, 19]. Additionally, excessive alcohol consumption hinders intestinal nutrient absorption and increases nutrient losses, further depleting vital antioxidant resources [20]. For optimal function, the brain relies on several nutrients, including biotin. Biotin serves as a crucial cofactor for various carboxylase enzymes, which play a role in the metabolism of carbohydrates, fatty acids, and amino acids. Biotin deficiency can lead to various neurological disorders, such as ataxia, developmental delays, hypotonia, seizures, and sensory and motor deficits [21]. Biotin has shown a protective effect against oxidative stress in the brain [22], particularly in neurodegenerative diseases [23]. This study aims to investigate the anxiolytic and antidepressant effects of biotin in rats exposed to EtOH in adolescence and undergoing withdrawal in adulthood.
2 Materials and Methods
2.1 Animals
In this study, 60 male Sprague–Dawley rats at 21 days postnatal age (PND 21) were obtained from the animal facility of Khatam Al-Nabieen University. The rats were housed in groups of 3–4 individuals in open-topped Plexiglas cages. The housing environment was controlled, maintaining a temperature of 22°C ± 2°C and a 12-h light–dark cycle (with lights on at 6:00 a.m.). They received a standard laboratory diet from Javaneh Khorasan (Mashhad, Iran) consisting of 46% nanofibrillated cellulose, 25% neutral detergent fiber, 19% protein, and 10% lipid. The animals had unrestricted access to drinking water throughout the experiment.
2.2 Drug and Experimental Design
EtOH with a purity of 99.5% (v/v) and biotin with a purity of ≥ 97% were purchased from Merck KGaA, Germany. The experimental EtOH solutions were prepared by diluting the 99.5% v/v EtOH stock solution with drinking water to achieve final concentrations of 5%, 10%, 15%, and 20% v/v. These different EtOH solutions served as the only drinking water for the experimental animals during the experimental period to induce progressive EtOH exposure. Biotin was dissolved in saline (0.9% sodium chloride solution) to prepare final dose solutions of 10, 100, and 500 mg/kg, each in a total volume of 1.5 mL. Animals were randomly assigned to six independent experimental groups of 10 rats each as follows: Group 1 (Vehicle): This group served as a control and received normal drinking water along with daily intraperitoneal (i.p.) injections of normal saline. Group 2 (EtOH withdrawal): Rats in this group were administered EtOH and received daily i.p. injections of normal saline, simulating the physiological effects associated with ethanol withdrawal. Groups 3–5 (EtOH + Biotin): These groups received the same EtOH administration as Group 2 and additionally received daily i.p. injections of biotin at doses of 10 mg/kg for Group 3, 100 mg/kg for Group 4, and 500 mg/kg for Group 5 during the EtOH administration. Group 6 (biotin): This group was administered biotin at a dose of 500 mg/kg via i.p. injection without exposure to EtOH.
The study employed an escalating EtOH exposure paradigm in adolescent rats to effectively mimic the binge drinking behaviors commonly seen in human adolescents. This approach was specifically designed to replicate the physiological and behavioral responses associated with acute ethanol consumption during critical developmental stages. From PND 24 to 25, rats assigned to the EtOH groups were given drinking water containing an initial concentration of 5% EtOH. This concentration was deliberately chosen to ensure controlled ethanol administration and minimize variability in exposure among subjects. To maintain consistent exposure levels across all experimental conditions, the EtOH concentration was incrementally increased by 5% after the rats in the ethanol groups consumed a volume of fluid equivalent to that of the control groups. The protocol was structured as follows: on days 24 to 25, the rats ingested a 5% EtOH solution; on days 26 to 27, the concentration was raised to 10% once the rats achieved fluid intake equivalent to the control group; and on days 28 to 29, the concentration was further increased to 15%, again ensuring parity in fluid intake. This gradual escalation of ethanol concentration not only emulates the binge drinking patterns observed in adolescents but also allows for the examination of physiological adaptations and potential behavioral changes resulting from increased ethanol consumption. Once all rats in the ethanol groups consistently consumed a 20% ethanol solution, this concentration was maintained from PND 30 to PND 60 [24, 25]. Throughout the study, interventions were conducted during the adolescent phase of the rats, specifically from PND 21 to 60. Following the treatment period, a subsequent 21-day drug-free interval was implemented for all groups (Figure 1). This interval was designed to enable the animals to eliminate any residual alcohol from their systems and to evaluate any withdrawal symptoms that may arise after the cessation of treatment.
