Administration of a Selective Antagonist for Pituitary Adenylate Cyclase-Activating Polypeptide Receptor in the Hippocampus Causes Anxiolytic Effects in the Male Rat
Funding: This study was supported by the Meiji University International Collaborative Research Promotion Project, Japan (Grant Numbers MU-OSRI-ICRPP2024-208 to MK) and JSPS KAKENHI (Grant Numbers 22H00396 to TM).
ABSTRACT
Pituitary adenylate cyclase-activating polypeptide (PACAP) affects rodents' stress-related behaviors, such as anxiety-like behavior or fear conditioning. However, previous studies have investigated the effect of intracerebroventricular, but not hippocampal, injection of this PAC1R-selective antagonist (PACAP-6-38) on anxiety-like behavior. However, it has been reported that administration of PACAP-6-38 to the dorsal hippocampus reduces the fear response in a fear conditioning test. Therefore, this study aimed to determine whether the effect of dorsal hippocampal PACAP-6-38 injection in Sprague–Dawley rats can affect anxiety-like behavior assessed by an elevated plus-maze test. As a result, rats treated with PACAP-6-38 spent longer time in open arms than those with saline, suggesting that this drug has an anxiolytic effect. In conclusion, the role of PACAP in the dorsal hippocampus can promote anxiety-like behavior.
1 Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide hormone belonging to the secretin-glucagon vasoactive intestinal polypeptide (VIP) family [1] and is originally isolated from sheep hypothalamic extracts. PACAP is widely expressed in the central and peripheral nervous systems in two forms, PACAP-38 and PACAP-27 [2, 3]. Both peptides are derived from the same precursor, and PACAP-38 is abundant in rat brain tissue [4, 5]. From PACAP isolation, three G protein-coupled PACAP receptor subtypes [PACAP type 1 receptor (PAC1R) and VIP-PACAP type 1/2 receptors (VPAC1/2R)] have been successively identified. PAC1R binds to the two forms of PACAP with high affinity and selectivity, whereas VPAC1R and VPAC2R bind PACAP and VIP with similar affinity [6].
The hippocampus is one of the most important brain regions responsible for regulating anxiety and fear [7-10]; however, it is also known for its functions in memory and spatial cognition. The dorsal hippocampus contributes specifically to cognitive learning, and the ventral hippocampus contributes to anxiety regulation, but the former is also involved in anxiety regulation [7]. PACAP and its receptors are highly expressed in the hippocampus [11, 12], and PACAP antagonist (PACAP6-38) administration directly into the dorsal hippocampus decreases fear responses [13]. Conversely, intracerebroventricular administration [14] and administration to the central nucleus of the amygdala of PACAP elicited anxiety [15]. However, the direct involvement of PACAP in anxiety in the hippocampus has not been confirmed.
This study aimed to evaluate whether PACAP in the dorsal hippocampus affects anxiety-like behavior using a specific PACAP antagonist, PACAP6-38, administered to the hippocampus. Schmidt et al. demonstrated that PACAP antagonist administration to the dorsal hippocampus reduced fear responses in a fear conditioning test [13], suggesting that PACAP signaling in this region may contribute to emotional regulation. Therefore, the dorsal hippocampus was targeted to investigate its role in anxiety-like behavior. PACAP6-38 is a fragment peptide with a sequence from the sixth amino acid from the N-terminus to the C-terminus of PACAP38. This antagonist is often used as a potent antagonist of PAC1R. Anxiety was evaluated using the elevated plus-maze (EPM) test. Its effect on fear conditioning was also tested to confirm the effect of the PACAP antagonist in the hippocampus, as reported previously [13].
2 Materials and Methods
2.1 Animals
Sprague–Dawley (SD) male rats (10 weeks old, 250–300 g) purchased from Charles River Japan (Kanagawa, Japan) were used. All animals were kept in a room with controlled temperature (24°C ± 2°C) and humidity (50% ± 5%) under a 12 h light/12 h dark cycle (light at 09:00–21:00). Food and water were provided to access ad libitum.
2.2 Placement Surgery of the Guide Cannula
Animals were anesthetized by isoflurane and placed in a stereotaxic apparatus (RWD Life Science, China) in a skull-flat position for the implantation of two stainless-steel guide cannulas in the left and right dorsal hippocampal areas. After the bregma was located, a rotary tool was used to drill into the skull and get two holes, according to the following sites: anterior, 3.8 mm; lateral, ± 2.7 mm; and ventral, 3.2 mm [16]. To maintain potency, a dummy cannula (AD-3.2, Eicom, Kyoto, Japan) was inserted into the guide cannula (AG-3.2, Eicom). Animals were allowed to recover from surgery for 7 days before behavioral observation. Animals were handled once daily for three consecutive days, and all behavioral observation procedures were conducted between 13:00 and 17:00.
