Modeling the Radical Batch Homopolymerization of Acrylamide in Aqueous Solution
Calista Preusser
Department of Chemical Engineering, Dupuis Hall, Queen's University, Kingston, Ontario, K7L 3N6 Canada
Search for more papers by this authorAnna Chovancová
Polymer Institute of the Slovak Academy of Sciences, Dúbravská cesta 9, 845 41 Bratislava, Slovakia
Search for more papers by this authorIgor Lacík
Polymer Institute of the Slovak Academy of Sciences, Dúbravská cesta 9, 845 41 Bratislava, Slovakia
Search for more papers by this authorCorresponding Author
Robin A. Hutchinson
Department of Chemical Engineering, Dupuis Hall, Queen's University, Kingston, Ontario, K7L 3N6 Canada
E-mail: [email protected]Search for more papers by this authorCalista Preusser
Department of Chemical Engineering, Dupuis Hall, Queen's University, Kingston, Ontario, K7L 3N6 Canada
Search for more papers by this authorAnna Chovancová
Polymer Institute of the Slovak Academy of Sciences, Dúbravská cesta 9, 845 41 Bratislava, Slovakia
Search for more papers by this authorIgor Lacík
Polymer Institute of the Slovak Academy of Sciences, Dúbravská cesta 9, 845 41 Bratislava, Slovakia
Search for more papers by this authorCorresponding Author
Robin A. Hutchinson
Department of Chemical Engineering, Dupuis Hall, Queen's University, Kingston, Ontario, K7L 3N6 Canada
E-mail: [email protected]Search for more papers by this authorAbstract
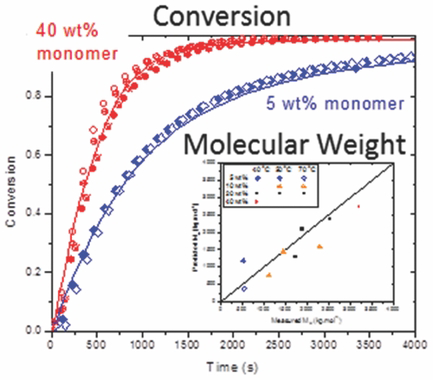
A kinetic model for the radical homopolymerization of acrylamide in aqueous solution is developed, incorporating propagation and termination rate coefficients as functions of monomer concentration and including the formation and reaction of midchain radicals based on the insights and measured rate coefficients from recent pulsed-laser studies. The model successfully represents the batch conversion profiles measured using an in situ NMR technique between 40 and 70 °C with initial monomer concentrations of 5 to 40 wt%, as well as the associated polymer molar mass distributions. In particular, the model captures the decreased rate that occurs at lowered monomer concentrations as a result of the formation of less-active midchain radicals by backbiting. Previous literature data are also well represented by the model.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| mren201500076-sup-0001-S1.pdf194.8 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1http://www.basf.com/group/corporate/en/. accessed Dec 2013.
- 2D. R. L. Vedoy, J. B. P. Soares, Can. J. Chem. Eng. 2014, 93, 888.
- 3T. Ishige, A. E. Hamielec, J. Appl. Polym. Sci. 1973, 17, 1479.
- 4H. Kattner, M. Buback, Macromolecules 2015, 48, 7410.
- 5I. Lacík, A. Chovancová, L. Uhelská, C. Preusser, R. A. Hutchinson, M. Buback, submitted to Macromolecules.
- 6V. F. Kurenkov, V. A. Myagchenkov, Eur. Polym. J. 1980, 16, 1229.
- 7C. Chachaty, A. Forchioni, J. Polym. Sci., Part A: Polym. Chem. 1972, 10, 1905.
- 8A. R. Mahdavian, M. Abdollahi, H. R. Bijanzadeh, J. Appl. Polym. Sci. 2004, 93, 2007.
- 9H. R. Lin, Eur. Polym. J. 2001, 37, 1507.
- 10F. S. Dainton, M. Tordoff, Trans. Faraday Soc. 1957, 53, 499.
- 11C. J. Kim, A. E. Hamielec, Polymer 1984, 25, 845.
- 12A. E. Hamielec, Chem. Eng. Commun. 1983, 24, 1.
- 13I. Lacík, S. Beuermann, M. Buback, Macromolecules 2003, 36, 9355.
- 14I. Lacík, S. Beuermann, M. Buback, Macromol. Chem. Phys. 2004, 205, 1080.
- 15I. Lacík, S. Beuermann, M. Buback, Macromolecules 2001, 34, 6224.
- 16S. Beuermann, M. Buback, P. Hesse, I. Lacík, Macromolecules 2006, 39, 184.
