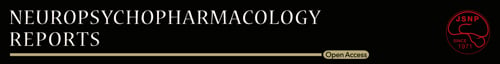Regulatory system of mGluR group II in the nucleus accumbens for methamphetamine-induced dopamine increase by the medial prefrontal cortex
Funding information
This work was supported by the grant-in-aid for Scientific Research (KAKENHI) (B) [JSPS KAKENHI Grant Number, 26293213, JP15H04662] (SM, AN), Challenging Exploratory Research [JSPS KAKENHI Grant Number, JP15K15050 and JP17K19801] (SM, AN), from Japan Agency for Medical Research and Development (AMED) [16mk0101076h0001] (AN), Uehara Memorial Foundation (AN), Tamura Memorial Foundation (AN), and Smoking Research Foundation Grant for Biomedical Research and Foundation (AN). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abstract
Aim
We previously reported that methamphetamine (METH)-induced conditioned place preference was attenuated by Shati/Nat8l overexpression in the medial prefrontal cortex (mPFC). Shati/Nat8l overexpression in the mPFC expressed lower levels of both glutamate and dopamine (DA) in the nucleus accumbens (NAc) and attenuated METH-induced DA elevation. We suggested a mechanism in which a decline of glutamate levels in the NAc decreases extracellular DA levels. However, the hypothesis has not confirmed.
Methods
We conducted a recovery experiments by pre-microinjection of an mGluR group II antagonist, LY341495, into the NAc shell of mPFC-Shati/Nat8l-overexpressed mice followed by METH injection and DA levels measurement by in vivo microdialysis.
Results
Pretreatment with LY341495 was able to restore METH-induced DA increase. Furthermore, mice injected with an adeno-associated virus vector containing GFP (AAV-GFP vector) in the mPFC expressed a colocalization of GFP with DARPP-32 a medium spiny neuron (MSN) marker. Next, co-immunostaining of DARPP-32 and neuronal nitric oxide synthase (nNOS: expressed in a subtype of gamma-Aminobutyric acid (GABA interneurons) in ventral tegmental area (VTA) showed a colocalization of nNOS and DARPP-32.
Conclusion
These results provided a proof that Shati/Nat8l attenuation of METH-induced DA increase is mediated by mGluR group II in the NAc. Moreover, immunohistochemical study showed a direct connection of mPFC projection neurons with NAc MSN and a connection of MSN projection neurons with a subtype of GABA interneurons in VTA.
1 INTRODUCTION
Shati/Nat8l is an N-acetyltransferase that generates N-acetylaspartate (NAA) from acetyl-CoA and aspartate.1 N-acetylaspartate binds to glutamate, mediated by N-acetylaspartylglutamate synthetase, and produces N-acetylaspartylglutamate (NAAG). Overexpression of Shati/Nat8l in the nucleus accumbens (NAc) increased both NAA and NAAG.2 NAAG is the most abundant neuropeptide in the brain.3 NAAG is also considered as an agonist of the metabotropic glutamate receptor mGluR group II.4 mGluR group II receptors are expressed in the presynaptic membrane of neurons.5
In our previous study, overexpression of Shati/Nat8l in the medial prefrontal cortex (mPFC) attenuated methamphetamine (METH)-induced conditioned place preference (CPP) and dopamine (DA) increase in the NAc.6 Moreover, Shati/Nat8l overexpression in the medial prefrontal cortex (mPFC) decreased both glutamate and DA extracellular levels in the NAc shell. These results are suggesting that Shati/Nat8l in the mPFC contributes to NAc function. mPFC is an important brain region that actively interacts with several brain areas.7 We have hypothesized a mechanism in which a decrease of DA was due to activation of mGluR group II by NAAG. However, this suggestion was not clarified. The reward system neuronal network is very complicated. There is no evidence of the histochemical colocalization of the direct connection of mPFC-projected neurons and medium spiny neurons (MSN) in the NAc, as much as the connection of MSN neurons from NAc with GABAergic interneurons in the ventral tegmental area (VTA). In this study, we investigated the implication of mGluR group II action on METH-induced DA response in the NAc in Shati/Nat8l-overexpressed mice in the mPFC. Furthermore, immunohistochemical studies were performed addressing the mPFC-NAc-VTA pathway. We attempted to clarify and examine the mPFC projection neurons to MSN neurons in the NAc. Finally, we attempted to detect a connection of MSN neurons with GABAergic interneurons in VTA.