2.3 Pharmacological Treatments
PACAP-6-38 (Tocris Biosciences, Bristol, UK) was used in this experiment. PACAP-6-38 is a nonstimulating competitive PACAP receptor (PAC)1 antagonist. The drug was freshly dissolved in 0.9% sterile saline. In the EPM test, microinjections (40 pg/side) [17] were carried out 30 min before the behavioral testing started. In the contextual fear conditioning (CFC) test, microinjections (40 pg/side) were carried out < 1 min after the training sessions. The animals were gently restrained by hand, and the injection cannula (AMI-4.2, Eicom) was fitted tightly into the guides, extending 1 mm from the tip of the guide cannula. The injection needle was connected to a 10 μL Hamilton microsyringe, and the infusions were performed at a rate of 0.5 μL/1 min. The microinfusion volume used was 1.0 μL/side into the dorsal hippocampus. At the end of the microinfusion, the injection needle was left in place for 1 min to allow the solution to diffuse away from the cannula tip, carefully withdrawn from both sides.
2.4 Behavioral Procedure: EPM Test
Anxiety-like behavior was measured using an EPM test with the open arms (L 500 × W 100 mm) and closed arms (L 500 × W 100 × H 450 mm), which were raised 50 cm above the ground. The closed arms had opaque walls, and the open arms were designed with a short ledge (H5 mm) [18]. During the EPM test, the light intensity in the four arms was adjusted to 100 ± 5 lx. Vehicle or PACAP6-38 was administered in the hippocampus 30 min before the behavioral testing started. The test began when the animals were faced with the open arms, with their heads always facing the open arms. Anxiety-like behavior was recorded for 5 min, and the time spent in the open arms, the number of entries into the open arms, and total moving distances were measured using a Noldus Ethovision XT version 15.0 system (Noldus Information Technology, Wageningen, The Netherlands).
2.5 Behavioral Procedure: CFC Test
We conducted the experiment 3 days after the EPM, by referring to a study that administered a PACAP antagonist to the hippocampus and performed fear conditioning tests [13]. The contextual fear conditioning system (MFS-01 M, Muromachi Kikai Co. Ltd., Tokyo, Japan) was placed in a soundproof chamber (length and width: 320 mm, height: 400 mm), and the illumination level at the floor of the chamber was adjusted to 100 lx. The rat behaviors were recorded with a video camera (HDR-CX720V, SONY, Tokyo, Japan) fixed to the ceiling of the soundproof box. On the training day, the animals were introduced into the conditioning chamber, habituated for 2 min, and given two foot shocks (0.6 mA, 1 s) that were delivered at a 30 s interval. Animals were removed from the conditioning chamber 30 s after the last foot-shock and immediately intrahippocampal infusions of vehicle (saline) or PACAP6-38 (40 pg/side). After 24 h, the animals were placed in the same apparatus for a 3-min retention test with no foot shocks. The freezing behavior was defined as a state in which no movement other than breathing and duration period of freezing was measured. Freezing was defined as a state in which no movement other than breathing was observed. Between the trials, the chamber and the grid floor were cleaned with 70% ethanol, followed by distilled water.
2.6 Cannula Placement Evaluation
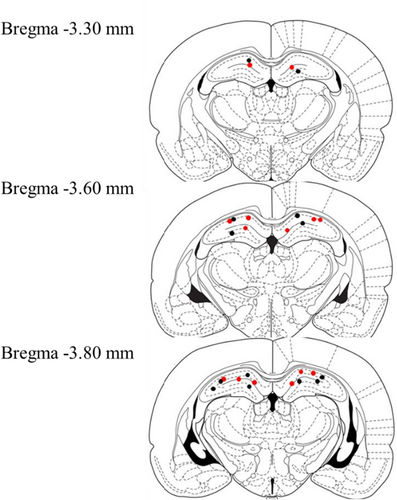
At the end of the behavioral experiment, rats were perfused intracardially with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer for fixation of the brains. The brains were removed, and cannula placement was verified on coronal sections (40 μm) made with a cryostat (CM 1510-3, Leica Instruments, Nussloch, Germany) and subsequent Nissl staining. Only rats with at least one cannula tip located in the area of the dorsal hippocampus were included in this study (n = 14; Figure 1).
2.7 Statistical Analysis
Statistical analyses were performed using Student's t-test for independent samples to compare the means between the treatment and control groups using BellCurve Excel Statistics for Windows (SSRI, Tokyo, Japan). The tests were two-tailed, and p < 0.05 was considered statistically significant.
3 Results
3.1 Effects of PACAP Antagonist in the EPM Test
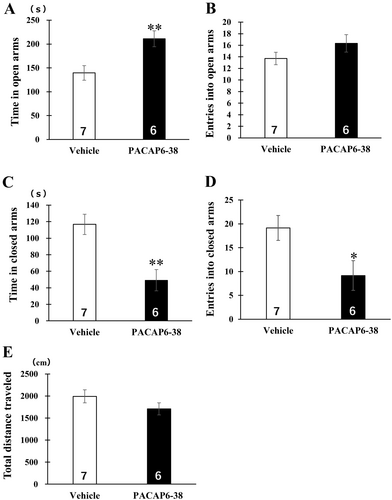
Vehicle or PACAP6-38 was administered into the dorsal hippocampus 30 min before the behavioral testing started. The animals that received PACAP6-38 intradorsal hippocampal infusions significantly increased the time spent in open arms of the EPM test [t (11) = 3.16, p < 0.01; Figure 2A]. Reductions by the antagonist were observed in the time spent [t (11) = 3.74, p < 0.01; Figure 2C] and the number of entries [t (11) = 2.39, p < 0.05; Figure 2D] in the closed arm. There were no significant differences in the number of entries into open arms [t (11) = 1.43, p = 0.17] and the total distance traveled [t (11) = 1.40, p = 0.19; Figure 2B,E].
