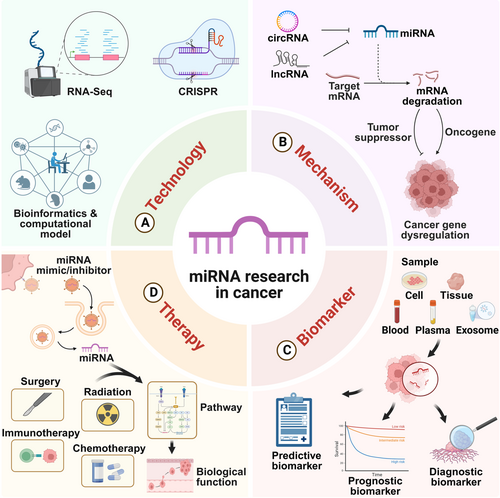
miRNA interplay: Mechanisms and therapeutic interventions in cancer
Zehua Wang and Hangxuan Wang contributed equally to this work.
Abstract
MicroRNAs (miRNAs) are key molecules that regulate gene expression. miRNAs regulate protein synthesis by binding to mRNA, influencing processes such as cell proliferation, metastasis, and apoptosis. They play a pivotal role in cancer development. Current research mainly explores miRNA mechanisms and applications, and the techniques underpinning this research are foundational to both basic science and clinical translation. However, no review has comprehensively examined miRNA mechanisms and applications from a technical perspective, creating a need for this work. Advances in RNA sequencing technology, CRISPR/Cas9 technology, and bioinformatics tools have deepened our understanding of miRNA interactions. miRNA can serve as a biomarker for cancer diagnosis and prognosis, with significant clinical potential. The development of miRNA mimics and inhibitors has brought new hope for cancer treatment, especially in reversing cancer drug resistance. This article reviews the vital role of miRNA interactions in cancer occurrence, development, diagnosis, and treatment, providing new perspectives and strategies for personalized medicine and cancer therapy.
Graphical Abstract
By integrating RNA sequencing, CRISPR, bioinformatics, and computational models, we have deepened understanding of MicroRNA (miRNA) interactions. miRNAs regulated by ceRNAs are closely linked to cancer development. They are detectable in various samples and serve as biomarkers for cancer diagnosis, prognosis, and prediction. miRNA-based therapies, combining mimics or inhibitors with conventional treatments, show promise in enhancing cancer treatment outcomes.
1 INTRODUCTION
miRNAs are single-stranded noncoding RNAs (ncRNAs) approximately 19–25 nucleotides long, known for their high evolutionary conservation.1 miRNA was first discovered in Caenorhabditis elegans in 1993, and since then, its presence has been identified in both plants and animals.2 The biogenesis of miRNAs involves several stages: transcription from DNA into primary miRNAs, processing into precursor miRNAs, and maturation into functional miRNAs.3 These mature miRNAs are predominantly found in the introns and exons of ncRNAs and the introns of pre-mRNAs.4 miRNAs inhibit the translation of messenger RNA (mRNA) by degrading mRNA or binding to the 3′ untranslated region (3′-UTR) of target mRNAs, thereby reducing the function of downstream protein-coding genes.5 Thus, miRNAs act as posttranscriptional micro-regulators of gene expression, finely tuning transcriptional and translational processes.3 miRNA can target more than 60% of human protein-coding genes, they play crucial roles in cell growth, differentiation, development, and apoptosis4, 6 and their dysfunction is closely linked to numerous diseases4, 7 miRNAs are especially impactful in cancer, as nearly all known cancer cells use them to regulate gene expression.2
The interplay between miRNAs is fundamental to their role in cancer. This interplay involves upstream ceRNAs (long noncoding RNA [lncRNAs] and circular RNAs [circRNAs]), downstream mRNAs, RNA-binding proteins (RBPs), and other miRNAs. Beyond their intracellular roles, miRNAs can be secreted into the extracellular fluid and transported to target cells via vesicles such as exosomes or in combination with specific proteins like argonautes (AGOs)3, 6 These extracellular miRNAs function as chemical messengers, mediating intercellular communication. These molecular interactions are also critical within the tumor microenvironment (TME), where miRNAs engage with various immune cells, expanding their influence on cancer progression. Advances in high-throughput sequencing and CRISPR/Cas9 technologies have enriched our understanding of miRNA mechanisms, propelling researchers to explore miRNA targets and functions further.
The complexity of miRNA functions, combined with advances in research technologies, offers new opportunities for cancer research. miRNAs have diagnostic potential as biomarkers, as their expression correlates with cancer diagnosis and prognosis. Emerging detection technologies, such as microfluidics and nanoparticle-based methods, are expanding the scope of miRNA diagnostics. Additionally, miRNAs hold promise as therapeutic agents. Early efforts in cancer treatment focused on miRNA mimics and inhibitors, but these have since evolved into nanoparticle-based delivery systems that enhance targeting specificity and improve treatment outcomes. CAR-T cells, as natural carriers, are also being explored for miRNA-based therapies. However, drug resistance remains a significant challenge in cancer treatment. miRNAs play a critical role in mediating cancer drug resistance, and targeting drug-resistant miRNAs is becoming a key area of research. With the integration of cutting-edge technologies, there is potential for breakthroughs in overcoming drug resistance.
This review will explore the foundational technologies used to study miRNA interactions and elucidate miRNA's role in cancer. In addition, we will highlight the importance of miRNAs in cancer diagnosis and treatment, summarizing recent diagnostic and therapeutic innovations. Our goal is to provide new insights and directions for future miRNA research and its clinical applications.
2 TECHNOLOGIES FOR STUDYING miRNA INTERACTIONS
In miRNA research, investigating miRNA interactions is critical to understanding their roles in cancer and other diseases. This chapter will explore various technologies, including RNA sequencing, CRISPR/Cas9 gene editing, and tools for predicting miRNA targets and interactions. Additionally, we will discuss how integrating multi-omics data can map miRNA networks in cancer, revealing their complex regulatory mechanisms.
2.1 Advancements and applications of RNA sequencing technology (RNA-Seq) in miRNA research
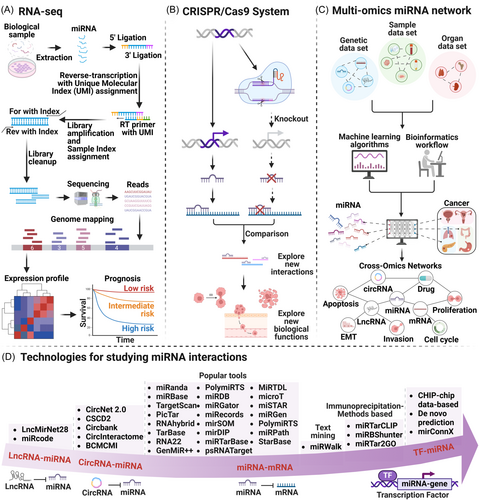
RNA-Seq is a high-throughput method for comprehensive analysis of all RNA molecules in biological samples.8 miRNA-seq, a specialized form of RNA-Seq for miRNAs, provides detailed expression profiles, highlights differences, and discovers new miRNAs, enhancing our understanding of gene regulatory networks (Figure 1A).9 Single-cell RNA-seq (scRNA-seq) studies cell subpopulation heterogeneity, offering insights into cell diversity, fate decisions, and disease mechanisms.10
Bulk RNA-seq data is widely used as a prognostic marker in cancer studies. In bladder cancer (BLCA), researchers developed a prognostic model based on three gene expression levels to predict patient survival.11 In pancreatic ductal adenocarcinoma (PDAC), RNA-seq analysis of 24 tissues revealed over 57,000 single-cell transcriptomes, uncovering links between ductal cell subpopulations and the inactivation state of tumor-infiltrating T lymphocyte (T cell). This study provided valuable insights into PDAC heterogeneity and tumor-immune interactions, aiding in the development of new immunotherapy strategies.10
Technological advancements and cost reductions12 have transitioned RNA-Seq from tissue-level to single-cell analysis, broadening its applications. High-throughput technologies like PANDORA-seq overcome RNA modification challenges, offering comprehensive maps of highly modified miRNAs.13 Nanopore MinION RNA-Seq, using Oxford Nanopore Technology, provides long-read sequences and single-molecule real-time sequencing, further advancing RNA-Seq.14
RNA-Seq technology provides rich data and analysis methods for miRNA research, deepening our understanding of miRNA interactions and regulatory networks. Its application in cancer research is particularly significant, offering new methods for selecting diagnostic and therapeutic biomarkers, and demonstrating substantial clinical potential.