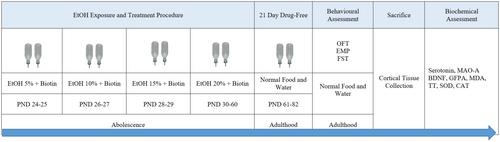
2.3 Behavioral Assessments
Behavioral assessments, including the Open Field Test (OFT), Elevated Plus Maze (EPM), and Forced Swimming Test (FST), were performed following the drug-free period. Prior to testing, all animals were acclimated to the experimental room for a minimum of 30 min to reduce stress. To further minimize anxiety, the rats underwent a 5-day treatment regimen before the experiments. On the day of testing, the rats were confined for 1 h prior to receiving either vehicle or diazepam via intraperitoneal injection. Lighting conditions were set to 150 lx for the EPM and 40 lx for the OFT to promote exploratory behavior. To prevent cross-contamination, previously tested rats were kept separate from those that had not yet been tested. Additionally, all behavioral apparatus was cleaned with a 10% ethanol solution between experiments to eliminate any residual odors.
2.3.1 Open Field Test
The OFT is a commonly used approach for assessing anxiety-related behaviors in rats [26]. In this study, the OFT was performed in a square arena measuring 100 × 100 × 40 cm, constructed from opaque materials, and divided into 25 equal quadrants, each measuring 20 × 20 cm. Each rat was placed in the center of the arena and given 5 min to explore this unfamiliar environment. Their movements were recorded and analyzed with video tracking software, specifically measuring the time spent in both the central and peripheral areas of the arena [27].
2.3.2 Elevated Plus Maze
After the OFT, the anxiolytic effects of biotin were assessed using the EPM, a plus-shaped device made of opaque gray wood positioned 50 cm above the ground. The closed arms were equipped with 40-cm-high walls to provide a safe environment. Each rat was placed individually in the center of the maze with an open arm and allowed to explore for 5 min. Their behavior was recorded and analyzed based on various parameters, including the time they spent in both the open and closed arms, which served as indicators of anxiety-like reactions.
2.3.3 Forced Swimming Test
In the FST, animals were placed in glass cylinders filled with water at a temperature of 24°C ± 2°C and a depth of 30 cm to facilitate swimming. The procedure included a 15-min pretest followed by a 5-min test conducted 24 h later. After each session, rats were removed, dried, and kept warm for 30 min before being returned to their cages for the remainder of the experiment. This standardized approach allowed the assessment of behavioral responses such as fighting, immobility, and swimming duration, which serve as common indicators of stress and depressive behavior. The 24-h interval between sessions was designed to assess the stability of observed behaviors over time [28].
2.4 Euthanasia
Following the behavioral experiments, animals were euthanized using a 95% CO2 method [29], specifically a step-fill technique in which CO2 was introduced into the euthanasia chamber at a rate of 30% of the chamber volume per minute [30]. The brains were then carefully removed, and the cortical tissue was dissected and frozen for later biochemical analysis.
2.5 Biochemical Measurements
Following the behavioral tests, we measured biochemical markers in cortical tissue samples, including serotonin, monoamine oxidase-A activity (MAO-A), brain-derived neurotrophic factor (BDNF), and glial fibrillary acidic protein (GFAP). Additionally, we assessed markers of oxidative stress, such as malondialdehyde (MDA), total thiol (TT), superoxide dismutase (SOD), and catalase (CAT) activity.
2.5.1 Measurement of Serotonin, MAO-A Activity, and BDNF, GFPA
Serotonin concentrations and MAO-A activity were assessed using an ELISA kit from MyBioSource (MBS713292), based in San Diego, CA, USA. Additionally, BDNF and GFAP concentrations were quantified using ELISA kits from CUSABIO (CSB-E04504r, CSB-E08602r, USA) specifically designed for rats. All tests were performed according to the manufacturer's guidelines. Absorbance values were recorded for quantitative analysis using a Biotech microplate reader (Winooski, Vermont, USA), and the results were compared to a standard curve prepared under consistent experimental conditions.