2 MATERIAL AND METHODS
2.1 Animals and drugs
Male C57BL/6J mice (8-week-old; 22-27 g; Nihon SLC, Inc.) were housed in a room with a 12-hour light/dark cycle. Lights were turned on at 7 am and turned off at 7 pm. All procedures followed the National Institute of Health Guideline for the Care and Use of Laboratory Animals and were approved by the Animal Experiments Committee of the University of Toyama (Permission Number A2015pha-21 and A2018pha10). Methamphetamine was purchased from Dainippon Sumitomo Pharmaceutical Co. Ltd. and was dissolved in saline (0.1 mg/mL). LY341495 was purchased from Tocris Bioscience. All other reagents were obtained from standard commercial sources.
2.2 Adeno-associated virus vector production and microinjection
The method used for the production and microinjection of adeno-associated virus (AAV) vector has been reported and described previously.6, 8, 9
Mice were anesthetized with a combination of anesthetics (medetomidine [0.3 mg/kg], midazolam [4.0 mg/kg], and butorphanol [5.0 mg/kg]) and were fixed in a stereotaxic frame (SR-5M; Narishige). AAV9 CMV 6xHis Shati/Nat8l or CMV 6xHis GFP vectors were injected (1010-1012 unit/0.7 µL/side for mPFC-Shati/Nat8l and mPFC-Mock, respectively). The suspension was injected bilaterally into the mPFC (AP 1.7; ML ± 0.3; DV 1.5) according to the mouse brain atlas.2, 10 The injection volume was set similarly to that used in previous studies.8 The injection rate was 0.05 µL/min, and the needle remained at rest at the injection site for 10 minutes after the end of the injection. Experiments were performed on mice 3 weeks after the injection procedure. The study was approved by the Board of Safety Committee for Recombination DNA Experiments of the University of Toyama (G2015PHA-12).
2.3 In vivo microdialysis
2.3.1 Dopamine measurement
In vivo microdialysis was performed as has been described previously.2, 6, 11, 12 The cannula was placed into the NAc shell (1.4 mm anterior and 0.5 mm lateral from the bregma, 3.2 mm below the skull surface) according to the atlas.10 The day after surgery, a dialysis probe (AI-4-1, 1 mm membrane length; Eicom) was inserted through the guide cannula and perfused with a ringer's solution (147 mmol/L NaCl, 4 mmol/L KCl, 2.3 mmol/L CaCl2) at a flow rate of 0.5 µL/min by a syringe pump (ESP-64; EICOM). The dialysis probe was inserted from 9 am. Dopamine was allowed to stabilize for about 3 hours. Dopamine baseline levels were measured for 1 hour after the stabilization.
2.3.2 LY341495 intra-NAc treatment
LY341495 (7 or 10 pmol/L), referred as LY in this article, was administered into the NAc shell through the microdialysis probe. LY administration was performed at a flow rate of 0.5 μl/min for 15 min in the same way as ringer solution (Veh).
2.3.3 Methamphetamine stimulation
Fifteen minutes after LY treatment, mice were injected with 0.5 mg/kg METH (subcutaneous s.c.) or saline and the DA levels were measured during 120 minutes after injection using in vivo microdialysis. Dopamine standard was purchased from SIGMA.
2.4 Immunohistochemistry
Immunohistochemistry was performed as previously described.6 Sections of 30 μm were placed in 4% PFA (paraformaldehyde) for 20 minutes, washed with PBS (Phosphate-buffered saline), and incubated with 0.25% Triton X-100 for 15 minutes. Sections were treated with 10 mmol/L citrate buffer (pH 6.00) for antigen retrieval at 100°C for 3 minutes, washed with Tris-buffered saline with Tween-20 (TBS-T), and then blocked in 10% goat serum for 1 hour. Sections were incubated with mouse antibody against GFP IgG (MBL), rabbit anti-DARPP-32 (Cell Signaling Technology), and rabbit anti-nNOS (Sigma-Aldrich) at 4°C overnight, washed with TBS-T, and then incubated with CF488 goat anti-mouse IgG (H + L; Biotium) and CF594 goat anti-rabbit (Biotium) at room temperature for 2 hours. After being washed and mounted, sections were observed under a ZEISS fluorescence microscope, LSM780 (Keyence Co.).