2.2 CRISPR/Cas9 revolutionizes miRNA research in cancer
CRISPR/Cas9 is a revolutionary gene editing tool that enables precise regulation of miRNA expression, offering specificity and long-term stability.15 By knocking out or knocking in specific miRNAs, CRISPR/Cas9 has advanced our understanding of miRNA functions and interactions (Figure 1B).16 However, the effectiveness and adaptability of CRISPR/Cas9 for treatment are limited by its toxicity and potential mutagenicity.17 The unique RNase activity of short CRISPR RNA (crRNA) (40 nt) processed into mature Cas12a pre-crRNA helps overcome the size challenge of delivery via viral vectors.18
In cancer research, CRISPR screening identifies key genes related to cancer development and treatment response, improving our grasp of drug resistance mechanisms and guiding new treatment strategies.19 The third generation of CRISPR technology has been continuously improved, achieving direct targeted DNA modification without generating DNA double-strand breaks, precise insertion of large DNA sequences, and gene regulation without any genome editing through epigenome engineering, thereby improving safety.20 The development of CRISPR-Cas9 and high-throughput sequencing technologies enhances our understanding of miRNA regulation in cancer, promoting targeted therapies.21
In colorectal cancer (CRC), scientists used CRISPR/Cas9 to inactivate miR-34a and miR-34b/c in the HCT116 cell line. The simultaneous inactivation enhanced cell migration and invasion, activated epithelial-mesenchymal transition (EMT), reduced chemotherapy sensitivity, and increased stress-induced autophagic flux, highlighting the complementary roles of miR-34a and miR-34b/c in regulating EMT and autophagy, providing new treatment insights.22
2.3 Tools and algorithms for predicting miRNA targets and interactions
In recent years, miRNA target prediction and interaction identification have advanced significantly. Tools and algorithms have evolved, integrating machine learning to align with disease development trends.23 These tools utilize miRNA-mRNA complementarity, pairing stability, and conservation to efficiently predict target genes. Popular tools for predicting miRNA–mRNA interactions include miRanda,24 miRBase,25 TargetScan,26 PicTar,27 RNAhybrid,28 TarBase,29 RNA22,30 GenMiR++,31 PolymiRTS,32 miRDB,33 miRGator,34 mirecords,35 mirSOM,36 mirDIP,37 miRTarBase,38 psRNATarget,39 MiRTDL,40 microT,41 miSTAR,42 miRGen,43 PolymiRTS,32 miRPath,44 and StarBase.45 Additional tools include miRWalk46 and databases based on immunoprecipitation methods (IB), such as miRTarCLIP,47 miRBShunter,48 and miRTar2GO.23, 49 Tools specific to lncRNA–miRNA interactions include LncMirNet2850 and miRcode,51 while circRNA–miRNA interaction tools include CircNet 2.0,52 CSCD2,53 Circbank,54 CircInteractome,55 and BCMCMI56 (Figure 1D).
The interplay between miRNAs and transcription factors (TFs) is pivotal. miRNAs target transcription factor mRNAs, while transcription factors reciprocally regulate miRNA gene expression, exerting regulatory roles pre- and post-transcriptionally.57 Tools for predicting TF–miRNA interactions are primarily based on gene expression profiles, sequence-based target predictions, experimentally validated target databases, and phylogenetic footprinting methods58, 59 Among these, De novo predictions utilize sequence-based methods, while experimentally validated databases often employ CHIP-chip technology to identify TF binding sites in DNA. Emerging experimental techniques, such as AgoHIST-CLIP, offer innovative approaches to miRNA target gene prediction.60 Additionally, some studies are developing a tool called mirConnX, which integrates regulatory networks of miRNA, TFs, and mRNA.61
However, improving the accuracy of miRNA target prediction remains a challenge. Researchers are integrating multiple biological features and leveraging single-cell sequencing data along with deep learning methods to enhance prediction accuracy.62 Analyzing extensive scRNA-seq data has unveiled critical insights into cancer biology, disease pathogenesis, and clinical outcomes, facilitating the development of prognostic models.11, 63
2.4 Integrating multi-omics data to map miRNA networks in cancer
The regulatory role of miRNA in cells is intricate and nuanced, capable of integrating multi-omics data to construct complex biological networks (Figure 1C). This approach offers researchers a comprehensive view to unravel its mechanisms and regulatory pathways.64
miRNA plays a crucial role in cancer research across multiple omics fields, including genomics, transcriptomics, proteomics, and metabolomics. By integrating and analyzing data from various omics, we gain a more comprehensive understanding of biological processes and disease mechanisms.65 At the genomic level, miRNA genetic variation can influence disease susceptibility and phenotypic diversity.66 At the transcriptomic level, bioinformatics, statistical methods, network inference, and experimental validation of mRNA networks systematically reveal miRNA's deep regulatory mechanisms in cell biology.67 Recent advances in spatial transcriptomics offer a novel way to study the distribution of miRNAs inside and outside cells, providing an intuitive understanding of their spatial dynamics.68 At the proteomic and metabolomic levels, miRNAs indirectly regulate protein expression by targeting specific genes, which in turn affects metabolite transfer and modulates metabolic pathways. Notably, miRNAs are also packaged into extracellular vesicles (EV-miRNA), playing a vital regulatory role.69
miRNA network analysis identifies pivotal regulatory hubs in cancer, highlighting potential biomarker Hub miRNAs. This insight not only enhances cancer diagnosis and treatment strategies but also sheds light on personalized medicine approaches.70 Integrating functional genomics data, such as miRNA and mRNA expression profiles, reconstructs regulatory networks for survival analysis,64 elucidating the critical role of miRNA–mRNA interactions in cancer prognosis, identifying miRNAs involved in the disease process.71
Furthermore, analyzing miRNA cooperation networks across different cancers elucidates shared and distinct mechanisms, offering comprehensive insights for cancer treatment and prevention.72 Computational simulations like miRNA-disease association prediction predict miRNA-disease associations, facilitating the discovery of novel therapeutic pathways and a deeper understanding of miRNA's role in disease pathogenesis.65 These studies not only advance our understanding of miRNA's regulatory roles in cells but also innovate new strategies for disease treatment and prevention.