2.5.2 Quantification of MDA, SOD, TT, and CAT
MDA levels were quantified by a method in which MDA reacts with thiobarbituric acid (TBA) to produce a red-colored complex, which was then measured spectrophotometrically [27]. The TT was evaluated using a colorimetric method using DTNB (5,5′-dithio-2-nitrobenzoic acid), which forms a yellow complex with maximum absorbance at 412 nm. SOD activity was measured using a colorimetric enzyme assay kit, where a standardized SOD activity unit indicates the amount of sample that can convert 1 μmol O2 to H2O and O2 in 1 min. The enzymatic activity of CAT was evaluated according to the Aebi protocol using hydrogen peroxide (30 mM) as a substrate [27].
2.6 Statistical Analyses
Data were analyzed using GraphPad Prism software (version 8.4.3.). Statistical methods used included one-way ANOVA accompanied by Tukey's post hoc tests. Results are presented as mean ± standard error of the mean (SEM). To assess statistical significance, a significance level of α = 0.05 was used, which corresponds to a confidence interval of 95%.
3 Results
3.1 Biotin Attenuates EtOH Withdrawal Induced Anxiety-Like Behavior
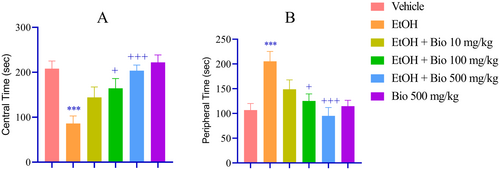
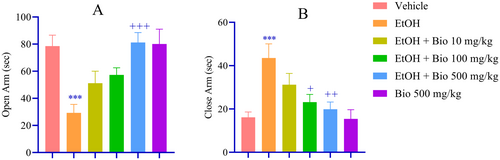
Animals subjected to EtOH withdrawal exhibited pronounced anxiety-like behaviors, demonstrated by a significant reduction in exploration of the central area during the OFT (p < 0.001; Figure 2A) and a corresponding increase in exploration of the peripheral area (p < 0.001; Figure 2B) compared to the vehicle-treated group. Pre-treatment with biotin at doses of 100 and 500 mg/kg led to a significant enhancement in central area exploration (p < 0.05 and p < 0.001, respectively; Figure 2A) and a decrease in peripheral area exploration (p < 0.05 and p < 0.001; Figure 2B) relative to the EtOH withdrawal group. Further validation using the EPM indicated that EtOH-deprived animals displayed significantly heightened anxiety-like behaviors, characterized by a marked reduction in the time spent in the open arms (p < 0.001; Figure 3A) and an increase in the time spent in the closed arms (p < 0.001; Figure 3B). Importantly, the higher dose of biotin significantly mitigated these anxiety-like behaviors, as reflected by an increased duration in the open arms (p < 0.001; Figure 3A) and a decreased duration in the closed arms (p < 0.05 and p < 0.01; Figure 3B) compared to the EtOH group.
3.2 Biotin Attenuates EtOH Withdrawal-Induced Depression-Like Behavior
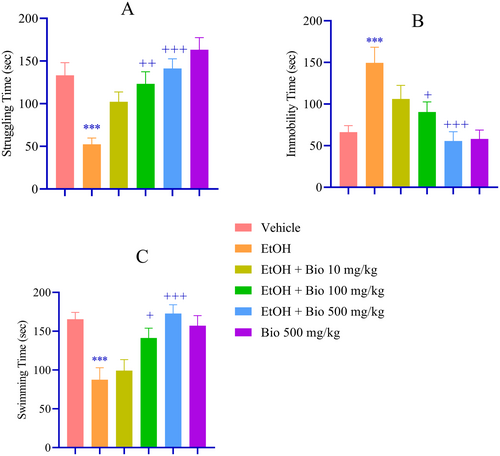
In the FST, EtOH withdrawal was associated with increased depressive-like behavior, as indicated by a significant reduction in struggling time (p < 0.001; Figure 4A), increased immobility (p < 0.001; Figure 4B), and shortened swimming time (p < 0.001; Figure 4C) compared to the vehicle group. Pre-treatment with biotin at doses of 100 and 500 mg/kg significantly increased fight time (p < 0.01 and p < 0.001, respectively; Figure 4A) and decreased immobility (p < 0.05 and p < 0.001, respectively; Figure 4B). and longer swimming time (p < 0.05 and p < 0.001, respectively; Figure 4C) compared to the EtOH group.