- 17S. Beuermann, M. Buback, P. Hesse, S. Kukucˇková, I. Lacík, Macromol. Symp. 2007, 248, 41.
- 18I. Lacík, L. Ucˇnˇová, S. Kukucˇková, M. Buback, P. Hesse, S. Beuermann, Macromolecules 2009, 42, 7753.
- 19M. Stach, I. Lacík, D. Chorvát, M. Buback, P. Hesse, R. A. Hutchinson, L. Tang, Macromolecules 2008, 41, 5174.
- 20V. F. Gromov, N. I. Galperina, T. O. Osmanov, P. M. Khomikovskii, A. D. Abkin, Eur. Polym. J. 1980, 16, 529.
- 21A. Chapiro, Eur. Polym. J. 1973, 9, 417.
- 22B.De Sterck, R. Vaneerdeweg, F. Du Prez, M. Waroquier, V.Van Speybroeck, Macromolecules 2010, 43, 827.
- 23S. A. Seabrook, M. P. Tonge, R. G. Gilbert, J. Polym. Sci., Part A: Polym. Chem. 2005, 43, 1357.
- 24A. N. Nikitin, R. A. Hutchinson, M. Buback, P. Hesse, Macromolecules 2007, 40, 8631.
- 25D. Konkolewicz, S. Sosnowski, D. R. D'hooge, R. Szymanski, M.-F. Reyniers, G. B. Marin, K. Matyjaszewski, Macromolecules 2011, 44, 8361.
- 26J. Barth, M. Buback, P. Hesse, T. Sergeeva, Macromol. Rapid Commun. 2009, 30, 1969.
- 27N. F. G. Wittenberg, C. Preusser, H. Kattner, M. Stach, I. Lacík, R. A. Hutchinson, M. Buback, Macromol. React. Eng. 2016, DOI: 10.1002/mren.201500017.
10.1002/mren.201500017 Google Scholar
- 28J. Barth, W. Meiser, M. Buback, Macromolecules 2012, 45, 1339.
- 29M. Buback, P. Hesse, I. Lacík, Macromol. Rapid Commun. 2007, 28, 2049.
- 30T. Junkers, C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 7585.
- 31C. Preusser, R. A. Hutchinson, Macromol. Symp. 2013, 333, 122.
- 32M. Wulkow, Macromol. Theory Simul. 1996, 5, 393.
- 33N. F. G. Wittenberg, M. Buback, R. A. Hutchinson, Macromol. React. Eng. 2013, 7, 267.
- 34C. A. Preusser, PhD Thesis, Queen's University 2015.
- 35R. A. Hutchinson, in Handbook of Polymer Reaction Engineering (Eds: T. Meyer, J. T. F. Keurentjes), Wiley-VCH Verlag GmbH, Weinheim 2005, pp. 153–212.
10.1002/9783527619870.ch4 Google Scholar
- 36A. N. F. Peck, R. A. Hutchinson, Macromolecules 2004, 37, 5944.
- 37W. Wang, R. A. Hutchinson, Macromol. Symp. 2010, 289, 33.
- 38J. Barth, M. Buback, G. T. Russell, S. Smolne, Macromol. Chem. Phys. 2011, 212, 1366.
- 39S. Santanakrishnan, L. Tang, R. A. Hutchinson, M. Stach, I. Lacík, J. Schrooten, P. Hesse, M. Buback, Macromol. React. Eng. 2010, 4, 499.
- 40G. Moad, D. H. Solomon, The Chemistry of Radical Polymerization, 2nd ed., Elsevier Ltd., Oxford 2006, pp. 251–263.
- 41A. N. Nikitin, R. A. Hutchinson, W. Wang, G. A. Kalfas, J. R. Richards, C. Bruni, Macromol. React. Eng. 2010, 4, 691.
- 42C. H. Bamford, R. W. Dyson, G. C. Eastmond, Polymer 1969, 10, 885.
- 43W. Chemicals, http://www.wako-chem.co.jp/specialty/waterazo/V-50.htm (accessed: Dec 2013).
- 44A. Giz, H. Çatalgil-Giz, A. Alb, J.-L. Brousseau, W. F. Reed, Macromolecules 2001, 34, 1180.
- 45M. W. Liberatore, S. Baik, A. J. McHugh, T. J. Hanratty, J. Non-Newton. Fluid Mech. 2004, 123, 175.
- 46N. M. Ahmad, F. Heatley, P. A. Lovell, Macromolecules 1998, 31, 2822.
- 47S. Aldrich, http://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Aldrich/General_Information/thermal_initiators.pdf. accessed Dec 2013.