3 miRNA-RELATED MECHANISMS OF CANCER OCCURRENCE
In recent years, miRNA interactions have become a key research focus, offering new insights into tumorigenesis mechanisms and the development of anticancer drugs.72 The role of miRNA in cancer is multifaceted, as it can act as both a tumor promoter and suppressor, influencing cellular pathways and biological functions. As an epigenetic modifier, miRNA can interact with other epigenetic regulators. Exosomol miRNAs are involved in cell-to-cell communication within the TME, contributing to complex mechanisms in cancer progression, such as metabolic reprogramming and tumor stem cell maintenance. Factors like genetic polymorphisms,73 epigenetic modifications74, 75 asymmetric strand selection, and interactions with RBPs or other RNA molecules76 all influence miRNA regulatory activity. A deeper understanding of these intricate interactions will shed light on cancer's molecular regulatory mechanisms, offering new strategies for diagnosis and treatment.77
3.1 miRNA interactions in cancer
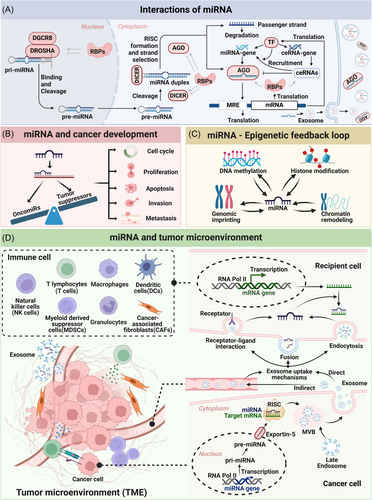
miRNA interacts with various molecules to exert its biological functions, categorized into upstream and downstream interactions. Downstream targets include mRNA, RBP, miRNA, and DNA, while upstream interactions involve competing endogenous RNAs (ceRNAs). Furthermore, miRNA can be secreted extracellularly, facilitating intercellular communication (Figure 2A).
miRNAs predominantly bind to specific sequences within the 3′ UTR of target mRNAs, leading to translational inhibition, mRNA deadenylation, or decapping.3 Alterations in the 3′ UTR length can affect RNA structure, impacting miRNA binding site accessibility.78 These binding sequences, known as MREs, determine the miRNA's effect—AGO-dependent degradation or translational inhibition of mRNA.3 A single miRNA can target hundreds of mRNAs, thereby influencing protein networks, while a single mRNA may be regulated by multiple miRNAs.76 miRNA binding sites are also present in the 5′ UTR, coding sequences (CDS), and promoter regions. miRNAs binding to the 5′ UTR and coding regions suppress gene expression, while promoter region interactions can activate transcription, mediating translational activation.79 Moreover, RNA editing can introduce changes in miRNA seed regions, altering target mRNA binding.78 Depending on the mRNA's location, miRNA-induced silencing complex (miRISC) can bind to ribosome- or ER-bound mRNA to inhibit translation.3 In tumorigenesis, synergistic miRNA effects on target mRNA, particularly involving multiple MREs, present viable therapeutic strategies.80 Additionally, feedback loops are formed when downstream mRNAs act as TFs, which can also regulate miRNA expression.21
The biogenesis of miRNA involves a series of processing steps facilitated by proteins such as Drosha, DGCR8, Exportin5, and Dicer, ultimately forming the miRISC.81 RBPs, which regulate gene expression posttranscriptionally, are critical in miRNA-mediated processes such as splicing, localization, and stability. Disruptions in RBP expression often impair miRNA processing, influencing target mRNAs related to cancer progression.82 Depending on the miRISC's location, it can promote mRNP degradation in the nucleus or maintain the mRISC:mRNP complex during cytoplasmic transport. miRNAs can also form complexes with proteins like FXR1, modulating adenosine/guanosine-rich elements (AREs) and activating translation.3 Additionally, miRNA expression levels are regulated by RBPs such as AUF1, DDX17, and ILF3.78 Recent research has highlighted the key role of interactions between miRNAs, RBPs, and exoribonucleases in tumorigenesis.83
miRNAs can interact with each other at different stages of biogenesis—directly at the pri- or mature-miRNA stages or indirectly by regulating TFs or miRNA regulators.84 Direct interactions involve complementary pairing between miRNAs, influencing their activity and expression, while indirect interactions involve targeting TFs, which then regulate downstream miRNAs. Global interactions allow a single miRNA to regulate entire families of miRNAs, providing a flexible mechanism for gene regulation.77
Nuclear-activated miRNAs (NamiRNAs), a newly discovered class, function as enhancers by binding to promoter and enhancer regions, promoting gene expression.85 NamiRNAs are enriched in active enhancers and alter chromatin states, activating homologous gene transcription. In cancer, NamiRNA networks have been shown to activate tumor suppressor genes, revealing novel mechanisms in cancer development and potential therapeutic strategies.86 Additionally, some miRNAs target DNA methyltransferases (DNMTs), influencing gene expression at both transcriptional and posttranscriptional levels. DNA methylation can regulate miRNA synthesis, but a reciprocal relationship exists when miRNAs modulate gene expression via methylation mechanisms. For instance, miR-211 silencing via DNA methylation reduces melanoma cell sensitivity to cisplatin, suggesting a potential role for epigenetic regulation in enhancing drug efficacy.87
miRNAs also interact with other ncRNAs, including lncRNAs, circRNAs, and pseudogenes, regulating various biological processes. ceRNAs modulate mRNA expression by binding to MRE sequences, counteracting miRNA-mediated inhibition of mRNA translation.88 miRNA–ceRNA interactions often form feedback loops, either amplifying or suppressing gene expression.89-91 ceRNAs can also recruit TFs to influence miRNA gene expression.57
miRNAs are secreted into extracellular fluid and transported to target cells via vesicles (such as exosomes) or bound to proteins like AGO.3 These extracellular miRNAs facilitate intercellular communication, remaining stable even under extreme conditions.78 They may bind to proteins such as high-density lipoprotein (HDL) and NPM1, further enhancing stability. miRNA transfer between cells can occur through exosomes, microvesicles, apoptotic bodies, or direct cell–cell contact.4 Extracellular miRNAs act as autocrine, paracrine, or endocrine regulators, modulating cellular activities.92 Notably, treating cells with extracellular miRNAs has consistently led to target mRNA repression, supporting the hypothesis that they play a significant role in intercellular communication.93
3.2 miRNA in tumorigenesis
The complex interaction of miRNA target molecules underscores the intricate role miRNAs play in regulating tumor suppressor genes and oncogenes (Figure 2B). Abnormal miRNA expression can result in dysregulation of these genes, leading to tumorigenesis.94 miRNA dysregulation impacts key aspects of tumor development, including cell cycle progression, proliferation, metastasis, invasion, EMT, apoptosis, DNA repair, angiogenesis, metabolic reprogramming, drug resistance, and immune modulation.95, 96 In cancer cells, miRNA genome rearrangements (such as deletions or amplifications), mutations in UTRs or miRNA sequences, epigenetic methylation, transcription factor dysregulation, altered miRNA biogenesis, and ceRNA activity can all lead to aberrant miRNA expression and function, creating a vicious cycle that exacerbates cancer progression.21, 97 Several common oncomiRs, including miR-21, let-7, miR-17-92, miR-155, and others. miR-21, notably overexpressed in many cancers, acts as a proto-oncogene and its elevated levels often correlate with poor prognosis.98 Conversely, miRNAs like Let-7, miR-34a/b/c, and miR-15a/miR-16-1 function as tumor suppressors. Let-7, the first discovered miRNA, suppresses tumors by targeting oncogenes such as Ras, Myc, HMGA2 (in lung cancer), and MYCN (in neuroblastoma).99
miRNAs influence various biological functions in cancer cells by targeting key mRNAs. For instance, critical mRNAs regulating cell cycle progression and proliferation include cyclin D1 (CCND1), E2F, and cyclin-dependent kinases (CDKs).100 At the EMT level, miRNAs can directly target EMT markers such as E-cadherin and Vimentin, or regulate EMT-associated TFs like Snail, ZEB, and Twist, influencing downstream signaling pathways such as Wnt, PTEN, TGF-β, and Ras, thereby promoting cancer progression.101 In apoptosis regulation, miRNAs target mRNAs such as APAF-1, BCL-2, and caspases.102 For DNA repair, key targets include ataxia telangiectasia mutated (ATM), essential for repairing DNA double-strand breaks.103 miRNA regulation of angiogenesis is primarily mediated through the PI3K/Akt pathway, affecting vascular endothelial growth factor (VEGF) expression.104 Metabolic reprogramming is influenced by miRNAs targeting oncogenes like c-Myc, HIF-1, and p53, which control processes such as the Warburg effect.105 Specifically, c-Myc regulates glucose transporters and pyruvate dehydrogenase, influencing tumor cell metabolism.106 miRNAs also modulate drug resistance by targeting multidrug resistance protein 1 (MDR1).107 At the immune modulation level, miRNAs regulate pathways like mTOR and NF-kB, which are involved in inflammatory and immune responses.108