3.3 Biotin Prevents EtOH Withdrawal-Induced Alterations in Cortical Serotonin Levels, MAO-A Activity, BDNF, and GFAP Concentrations
EtOH withdrawal resulted in a significant decrease in serotonin levels, accompanied by an increase in MAO-A activity, a decrease in BDNF, and an increase in GFAP levels (p < 0.001; Figure 5) compared to the vehicle group. Administration of biotin at doses of 100 and 500 mg/kg significantly increased serotonin levels (p < 0.05 and p < 0.001, respectively; Figure 5A). Furthermore, the biotin dose of 500 mg/kg significantly reduced MAO-A activity (p < 0.001; Figure 5B) and effectively prevented the decrease in BDNF (p < 0.001; Figure 5C) and decrease in GFAP levels (S < 0.05; Figure 5D) observed in the EtOH withdrawal group.

3.4 Biotin Improves EtOH Withdrawal-Induced Oxidant/Antioxidant Imbalance
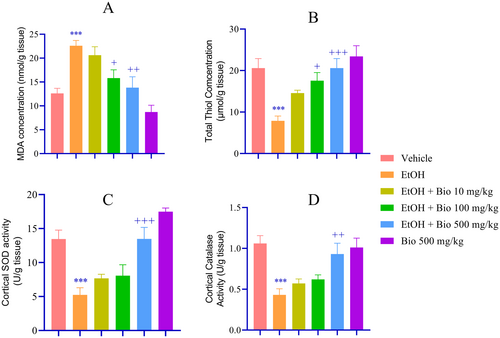
Biochemical analyses showed that EtOH-deprived animals had increased MDA levels, decreased thiol concentrations, and decreased SOD and CAT activity compared to the vehicle group (p < 0.001; Figure 6). Pre-treatment with biotin at doses of 100 and 500 mg/kg significantly decreased MDA levels (p < 0.05 and p < 0.01, respectively; Figure 6A) and increased TT concentrations (p < 0.05 and p < 0.001, respectively; Figure 6B). Notably, biotin at a dose of 500 mg/kg increased SOD (p < 0.001; Figure 6C) and CAT activity (p < 0.01; Figure 6D) compared to the EtOH group.
4 Discussion
Extended ethanol consumption during developmental stages [31, 32], followed by withdrawal, leads to various behavioral impairments, particularly characterized by anxiety and depression [33].
The findings of our study demonstrate that EtOH consumption during adolescence, followed by cessation, results in heightened anxiety and depressive-like behaviors in adulthood in rats. This is substantiated by a significant decrease in exploratory behavior within the central zone of the OFT (Figure 2), a markedly reduced duration spent in the open arms of the EPM (Data S1 and Figure 3), and reduced struggling and swimming time and increased immobility time in the FST (Data S1 and Figure 4). Interestingly, pretreatment with biotin at doses of 100 and 500 mg/kg significantly improved exploration of the central area, suggesting a reduction in anxiety-like behaviors. This is further supported by the results of the EPM, where biotin resulted in a longer time in the open arms and a shorter time in the closed arms, confirming its anxiolytic effect. Moreover, biotin at doses of 100 and 500 mg/kg significantly improved struggling and swimming time and reduced immobility time in FST, confirming its anti-depression effect.