2.5 Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). To analyze the development of in vivo microdialysis, statistical differences were determined by a two-way ANOVA with repeated measurement, followed by the Bonferroni's post hoc tests (using Prism version 5).
3 RESULTS
3.1 The inhibitory effects of Shati/Nat8l in the mPFC on METH-induced DA attenuation are mediated by mGluR group II
As previously described in the introduction, Shati/Nat8l overexpression in the mPFC was able to suppress METH-induced CPP and attenuate METH-induced DA increase. Both DA and glutamate were decreased in the NAc.6 In these experiments, we aim to verify whether Shati/Nat8l effect is mediated by NAAG, so we blocked mGluR group II receptors using LY. However, LY by itself can increase DA levels so we first assessed the highest LY concentration that does not affect DA levels in the NAc in wild-type mice (WT) by intra-NAc treatment of LY by perfusion for 15 minutes. At these conditions, 10 pmol/L was enough to induce a significant difference while 7 pmol/L did not affect DA levels (Figure 1A). FInteraction(11,44) = 3.12; P < 0.01, Fdrug(1,4) = 8.69; P < 0.05, Ftime(11,44) = 1.54; P > 0.05. In the next experiments, we used 7 pmol/L on mPFC-Shati/Nat8l mice. We treated mPFC-Shati/Nat8l with LY 7 pmol/L (or Veh) for 15 minutes: from t-30 to t-15 (minutes). Fifteen minutes later, METH (or saline) was injected to the mice at t-0. Interestingly, mPFC-Shati/Nat8l mice treated with LY 7 pmol/L prior to the METH injection showed an increase of DA levels compared with mice treated only with ringer solution prior to the METH injection (Figure 1B). FInteraction(26,143) = 3.49; P < 0.0001, Fdrug(2,11) = 21.50; P < 0.001, Ftime(13,143) = 1.08; P > 0.05. These results confirm the implication of mGluR group II in mPFC-Shati/Nat8l suppressive effect on METH-induced DA increase in the NAc.
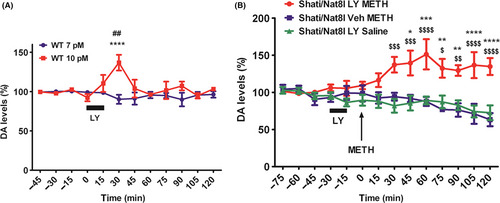
3.2 Colocalization of mPFC neuron projection with MSN neurons in the NAc shell
Consecutively, we injected AAV-GFP vector into the mPFC (Figure 2A). Three weeks later, we performed immunohistochemistry of GFP and DARPP-32 in the NAc shell. Dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) is a protein specifically identified in MSN neurons expressing D1 and D2 receptors.13, 14 Firstly, we checked the expression of DARPP-32 in MSN-specific areas (striatum and NAc). DARPP-32 is expressed in both striatum (STR) and NAc (Figure 2B). Moreover, we found that DARPP-32 and GFP are co-expressed in the NAc shell (Figure 2C). This implies that mPFC neurons projecting to the NAc shell connect directly to MSN neurons.