Moreover, miRNAs packaged in exosomes facilitate intercellular communication, influencing carcinogenesis in recipient cells. Their regulation is intricately linked to epigenetic modifications, transcriptional and posttranscriptional processes. Target genes of miRNAs involved in these regulatory mechanisms can form feedback loops, achieving precise spatiotemporal control of gene expression crucial for cancer initiation and progression.109
3.3 Epigenetic regulation of miRNA in cancer
Epigenetics encompasses inheritable changes in gene expression during cell division without altering DNA sequence.110 miRNAs play a crucial role in this process by directly modulating gene expression or targeting epigenetic enzymes to influence DNA methylation, histone modification, genomic imprinting, and chromatin remodeling (Figure 2C).109
Conversely, epigenetic modifications profoundly impact miRNA expression,21 creating a complex miRNA-epigenetic feedback loop crucial for gene regulation. Dysregulation of this loop is closely linked to cancer development.109 A specific subset known as “epi-miRNAs” regulates epigenetic elements, containing DNMTs, histone-lysine N-methyltransferase (EZH2), histone deacetylases (HDACs), which forms several regulatory circuits.108 For example, miRNA biogenesis and function are regulated by DNA and mRNA methylation, while miRNAs can, in turn, target DNMTs, influencing genome-wide methylation patterns.66 Dysregulated epi-miRNAs contribute significantly to cancer initiation and progression.111 Methylation of miRNAs represents a key epigenetic modification, adding complexity to the posttranscriptional regulation of gene expression.112
miRNAs influence various epigenetic modifications. For instance, the miR-29 family targets DNMT3A and 3B to modulate DNA methylation, influencing lung tumor development.113, 114 miR-29b targets histone deacetylase 4 (HDAC4) to enhance histone and tubulin acetylation, inhibiting multiple myeloma progression.115 In genomic imprinting, miRNAs in imprinted regions influence DNA methylation processes, impacting gene expression and function. Notably, imprinted miRNAs from C2MC and C19MC clusters regulate PTEN, crucial for growth and metabolism.116 Additionally, miR-223 indirectly regulates chromatin structure by targeting chromatin remodeling complexes like SWI/SNF.110, 117
Overall, miRNAs orchestrate a broad spectrum of molecular activities through epigenetic control, forming intricate regulatory networks with significant potential in cancer therapy. Further exploration of miRNA–epigenetic interactions is essential for advancing clinical applications, potentially reversing abnormal gene expressions and enhancing immune responses.109
3.4 miRNA and Tumor Microenvironment (TME)
The TME is crucial for tumor growth and metastasis, being closely linked to processes like immune evasion, extracellular matrix (ECM) remodeling, hypoxia, abnormal angiogenesis, and metabolic reprogramming, ultimately reshaping the TME itself.118 In cancer, miRNA levels undergo significant changes influenced by complex interactions within the TME, involving a dynamic network of factors.119 As depicted in Figure 2D, miRNAs not only target critical regulatory molecules in cancer cells but also contribute to intricate signaling networks between cancer cells and their microenvironment.119 Various cytokines within the TME can alter miRNA expression, activating pathways that help cancer cells evade immune surveillance while promoting proliferation, metastasis, and invasion.120 Thus, miRNAs play a significant role in modulating cell-cell interactions within the TME, influencing these critical processes.
Exosomal miRNAs are pivotal in tumor progression, modulating immune responses, remodeling the TME, promoting angiogenesis, and facilitating tumor cell metastasis through intercellular communication.121, 122 Moreover, miRNAs are secreted via microvesicles or exosomes, further influencing cancer cell growth and metastasis.119 Notably, miRNAs regulate immune cell function, thereby shaping the tumor-immune microenvironment, which profoundly impacts tumor progression.123
The TME transforms miRNA into a bridge connecting diverse cell types, allowing tumor cells to evade immune surveillance, facilitating infiltration, and promoting metastasis. miRNAs primarily impact tumor development by modulating various immune cells such as macrophages, myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs), and natural killer cells (NK cells). Recent studies have highlighted miRNA's role in regulating Treg cells and granulocytes, further emphasizing its broad impact on cancer.123 Epstein-Barr virus (EBV) infections can release miRNAs targeting FOXP1 and PBRM1, which bind to enhancer regions of programmed cell death 1 ligand 1 (PD-L1), thereby inhibiting PD-L1 expression and promoting immune escape.124 Beyond immune cells, cancer stem cells (CSCs) are also pivotal in the TME. In BLCA (UBC), cancer-associated fibroblast (CAF)-derived miR-146a-5p mediates tumor-stroma crosstalk, promoting CSC proliferation and chemotherapy resistance.125 miRNAs also regulate hematopoietic stem cells (HSCs) in the blood. For instance, EV miR-181a-5p from CRC activates HSCs by modulating the IL6/STAT3 pathway. Activated HSCs secrete chemokine CCL20, which further amplifies miR-181a-5p activity, creating a positive feedback loop.126
Besides modulating immune cells, miRNAs play a role in other specific TME processes. From an ECM perspective, tumor cells can secrete exosomal miRNAs that transform normal fibroblasts (NFs) into CAFs. These CAFs secrete growth factors and cytokines that activate adjacent ECM components.127-129 Collagen synthesis and degradation are vital for ECM remodeling. Exosomal miRNA-29a inhibits the TGF-β2/Smad3 pathway, reducing type I and type III collagen expression.129 Hypoxia, a hallmark of the TME, promotes the Warburg effect, which enhances glycolysis and metabolite production, leading to an acidic environment that fosters tumor invasion and metastasis. miRNAs can inhibit hypoxia-inducible factor 1α (HIF-1α) translation, inducing its degradation.130 Additionally, EV-miR-21-5p secreted by hypoxic papillary thyroid cancer cells enhances angiogenesis in the TME by targeting TGFBI and COL4A1.131 miRNAs also target proteins like TSGA10, KLF2, and PTEN, activating molecules such as vascular endothelial growth factor receptor 2 (VEGFR2), p-AKT, and p-ERK, to promote angiogenesis by regulating the quantity and function of local blood vessels.122
Metabolic reprogramming is another key cancer hallmark regulated by miRNAs. miRNA-mediated regulation of COL1A1 activates MYC signaling, inducing metabolic reprogramming in CAFs, which adapt to different metabolic environments and support tumor growth.132 Mitochondrial reprogramming is often associated with metabolic adaptation, crucial for energy production and biosynthesis.133 miRNAs can also affect adipocytes and CAFs. For instance, Kaposi's sarcoma-associated herpes virus-derived EVs transfer miRNAs to uninfected cells, reducing mitochondrial biogenesis and respiration while inducing aerobic glycolysis.133
Overall, EV miRNAs act as communication molecules within the TME, regulating various critical processes through cell–cell interactions. miRNA often compromises effective antitumor immune responses, potentially promoting tumor development. Exosome-mediated delivery of miRNAs presents a promising strategy to counteract tumor-immune evasion and metastasis.134 miRNA serves as a crucial molecular mediator of communication between tumor cells and immune cells, underscoring its significance in advancing tumor immunotherapy through deeper investigation.135
4 miRNA AS BIOMARKER IN CANCER RESEARCH
In cancer research, miRNAs have emerged as important biomarkers due to their specificity and stability. This chapter will introduce miRNA detection methods, covering traditional techniques and recent advancements aimed at improving sensitivity and specificity. We will also discuss miRNA's practical applications in cancer diagnosis, prognosis assessment, and treatment, highlighting its potential for clinical implementation.