Although this finding has not been reported previously, research has indicated that biotin metabolism influences the central nervous system [34], and maintaining biotin homeostasis is crucial for proper bodily functions, particularly in the brain [35]. Increased biotin intake is associated with reduced occurrence of depressive symptoms [36]. Magnesium biotinate also reported significant improvements in anxiety-like behaviors and cognitive impairment in animal models exposed to propionic acid, a neurotoxin [37]. Additionally, a randomized controlled trial showed that biotin supplementation combined with probiotics demonstrated positive effects on psychiatric symptoms in individuals with major depressive disorder. The study suggested that biotin may influence the gut microbiota, which is linked to mental health through metabolic and anti-inflammatory pathways [38]. Such results confirm existing literature suggesting that nutritional supplementation can positively influence anxiety behavior [36, 39].
In this study, we further conducted biochemical experiments on cortical tissue to assess the effects of EtOH and biotin on serotonin metabolism, inflammatory response, neurotrophic factor, and oxidative stress balance. The cortex is a critical area of the brain involved in various higher-order functions, including cognition, emotion regulation, and sensory processing [40]. Additionally, the cortical region is especially vulnerable to the effects of alcohol, particularly oxidative stress [41] and neuroinflammation [42], especially during key developmental stages. Studies have shown that exposure to ethanol can result in considerable neuronal loss and structural alterations within the cerebral cortex, especially in developing brains [43]. Furthermore, alcohol consumption is linked to changes in gene expression in the prefrontal cortex, a region vital for cognitive functions and impulse regulation [44]. Adolescents, in particular, are at heightened risk for alcohol's impact on the frontal cortex, which can disrupt synaptic connections and result in enduring behavioral and cognitive impairments [45]. By focusing on the cortex, we aimed to gain insights into the specific mechanisms through which biotin may exert its effects on anxiety and depressive-like behaviors. The results showed that EtOH withdrawal is associated with reduced cortical serotonin levels and elevated MAO-A activity (Data S1 and Figure 5A,B), both of which contributed to the depressive symptoms noted in the FST. Elevated MAO-A activity is known to lead to decreased serotonin levels, exacerbating mood disorders [46, 47]. It has been demonstrated that inhibiting MAO-A can produce antidepressant effects. For instance, one study identified novel MAO-A inhibitors that have the potential to facilitate the development of effective antidepressants with fewer side effects, underscoring the enzyme's significance as a therapeutic target in the treatment of major depression and anxiety disorders [48]. Likewise, additional research into monoamine oxidase inhibitors indicates their potential to elevate serotonin levels, which may enhance mood and alleviate anxiety symptoms [49]. In this study, the administration of biotin at doses of 100 and 500 mg not only resulted in behavioral improvements but also positively affected key neurobiological parameters. Specifically, biotin pre-treatment was associated with increased serotonin levels and decreased MAO-A activity, as illustrated in Figure 4B. Consistent with our previous study [50], EtOH withdrawal was associated with a decrease in BDNF levels and increased GFAP concentrations. The rise in GFAP levels in the EtOH group signifies reactive astrogliosis, which is the response of astrocytes to injury or stress, such as that induced by ethanol exposure. This increase indicates that astrocytes are becoming more active, likely as a protective measure for neurons and an effort to restore balance in the brain [51]. BDNF is crucial for neuroplasticity and neurological survival. Lower levels of BDNF have been associated with major depressive disorder and anxiety, suggesting that reduced neurotrophic support may contribute to the pathophysiology of these disorders [52]. Increased levels of BDNF, often stimulated by physical activity and certain antidepressants, may help relieve symptoms of depression and anxiety [53]. GFAP is being investigated as a potential biomarker for neurotoxicity and neuroinflammation, as its high levels have been found to correlate with the severity of neuropsychiatric symptoms [54], particularly anxiety and depression, suggesting a possible role of astrocytes in the neuroinflammatory response associated with these disorders [55]. In this study, biotin not only prevented the declines but also increased BDNF levels and decreased GFPA concentrations, suggesting a possible restoration of astrocyte functionality and a decrease in the reactive state caused by ethanol. This change does not necessarily imply a reduction in the number of astrocytes but rather indicates a return to typical activity and function [56]. Biotin has been investigated for its role in improving the delivery and effectiveness of BDNF in therapeutic applications. It has been shown that a biotin-conjugated version of BDNF, administered intravenously, successfully restored neuronal density in rats. This finding indicates that biotin may aid in the transport of BDNF across the blood–brain barrier, thereby enhancing its neuroprotective effects [57]. Although biotin is recognized for its involvement in various metabolic functions and its potential neuroprotective properties [23, 58], direct studies connecting biotin to GFAP expression or function are currently insufficient. Nonetheless, GFAP is frequently utilized as a biomarker for neurotoxicity and gliosis [59], suggesting that any neuroprotective effects of biotin might affect GFAP levels by modulating neuroinflammatory processes. On the other hand, as demonstrated in our previous study [50], the results indicated that EtOH withdrawal worsens the oxidative stress profile, as evidenced by elevated levels of the oxidative marker MDA and reduced levels and activity of antioxidant indicators, including total thiol content and the activities of SOD and CAT. The brain is particularly susceptible to oxidative stress due to its high oxygen consumption, limited antioxidant defenses, and lipid-rich structure [60]. Oxidative stress is linked to various neurological conditions, including neurodegenerative and neuropsychiatric diseases [61], neuroinflammation [62, 63], and disrupted neurotransmitter systems [64, 65], suggesting it may play a role in their pathogenesis. Reducing oxidative stress in the brain can significantly restore neurotransmitter levels, including serotonin, which often declines during stress or deprivation. For example, studies have demonstrated that administering antioxidants can elevate serotonin levels in animal models experiencing oxidative stress [27, 66]. Normalizing serotonin levels through the reduction of oxidative stress has been linked to improved behavioral outcomes. For example, treatments that mitigate oxidative stress have been associated with reduced anxiety and depression and enhanced cognitive performance in various animal studies [27, 50, 67, 68]. Increased oxidative stress is associated with decreased BDNF levels. For example, in patients with bipolar disorder, higher levels of oxidative stress markers correlated negatively with BDNF levels, suggesting that reducing oxidative stress may help normalize BDNF [69]. Oxidative stress can stimulate BDNF release under certain conditions. For instance, in neuronally differentiated PC12 cells, exposure to oxidative stress increased BDNF secretion [70]. Antioxidant treatments have been shown to increase BDNF expression in various models. For example, in PC-12 cells subjected to oxidative stress, the use of specific compounds reduced oxidative damage and promoted BDNF expression, highlighting the protective role of antioxidants in enhancing BDNF levels [71]. Our results indicated that administration of biotin at doses of 100 and 500 mg/kg led to a significant reduction in MDA levels. Simultaneously, we observed an increase in antioxidant activity, further underscoring biotin's potential as a powerful antioxidant [72]. These findings suggest that biotin may be instrumental in mitigating oxidative stress, particularly during periods of EtOH withdrawal, which is often accompanied by heightened oxidative damage.
5 Conclusion
Our findings demonstrated that EtOH exposure in adolescents, followed by abstinence, negatively impacted behaviors related to anxiety and depression by altering various biochemical processes. These included changes in serotonin metabolism, MAO-A activity, increased neuroinflammation, and oxidative stress in the cortex. Biotin pre-treatment effectively mitigated the harmful effects of alcohol withdrawal by restoring disrupted biochemical parameters. Thus, we recommend biotin administration as a safe, cost-effective, and efficient approach to prevent or alleviate cognitive issues linked to alcohol abstinence in adolescents. Further research is needed to explore how biotin may reverse biochemical changes induced by EtOH withdrawal at the receptor and signaling levels.
Author Contributions
Murtaza Haidary: supervision, conceptualization, methodology, formal analysis, writing – review and editing. Dawood Hossaini: investigation. Adam Khan Alipour: investigation, review and editing. Meysam Sajjadi: investigation. Mustafa Ansari: data curation.
Acknowledgments
The authors express their gratitude to the Medical Sciences Research Center of Khatam Al-Nabieen University for their valuable collaboration and provision of the essential resources to carry out this study.
Ethics Statement
All procedures of the study were approved by the Animal Ethics Committee of Khatam Al-Nabieen University (AF, knu.edu.af.rec 14–10 May 2024) and conformed to the Guide for the Care and Use of Laboratory Animals [73].
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data generated and analyzed during this study are freely available to the public.