3.2 Effects of PACAP Antagonist in the CFC Test
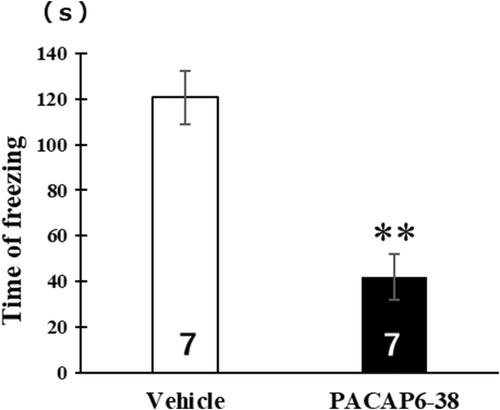
The animals that received PACAP6-38 intradorsal hippocampal infusions after the contextual fear conditioning training exhibited lower levels of freezing than the vehicle group during the retention test [t (12) = 4.61, p < 0.01] (Figure 3). Freezing was scored and converted into a percentage.
4 Discussion
PACAP is a neuropeptide involved in learning, memory, and emotion regulation [19, 20, 26]. In this study, PACAP acted via the hippocampal PAC1R to increase anxiety-like behavior in the EPM test by administering a PAC1R-selective antagonist within the dorsal hippocampus. The current CFC test was performed the same way as the experiment conducted by Schmidt et al. [13] and reproduced the same results of suppressing freezing as in Schmidt et al. Therefore, it was confirmed that administration of the PAC1R-selective antagonist to the hippocampus in this study was as effective as reported previously. However, no significant differences were observed in the number of entries into the open arms between the vehicle and PACAP antagonist groups. One explanation is that the PACAP antagonist group may have simply spent more time in the open arms without increasing the frequency of entries, which is supported by the finding that no significant difference occurred in the total distance traveled between the two groups, suggesting that the PACAP antagonist did not affect the overall locomotor activity. Therefore, the increased time spent in the open arms by the PACAP antagonist group may reflect reduced anxiety-like behavior rather than altered general activity.
Activation of intrahippocampal PAC1R stimulated the phospholipase C/protein kinase C/Pyk2/Src signaling pathway and enhanced N-methyl-D-aspartate receptor (NMDAR) function in hippocampal neurons [21]. Hippocampal NMDARs play an important role in the expression of anxiety-like behaviors, and studies using transgenic mice lacking hippocampal-specific NMDAR subunits have shown that these mice spend more time in the open arms in the EPM than wild-type mice and exhibit less anxiety-like behavior [22]. In Schmidt et al.'s experiment, the reduction of fear responses by PACAP6-38 was also suggested to involve NMDARs. Considering the current results, it is likely that the hippocampal PACAP antagonist mitigated the enhancement of NMDAR function, thereby contributing to anxiolytic effects. In contrast, PACAP-38 promoted hippocampal synaptic transmission, which is involved in emotional regulation by activating the cholinergic system through muscarinic receptors [23]. However, increasing cholinergic levels in the dorsal or ventral hippocampus significantly reduced anxiety-like behavior as measured by the EPM and shock-probe tests [24], contradicting the results of this study where the PAC1R-selective antagonist reduces anxiety-like behavior. Therefore, it is likely that the reduction in anxiety-like behavior induced by the PAC1R-selective antagonist is not mediated by the cholinergic system.
PACAP plays a role in responding to various threats and stressors, and because PACAP signaling has multiple functions across various organs and physiological systems, it is crucial to consider methods that specifically target the PACAP pathway when developing therapeutic approaches [25]. Therefore, it is necessary to further investigate the hippocampal pathways through which PACAP induces anxiety-like behavior. This study newly demonstrated that PACAP is involved in the expression of anxiety-like behavior in the dorsal hippocampus, consistent with the results showing that intracerebroventricular administration of PACAP agonists increases anxiety [14]. Additionally, this research provides new insights into the brain pathways of PACAP in stress-related responses. However, it is important to note that the sample size in this study was relatively small, which may limit the statistical power and generalizability of the findings.
Author Contributions
K.S., S.C., T.M., G.W., and M.K. conceived and designed the experiments and wrote the article. K.S., T.H., and G.W. performed the behavioral experiments and analyzed the data.
Ethics Statement
All experiments in this study were approved by the Animal Care and Use Committee of Meiji University (MUIACUC2022-03).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data supporting the findings of this study are available in the Supporting Information of this article (Table S1).