4.1 Characteristics and techniques for miRNA detection
miRNAs are a class of small ncRNAs that play crucial regulatory roles in organisms, exhibiting several notable characteristics that hold significant implications for biological research and medical applications. First, miRNAs demonstrate a high degree of conservation, suggesting that they may perform similar regulatory functions across diverse biological developmental processes.136 Second, miRNA expression shows tissue-specific patterns, with varying levels of expression in specific cell types or tissues.137 Additionally, miRNA expression exhibits tissue specificity, with significant variations in expression levels across different developmental stages and tissues, indicating dynamic regulation.138 These characteristics collectively reveal the complexity and precision of miRNAs in gene regulation, with profound implications for understanding their biological functions and their potential applications in disease diagnosis and treatment.
miRNAs exist in various forms, including pre-miRNA, pri-miRNA, mature miRNA, and intermediate products derived during miRNA processing within tumor cells. Liquid biopsy is a noninvasive diagnostic technique that provides disease information by analyzing biomarkers (cfDNA, mRNA, circulating tumor cells, EVs, metabolites, miRNA) present in body fluids, such as blood, urine, and saliva.139, 140 Currently, circulating free miRNAs (cf-miRNAs) have been detected in the blood through liquid biopsy. Once these miRNAs leave the intracellular environment, they exist in association with carriers, such as Ago proteins, nucleophosmin 1 (NPM1), and HDL. However, the most common form is their presence within EVs (exosomes, microvesicles, apoptotic bodies).141 cf-miRNAs are stable in body fluids and show specific abnormal expression in various cancers, making them promising markers for cancer diagnosis with higher sensitivity than traditional protein markers142 (Figure 3A and Figure 3B). Compared to total circulating RNAs, EV-miRNA often targets specific cells, so detection of EV-miRNA is more likely to help physicians predict cancer prognosis.129, 143 Specifically, cell-free immune-related miRNAs (cf-IRmiRNAs) are recognized for their stability and abundance, making them suitable for liquid biopsies to detect cancer early.144, 145 These findings underscore miRNAs' value in early cancer diagnosis, potentially improving early detection rates and patient survival.
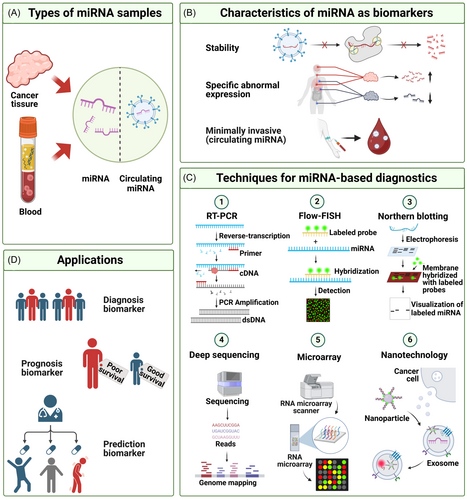
With the advancement of scientific research and technology, miRNA-based cancer detection methods are poised to make significant strides in clinical practice. Various miRNA quantification technologies are emerging, each with distinct advantages. These include quantitative reverse transcription polymerase chain reaction (qRT-PCR), in situ hybridization,146 Northern blot,147 microarray chips,148 NGS, and nanotechnology149 (Figure 3C).
qRT-PCR, which combines reverse transcription and real-time quantitative PCR, is a precise technique for quantifying miRNA and analyzing gene expression. It is known for its high specificity and sensitivity and can be classified based on primer types into Linear, T4 RNA ligase, stem-loop, and poly(A)-tailing-based methods.150 Detection methods for PCR products include synergy brands and TaqMan methods. Digital PCR, an emerging absolute quantitative technique, divides single analyte miRNA molecules and PCR reagents into individual water–oil emulsion droplets, offering high sensitivity for low-abundance miRNA samples.151
In situ hybridization, a hybridization-based detection method, displays the spatial distribution and transport of miRNAs within cells, providing a visual representation of miRNA expression. However, due to the short sequence of miRNAs, creating specific probes is challenging.152 To overcome this, specially chemically modified LNA probes, which offer higher thermodynamic stability and reduced signal loss, are often used. Some studies utilize LNA probes with 20-O-methyl RNAs to enhance specificity and sensitivity. DIG-labeled LNA probes can be detected quantitatively using alkaline phosphatase (AP)-conjugated antibodies, while fluorescence in situ hybridization employs horseradish peroxidase-conjugated secondary antibodies to produce fluorescence.149 Northern blot, a molecular biology technique, detects specific RNA sequences by transferring RNA from a gel to a membrane and hybridizing with a labeled probe. Although it confirms new miRNAs, it is time-consuming and does not quantify miRNA.147
Northern blot is based on the hybridization between target miRNAs and complementary probes. It involves the separation of miRNA and pre-miRNA through electrophoresis, followed by transferring the miRNA onto a solid membrane. The membrane is then hybridized overnight with complementary oligonucleotide probes. The greatest advantage of Northern blot is its high specificity, allowing for the determination of the target miRNA's sequence and length, and it is relatively straightforward to perform in the laboratory. However, miRNAs are prone to degradation by external factors such as RNase during the experiment, and Northern blot requires a large amount of miRNA sample for detection.147
Microarray chips, which detect miRNAs at high throughput, are not suitable for discovering new miRNAs or providing absolute quantification. Hydrogel microfluidic chips can capture Ago2-miRNA protein complexes from serum, enabling PCR and quantitative miRNA detection. Microfluidic surfaces can also integrate with surface-enhanced Raman spectroscopy (SERS) to enhance detection sensitivity.153
Next-generation sequencing (NGS) offers high throughput and sensitivity for detecting and identifying new miRNAs but involves complex library preparation and data processing, with challenges related to cost and absolute quantification.154 NGS can also analyze miRNA expression levels and genetic mutations, which aids in selecting specific miRNA biomarkers.155
Nanotechnology plays a crucial role in quantitative miRNA detection, with methods including nanoparticle solution methods, nanostructure surface-based methods, and methods combining nanoparticle materials with solid carriers. Optical signal-based strategies using nanoparticles such as gold nanoparticles (AuNPs), quantum dots (QDs), and silver nanoclusters are common. Magnetic nanoparticles can also be used in nuclear magnetic resonance measurements.156
In summary, RT-qPCR remains the most widely used method for exosomal miRNA detection due to its sensitivity and specificity. Microfluidics and deep sequencing have improved sample analysis efficiency with high throughput. Microarray chips are useful for comparing miRNA expression levels, while NGS offers advantages in discovering new miRNAs and characterizing miRNAomes. Despite its benefits, each detection technology has limitations, and developing more economical, rapid, and high-throughput methods, especially for circulating miRNAs, remains a key research direction.150
4.2 Improvements and innovations in miRNA detection technology
Specificity and sensitivity are the two most critical factors that require enhancement in miRNA detection technologies. Currently, most advancements focus on optimizing these two performance metrics. Established methods for miRNA quantification, such as microarray chips, qRT-PCR, and NGS, rely heavily on various probe designs and labeling technologies to improve detection sensitivity and specificity.150 To further enhance these aspects, miRNA detection is increasingly adopting complex sensor systems, with CRISPR, enzymes, and amplification technologies playing crucial roles in constructing miRNA biosensors. Portable miRNA detection sensors also offer promising solutions for point-of-care testing (POCT).