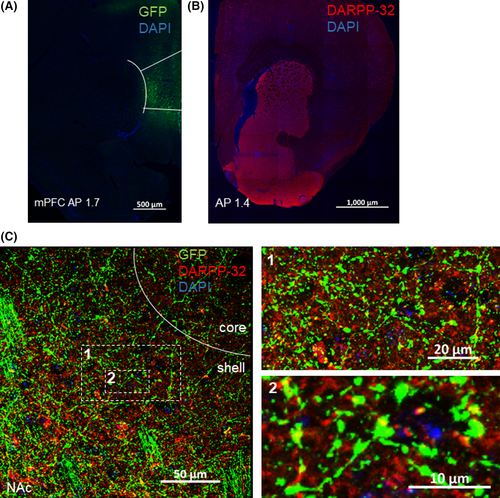
3.3 Nucleus accumbens shell neurons projection to the VTA and colocalization of MSN neurons with nNOS GABAergic interneurons subtype
Next, we injected AAV-GFP vector into the NAc shell and checked the expression of GFP protein in the VTA (Figure 3A). In concert with previous reports, NAc shell projects to VTA.15, 16
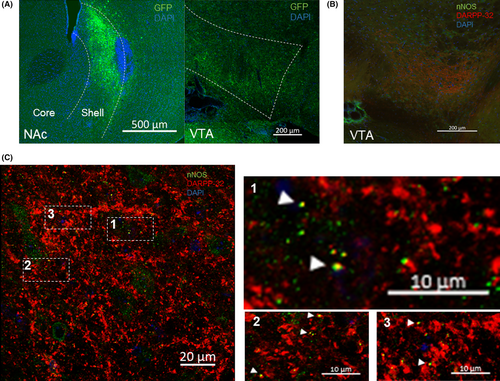
Then, we tried to identify a connection of MSN neurons to VTA interneurons. For this purpose, we performed DARPP-32 and nNOS (neuronal nitric oxide synthase) immunohistochemistry in VTA (Figure 3B,C). nNOS is expressed in a subset of interneurons in the VTA.17 Interestingly, we found some colocalization of DARPP-32 and nNOS in VTA as indicated by the white arrowheads (Figure 3C). These results provide an immunohistochemical proof that MSN neurons target GABAergic interneurons in the VTA.
4 DISCUSSION
In the previous article, we demonstrated that Shati/Nat8l overexpression in the mPFC attenuates METH-induced conditioned place preference and DA increase in the NAc. Furthermore, Shati/Nat8l overexpression in the mPFC decreased both DA and glutamate levels in the NAc.6 We proposed a mechanism in which metabotropic receptor mGluR group II in synaptic terminals from mPFC to NAc was implicated in this effect. In this study, we tested this idea by using intra-NAc administration of mGluR group II antagonist. We were able to confirm that this suppressive effect is mediated by mGluR group II in the NAc. Furthermore, we explored the circuitry involved in this mechanism by immunohistochemistry study. mGluR group II is a class of glutamate receptors (mGluR2/3) that are localized in the presynapse.18, 19 Its activation inhibits neurotransmitter release.20 In Shati/Nat8l-overexpressed brain regions of mice, both NAA and NAAG were increased.2 Next, NAAG binds to the presynaptic mGluR group II in the NAc. Heretofore, intraperitoneal injection of LY to mice overexpressing Shati/Nat8l in the NAc restored METH-induced DA increase in the NAc.2 Here, we proved that intra-NAc treatment with LY recovered the response of mPFC-Shati/Nat8l mice to METH by increasing DA levels in the NAc after single METH stimulation (Figure 1B). These results reinforce our previous theory suggesting that Shati/Nat8l in the mPFC affects NAc DA through the glutamatergic neurons projection from the mPFC to the NAc.