For improving specificity, clustered regularly interspaced short palindromic repeats (CRISPR) technology—a gene-editing system originally found in bacteria—has shown promise. It uses RNA molecules to precisely recognize and cut specific DNA sequences, thus targeting and modifying particular locations in the genome. The CRISPR/Cas12a system has recently been reported for direct miRNA detection,157 where the CRISPR sequence provides targeting information and the enzyme executes the cutting action, significantly enhancing specificity.158
Sensitivity improvements largely focus on nucleic acid and signal amplification techniques, with nanoparticles being commonly employed for signal amplification. Current amplification strategies include rolling circle amplification (RCA), exponential amplification reaction (EXPAR), catalytic hairpin assembly (CHA), hybridization chain reaction (HCR), duplex-specific nuclease signal amplification (DSNSA), and loop-mediated isothermal amplification (LAMP).159 Unlike traditional qRT-PCR, these methods maintain a constant temperature during analysis and quantification,160 which can be further enhanced by combining different amplification techniques. For instance, EXTRA-CRISPR can be combined with RCA,161 while CHA can be integrated with DSNSA.162
Nanotechnology-based miRNA detection techniques are relatively sophisticated, involving nanoparticles, nanostructured surfaces, and solid carriers. Nanoparticles are particularly useful for amplifying signal carrier material functions. For instance, nano-morphological DNA cage-based thermophoretic assays can selectively analyze miRNA in exosomes in situ, offering high sensitivity, selectivity, and in situ detection.163 Additionally, the combination of SERS tags and magnetic separation with nanotechnology enhances sensitivity and response speed, making it particularly effective for detecting trace amounts of miRNA.164 Surface polymerization technology, when combined with surface plasmon resonance imaging (SPRI), leverages changes in the refractive index between light and electrons on the nanoparticle surface to reflect miRNA-probe binding.165 Moreover, electrochemical nanobiosensor technology is gaining traction, with photoelectrochemical (PEC) biosensors based on MoS2@Ti3C2 nanohybrids enabling ultra-sensitive miRNA detection.166
Hydrogels, particularly DNA hydrogels, are also emerging as vital tools for miRNA detection. DNA hydrogels, formed through chemical cross-linking or physical entanglement of DNA chains into a three-dimensional network structure, respond to specific biochemical or physical signals by undergoing structural changes. These changes allow for the specific release of miRNA, which significantly enhances detection specificity and sensitivity.167 DNA hydrogels can be engineered into various forms, such as microcapsules, particles, membranes, or microneedle patches, and are integral to the development of portable biosensors tailored to different detection needs and application scenarios.168 The responsiveness of DNA hydrogels can also be integrated with CRISPR technology.169
Additionally, biosensing, microfluidics, and machine learning represent a novel strategy for miRNA detection.170 Ramshani et al. developed an integrated system combining a lysing chip with a concentration/sensing chip. This system eliminates the need for pretreatment or analyte transfer, significantly reducing measurement time.171 Such microchip systems, constructed using high-performance materials to create nanobiosensing platforms, amplify multiple signals for rapid, sensitive, and specific exosomal miRNA detection, showing immense potential for future applications.
4.3 Application of miRNA as biomarker
miRNAs have emerged as valuable biomarkers for cancer diagnosis. Specific miRNAs are integral to cancer progression, leading to the development and application of miRNA-based diagnostic tools for cancer screening and early detection.172, 173 Furthermore, miRNAs can assist in clinical staging of cancer.154 Exosomal miRNAs, which facilitate intercellular communication, play a crucial role in cancer development.174 Since blood is often used as a sample for exosomal miRNA detection, this method offers the advantage of being minimally invasive.175 With advancements in portable POCT devices, cancer diagnosis can now be expedited through miRNA detection (Figure 3D).176, 177
The role of miRNAs in cancer prognosis assessment is increasingly prominent. Abnormal miRNA expression is a potential biomarker for overall survival and disease-free survival in cancer post-neoadjuvant chemotherapy.178 Changes in miRNA levels can predict cancer recurrence risk and provide crucial insights into cancer prognosis. By constructing mRNA–miRNA–lncRNA networks and performing functional enrichment analysis, hub gene identification, and prognostic correlation analysis, we can accurately identify miRNAs linked to cancer prognosis.179 Additionally, analysis of the TCGA database and the Cancer Gene Atlas database, combined with risk survival curves, ROC curves, and survival status diagrams, has successfully identified miRNAs closely related to cancer prognosis, establishing corresponding prognostic risk models 180, 181 certain miRNA expression levels correlate with clinical stage, lymph node metastasis, and patient survival.182 A novel computational approach, ProModule, aims to systematically identify miRNA-mediated modules with prognostic value using clustering methods.183 Additionally, deep learning shows promise in integrating multi-omics data to predict hepatocellular carcinoma (HCC) survival rates, offering new insights for clinical cancer treatment.64
miRNAs can also serve as biomarkers for predicting and dynamically monitoring the therapeutic efficacy of cancer treatments. In predicting efficacy, AI-driven pan-cancer analysis has highlighted miRNA's unique role in cancer staging prediction.184 In breast cancer, miRNAs like miR-30c, miR-187, and miR-339-5p predict treatment outcomes.185 Specifically, miR-21's expression level correlates with breast cancer invasiveness and chemotherapy resistance, providing a reference for predicting chemotherapy response and guiding personalized treatment.186 In immunotherapy, miRNAs influence PD-L1 expression, a key factor in tumor-immune escape. Hsa-miR-200 is associated with high PD-L1 expression, while hsa-miR-197 is negatively correlated with PD-L1 expression.187 In the dynamic monitoring of treatment effect, miRNAs are crucial in optimizing treatment strategies by regulating drug resistance and affecting metabolic pathways and gene expression related to cancer metabolism. For example, distinct mutational signatures of 13 miRNAs have been associated with disease progression in chronic lymphocytic leukemia.97 In addition, tools like the miRNA signature classifier (MSC) and MIR-Test have demonstrated high specificity and sensitivity in reducing the false-positive rate of low-dose computed tomography (LDCT).188 MSC has shown promising results in detecting disease recurrence in postoperative plasma samples and is currently undergoing prospective validation.189
Research indicates that genetic and epigenetic modifications of miRNAs can serve as both diagnostic and prognostic markers in cancer. Single nucleotide polymorphisms (SNPs) in miRNAs are linked to cancer susceptibility, while ADAR proteins, which mediate A-to-I editing, influence the processing, expression, and activity of miRNAs in cancer, making them important diagnostic and prognostic markers.190, 191 Additionally, N6-methyladenosine (m6A)-modified miRNAs represent another key epigenetic modification associated with cancer diagnosis and prognosis.192 With new technologies such as MeRIP-Seq (m6A-seq), it is now possible to identify m6A-modified miRNAs. As detection technologies continue to advance, newly discovered miRNAs and their modifications are being evaluated as potential diagnostic and prognostic markers in clinical trials.193 Increasingly, studies are identifying or testing miRNA biomarkers in cancer efficacy monitoring, with many clinical trials completed or underway (Table 1).
| Application type | miRNA | Cancer | Clinical trial number(s) | References |
|---|---|---|---|---|
| Diagnosis | miR-155 | Nonmuscle-invasive bladder cancer | NCT03591367 | [193] |
| Diagnosis | 84 miRNAs | Multicentric breast cancer | NCT04516330 | [193] |
| Diagnosis | let-7a and miR-124 | Non-Hodgkin's lymphoma and acute leukemia | NCT05477667 | [193] |
| Diagnosis | miR-122 and miR-21 | Lung cancer | NCT02247453 | [194] |
| Diagnosis | Circulating miRNAs | Gynecologic cancer | NCT03776630 | [193] |
| Diagnosis | miR-421 and miR-27a-3p | Colorectal cancer | NCT05346757 | [193] |
| Diagnosis | Circulating miRNAs | Gastric carcinogenesis | NCT05544396 | [193] |
| Diagnosis | 10 miRNAs | Thyroid cancer | NCT04285476 | [193] |
| Diagnosis | 578 miRNAs | Gastric cancer | NCT04329299 | [195] |
| Diagnosis | miR-222-3p, miR-24-3p, and miR-30c-5p | Prostate cancer | NCT05767307 | [196] |
| Diagnosis | miR-642b-3p | Pancreatic adenocarcinoma | NCT06497777 | — |
| Diagnosis | Circulating miRNAs | Breast cancer | NCT06439940 | — |
| Diagnosis | miR-371 | Active germ cell tumors | NCT06060873 | — |
| Diagnosis | Circulating miRNAs | Non-Hodgkin's lymphoma | NCT05921812 | — |
| Diagnosis | miR-20a, miR-21, miR-106b, miR-199a, and miR-223 | Gastric cancer | NCT05901376 | — |
| Diagnosis | miR-371 | Testicular cancer | NCT04914026 | — |
| Diagnosis | miR-371 | Germ cell tumors | NCT04435756 | — |
| Prognosis | Circulating miRNAs | Colorectal cancer | NCT01762813 | [197] |
| Prognosis | miR-10b | Gliomas | NCT01849952 | — |
| Response to treatment (chemotherapy) | Circulating miRNAs | Metastatic castration-resistant prostate cancer | NCT04662996 | [193] |
| Response to treatment (adjuvant chemotherapy) | miR-21, miR-20a, miR-103a-3p, miR-106b, miR-143, and miR-215 | Colon cancer | NCT02466113 | [193] |
| Response to treatment (immunotherapy) | Exosomal miRNAs | Nonsmall cell lung cancer | NCT04427475 | [193] |
| Response to treatment (chemotherapy) | miR-371a-3p | Testicular germ cell tumors and prostate cancer | NCT05529251 | [193] |
| Response to treatment (radiotherapy) | miR-141 and miR-375 | Prostate cancer | NCT02391051 | [193] |
| Response to treatment (chemotherapy) | Circulating miRNAs | Triple-negative breast cancer | NCT04771871 | [193] |
| Response to treatment (chemotherapy) | miR-30A, miR-122, miR-125B, miR-200A, miR-374B, miR-15B, miR-107, miR-320, and miR-645 | Hepatocellular carcinoma | NCT01774344 | [198] |
| Response to treatment (chemo-immunotherapy) | miR-125b, miR-15b, and miR-181c | B-chronic lymphocytic leukemia | NCT01370772 | [199] |
| Response to treatment (chemoradiotherapy) | Circulating miRNAs | Rectal cancer | NCT03962088 | [200] |
| Response to treatment (chemotherapy) | 26 miRNAs | Metastatic soft tissue sarcoma | NCT00413192 | [201] |
| Response to treatment (chemotherapy) | miR-652-3p | Metastatic colorectal cancer | NCT03010722 | [202] |
| Response to treatment (monitor recurrence) | Circulating miRNAs | Diffuse glioma | NCT06203496 | — |
| Response to treatment (chemo-immunotherapy) | Exosomal miRNAs | Lung squamous carcinoma | NCT05854030 | — |
| Diagnosis and Response to treatment (monitor recurrence) | Circulating miRNAs | Hepatocellular carcinoma | NCT05148572 | — |
- Note: NCT, numbered trials are registered at ClinicalTrials. gov.
5 THERAPEUTIC APPLICATIONS OF miRNA
The use of miRNA in cancer therapy is gaining increasing attention. This chapter will explore the development of miRNA mimics and inhibitors for cancer treatment, along with advancements in nanoparticle-based miRNA delivery systems to enhance therapeutic efficacy. Additionally, we will examine combination therapy strategies that integrate miRNA with traditional treatments, as well as miRNA drugs currently in clinical trials.
5.1 Development and use of miRNA mimics and inhibitors in cancer treatment
Dysregulated miRNA expression, acting as tumor suppressors or oncogenes (oncomiRs), plays a crucial role in many cancers. Targeted therapies against miRNAs, including miRNA mimics and inhibitors, have shown great potential in preclinical studies, as shown in Figure 4A.96, 203, 204 These therapeutic strategies involve using anti-miRNA molecules to inhibit upregulated miRNAs causing pathological conditions and employing miRNA mimics to replace and restore the activity of downregulated miRNAs.205
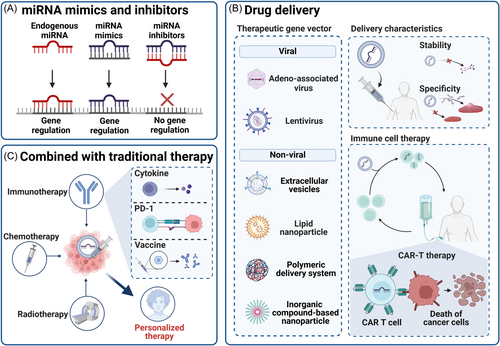
miRNA mimics are synthetic RNA duplexes that replicate endogenous miRNA sequences. They integrate into the RISC and restore miRNA expression in disease states.206 This approach has demonstrated therapeutic value in clinical trials, paving new avenues for cancer treatment.207 Additionally, using small miRNA inhibitors/mimics as adjuvants in vaccination strategies is an innovative method to enhance antibody responses.208 Although initial progress has been made in clinical trials, challenges such as improving stability, achieving specific delivery, and ensuring safety remain.209 To counter endonuclease degradation in the blood, strategies like chemical modification or conjugation enhance miRNA mimics' stability.203 Rigorous preclinical studies and long-term follow-up experiments are essential to evaluate safety and manage potential immune and toxic reactions.210
miRNA inhibitors can alleviate the overexpression of target mRNA and restore cell phenotypes, making them a promising therapeutic strategy. These include natural miRNA inhibitors (such as lncRNA, circRNA, and other ceRNA) and artificial miRNA inhibitors (such as Anti-miRNA oligonucleotides (AMOs) and microRNA sponges).211 MicroRNA sponges, once transfected and stably expressed in cells, can produce long-term or permanent inhibition of the target miRNA.212 Other small molecule inhibitors can directly target oncomiRs and Dicer (an enzyme important in miRNA generation) to achieve tumor suppressor effects. Additionally, CRISPR can be used to directly edit oncomiRs for tumor suppression.17
However, the therapeutic potential of natural miRNAs is often limited due to degradation by RNAse or activation of toll-like receptors, which stimulate the innate immune system.154 To improve miRNA stability, chemically modified nucleic acids, such as locked nucleic acids (LNAs), are employed. LNAs contain a methylene bridge between the 2’-O and 4’-C of the sugar, creating a stable, “locked” ring conformation with enhanced stability. For the issue of miRNA reduction after transfection, viral vectors can provide targeted delivery at specific times, and caged miRNAs, connected by photocleavable linkers, can be specifically released via light.213, 214 Several miRNA-based antitumor drugs have entered clinical trials, as shown in Table 2, offering promising new directions for cancer treatment.220, 221
| miRNA drug name | miRNA | Mode of action | Cancer | Vector | Stage of Development | Clinical trial number(s) | Ref |
|---|---|---|---|---|---|---|---|
| TargomiR | miR-16 | miRNA mimic | MPM, NSCLC | EDVs | Phase 1 | NCT02369198 | [215] |
| [216] | |||||||
| MRX34 | miR-34a | miRNA mimic | LIHC, SCLC, Lymphoma, RCC, NSCLC, HCC, Melanoma | LNP | Phase 1 | NCT01829971 | [217] |
| NCT02862145 | [218] | ||||||
| INT-1B3 | miR-193a-3p | miRNA mimic | TNBC, NSCLC, CC, HCC, Melanoma | LNP | Phase 1 | NCT04675996 | [219] |
| Cobomarsen(MRG-106) | miR-155 | Anti-miR | ATLL, CTCL | Locked nucleic acid-modified | Phase 1/2 | NCT02580552 | [220] |
| NCT03713320 | [221] | ||||||
| NCT03837457 | |||||||
| TTX-MC138 | miR-10b | Anti-miR | MBC, GBM, PC, SCLC, Osteosarcoma | Dextran-coated iron oxide nanoparticles | Phase 1/2 | NCT06260774 | [193] |
| RGLS5579 | miR-10b | Anti-miR | GBM | Unknown | Preclinical | N/A | [221] |
- Abbreviations: ATLL, adult T-cell leukemia/lymphoma; CC, colon cancer; CTCL, cutaneous T-cell lymphoma; EDV, enveloped delivery vehicles; GBM, glioblastoma; HCC, hepatocellular carcinoma; LIHC, liver cancer; LNP, Lipid nanoparticle; MBC, metastatic breast cancer; MPM, malignant pleural mesothelioma; NCT, numbered trials are registered at ClinicalTrials. gov; NSCLC, non-small cell lung cancer; PC, pancreatic cancer; RCC, renal cell carcinoma; TNBC, triple negative breast cancer.