It is considered that DA is critical for acute and initiation for METH. However, glutamate also plays an important role in dependence after chronic treatment of METH. Therefore, prefrontal glutamatergic innervation to the NAc promotes the compulsive behavior of drug seeking.21
Chronic drug intake leads to the disruption in the release of glutamate from the PFC to the NAc, which eventually affects glutamate in the NAc, leading to an enhancement in addictive behavior.22 Moreover, increased sensitivity to glutamate in the NAc, and the increased glutamate release from the PFC to the NAc, mediates craving, and relapse in response to drug-related cues.21, 23 Systemic administration of an mGluR group II agonist inhibits cue-induced and drug-primed reinstatement of METH seeking.24
Acute METH administration induces an increase in DA release to the NAc and a decrease of GABAB receptor signaling in VTA.25, 26 This DA release is associated with the experience of reward or euphoria.27 In the NAc, microdialysis studies showed that blockade of mGluR group II increases extracellular glutamate levels, whereas their activation has the opposite effect.28 Intra-NAc shell administration of mGluR group II antagonist for 20 minutes induced extracellular DA level increase in the NAc shell.29
Subsequently, immunohistochemistry study in the NAc shell following AAV-GFP vector injection in the mPFC exhibited a colocalization of GFP and DARPP-32 (Figure 2C) which is a confirmation that mPFC neurons project to the NAc MSN neurons. To note, DARPP-32 is not only expressed in cell body but also in dendrites.30 In another hand, NAc MSN neurons project to VTA. Many studies showed the involvement of NAc MSN neurons projecting to VTA in reward behavior by disinhibition of VTA dopaminergic neurons as observed in repeated cocaine exposure.(31, 32) Likewise, whole-cell recordings showed that stimulation of MSN neurons induced strong inhibition on VTA GABA neurons.(31)
In accordance with previous reports,15, 16 our experiments showed that NAc shell projects to VTA (Figure 3A). Optogenetic studies showed that MSN neurons from the NAc targeted VTA interneurons.32 One third of VTA neurons are interneurons.33, 34 However, no colocalization was found in MSN neurons projections with VTA interneurons. No colocalization would be resulted from the nonexpression of most of interneurons in VTA in comparison with other brain areas or to the nonavailability of a specific interneurons marker; indeed, DA neurons in VTA also express different interneurons markers, including neuropeptide Y, calretinin, cholecystokinin, and vasoactive intestinal peptide.(35-39) Other markers such as VGAT and GAD65/67 are also expressed in any type of GABAergic neuron which is difficult to discriminate local interneurons and MSN projection terminals in VTA. In fact, VGAT is expressed in GABAergic terminals and GAD67 is also expressed in presynaptic clusters.40, 41
Remarkably, a study showed that neuronal nitric oxide synthase (nNOS) is also another marker of interneurons in VTA. nNOS was identified as a marker of VTA GABA interneurons that is not expressed in VTA DA neurons, and putatively expressed in glutamatergic projecting neurons, mostly in the rostral linear nucleus of the raphe (RLi).17 Although, glutamatergic neurons represent only 2%-3% of total VTA neurons.33 We performed immunohistochemistry of nNOS and DARPP-32 and detected a colocalization of DARPP-32 and nNOS (Figure 3C). DARPP-32 projection neuron innervation is found in VTA (Figure 3B) and represents the projection of NAc MSN neurons to VTA. The results suggest that MSN neurons target GABA interneurons expressing nNOS. Nonetheless, future studies are needed to determine the involvement of this subset of interneurons in reward and a histological colocalization of MSN projection neuron innervation with other subsets of VTA interneurons.
In conclusion, Shati/Nat8l overexpression in the mPFC suppressed METH-induced DA increase in the NAc via mGluR group II. Together, mPFC neurons projecting to NAc directly connect with NAc MSN neurons. In another hand, MSN projections to VTA also connect directly to nNOS GABAergic interneurons.
ACKNOWLEDGMENTS
We thank Naomi Takino and Mika Ito for technical assistance in producing the Shati/Nat8l AAV vectors. This work was supported by the grant-in-aid for Scientific Research (KAKENHI) (B) [JSPS KAKENHI Grant Number, 26293213, JP15H04662] (SM, AN), Challenging Exploratory Research [JSPS KAKENHI Grant Number, JP15K15050 and JP17K19801] (SM, AN), For Young Scientists (B) [JSPS KAKENHI Grant Number JP16K18933] (KU) from the Japan Society for the Promotion of Science JSPS Core-to-Core Program, Japan Agency for Medical Research and Development (AMED) [16mk0101076h0001] (AN), Uehara Memorial Foundation (AN), Tamura Memorial Foundation (AN), and Smoking Research Foundation Grant for Biomedical Research and Foundation (AN). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
MH, KU, and AN conceived the projects. MH and KU designed research. MH, KU, and KH performed research. MH analyzed the data. SM provided the AAV-Shati vectors. MH wrote the draft of the manuscript, and AN confirmed the final version.
ETHICAL APPROVAL
All experiments were approved by the Animal Experiments Committee of the University of Toyama (Permission Number A2015pha-21 and A2018pha10).
Open Research
DATA ACCESSIBILITY
We have made our data publicly available through directly submitting as the Supporting Information.