5.2 Nanoparticle-based miRNA therapy delivery system
In addition to improving drug stability and specificity by changing the state of the nucleic acid itself, miRNA mimics and inhibitors can also be delivered into the body through various methods, including vector delivery222 and microinjection.223 Delivery vectors include viral vectors (adeno-associated virus and Lentivirus) and nonviral vectors (EVs, LIPID NANOPARTICLE, POLYMERIC DELIVERY SYSTEM, INORGANIC COMPOUND-Based nanoparticle)224 (Figure 4B).
Nanoparticles, as nonviral vectors, show significant advantages in delivering miRNA to specific locations.210, 225 Compared to naked miRNA delivery, nanoparticles reduce adverse off-target effects and immune responses while improving cellular uptake rates and half-life.226 To optimize nanoparticles, researchers focus on material selection, design rationality, and particle modification to achieve high stability, targeting specificity, and controlled release.227
Recent developments include lipid nanoparticles, SEND RNA delivery platforms, and Fusogenix protein-lipid carriers. These particles improve stability, facilitate storage and transportation, and enhance cellular uptake efficiency.227 LNPs are a common nanoparticle carrier. In addition to their raw materials and surface modifications, barcoded nanoparticles, introduced by Dr. Dahlman, significantly enhance high-throughput screening of LNPs, accelerating the development of targeted drug delivery systems.228 A poly-l-lysine complex, a positively charged polymerized amino acid, can also be used as a specific carrier for miRNA delivery. It forms a complex with negatively charged miRNA molecules, enhancing the stability and cellular uptake of miRNAs.229 miRNA-peptide nanoparticles can be combined with materials like hydrogels to improve stability and facilitate local drug delivery.230 Additionally, dual-responsive polypeptide nanovectors can be engineered to achieve pH-specific miRNA release in the TME.231 Poly(l-lysine)-modified polyethyleneimine (PEI-PLL) copolymers, which combine the high transfection efficiency of PEI with the biodegradability of poly-l-lysine, have been shown to effectively deliver miR-21 with reduced toxicity.232
Exosomes, natural EVs secreted by cells, also show promise due to their superior biocompatibility and targeting capabilities.233 Chemical modification and optimized drug delivery systems can offer advantages like rapid synthesis, controlled release, and targeted administration through.224 For instance, MRX34, a synthetic double-stranded miR-34a mimic encapsulated in liposome nanoparticles, is undergoing phase 1 anticancer clinical trials and has shown apoptosis-inducing effects on tumor cells.205 Mesomir, a miR-16 mimic targeting malignant pleural mesothelioma or non-small cell lung cancer, has also entered phase I clinical trials.234-236
However, challenges remain in applying miRNA in cancer delivery therapy. Researchers use anti-fouling molecules to improve in vivo stability, reducing protein corona formation and aggregation and enhancing colloidal stability.237 Controlled and sustained miRNA release is achieved by combining controlled triggering and stimulus-responsive materials, extending the half-life.238 Additionally, proton-accepting materials can prevent miRNA complexation and degradation after cytoplasmic release.239 Functionalizing nanoparticles with specific cell-targeting ligands improves targeting specificity.240 Collaboration across molecular biology, bioengineering, and clinical oncology is crucial to improve miRNA delivery's stability and specificity. Most studies are still in the preclinical stage, necessitating further toxicity research on nanoparticles.226
5.3 Combination therapy of miRNA and traditional therapy
As shown in Figure 4C, miRNA therapy has unique advantages in cancer treatment and can synergize with various traditional therapies to significantly enhance therapeutic effects. Specifically, miRNA therapy can increase sensitivity to traditional therapies, overcome drug resistance, and inhibit tumor angiogenesis, improving therapeutic outcomes.241
In chemotherapy, miRNA can be combined with drugs to target specific molecules or signaling pathways accurately, reducing damage to normal cells and enhancing tumor cell killing.241 miRNA therapy can also be combined with radiosensitization. Before and after surgery or radiotherapy, miRNA therapy can reduce tumor recurrence risk or increase radiotherapy sensitivity.241 For instance, miR-34a (MIRX34) has shown a radiosensitization effect in NSCLC by binding to the DNA damage response gene RAD51.242
In immunotherapy, miRNAs play diverse roles. They can directly target cancer cells for immunotherapy, regulate immune checkpoint molecules, enhance the response to immunotherapy, and fine-tune immune responses by affecting the immunosuppressive microenvironment and antigen presentation function of DCs.208, 209, 243 In cytokine therapy, miRNAs participate in interferon treatment responses as adjuvants, verified in clinical trials.244 miRNA can enhance T cell receptor sensitivity, T cell fitness, and effector function.245 Additionally, combining miRNA therapy with adoptive cell immunotherapy is a research hotspot. Overexpression of miR-143 supports memory T cell differentiation and tumor metabolic reprogramming regulation, enhancing HER2-CAR T cells' specific killing effect.246 A novel miRNA cluster and epidermal growth factor receptor (EGFR) variant III lentiviral vector CAR-T cells significantly improved persistence and efficacy in treating glioblastoma.247 miR-1258 targets programmed death ligand 1 (PDL1) and affects myeloma treatment by inhibiting PDL1.248 Despite promising clinical trials linking T cell persistence and tumor treatment, miRNA adoptive immunotherapy is still mainly in preclinical trials.245 Future breakthroughs in clinical trials are expected to provide more effective cancer treatment strategies.
6 miRNA AND DRUG RESISTANCE
Drug resistance remains a significant challenge in cancer treatment, and miRNA plays a crucial role in this process. This chapter will investigate how miRNAs contribute to drug resistance in cancer cells and explore strategies to target drug resistance-associated miRNAs. We will also highlight emerging technologies for studying miRNA-related drug resistance, offering new avenues for more effective treatments.
6.1 Mechanisms by which miRNA promotes drug resistance in cancer cells
Chemotherapy, as a systemic treatment, is primarily targeted at tumors prone to spreading and metastatic advanced tumors, showcasing its indispensable therapeutic value.249 However, the development of drug resistance has become the primary factor in the failure of chemotherapy for advanced cancers.250 Acquired drug resistance refers to resistance emerging in previously drug-sensitive tumor populations, leading to tumor recurrence. This resistance often results from genetic, epigenetic, transcriptomic, and proteomic alterations in cancer cells, driving tumor heterogeneity. Many tumors exhibit resistance through a combination of intrinsic and acquired factors.251 Moreover, drug-sensitive tumors containing resistant clones can acquire resistance through clonal expansion.252
In this context, the regulatory role of miRNA is particularly significant. miRNA can precisely target and regulate drug-related mRNA, and its mutation, abnormal expression, or processing may cause aberrant expression levels of target gene proteins.253 When these target genes are closely related to the sensitivity of tumor cells to drugs, abnormal miRNA changes will directly affect cancer's response to chemotherapy drugs.2 miRNAs are closely linked to drug resistance in hematologic malignancies. EV-miRNAs can be transferred to sensitive cells, promoting the formation of drug-resistant tumor cells. Identifying miRNAs that regulate cancer cell resistance has become a focal point in hematologic tumor research.252
Key target genes involved include anticancer drug target-related genes, pharmacokinetics-related genes, key genes in drug action signaling pathways, and DNA damage repair-related genes. As shown in Figure 5B, they collectively constitute a regulatory network for chemotherapy sensitivity.250 Specifically, miR-192 and miR-215 target thymine synthase (TS), the main target of fluoropyrimidine drugs used to treat colorectal cancer. By downregulating TS expression, miR-192 and miR-215 significantly increase the sensitivity of colon cancer cells to 5-FU.254 Conversely, the multidrug resistance-associated protein 1 (MRP1) is a key efflux transporter, and its overexpression often leads to chemotherapy resistance in breast cancer. miR-145 can target MRP1 and enhance the sensitivity of breast cancer cells to doxorubicin by inhibiting its expression.255 Key genes involved in DNA repair, such as excision repair cross-complementation group 1 (ERCC1) and XPC, are regulated by miR-149 and miR-488, respectively.256
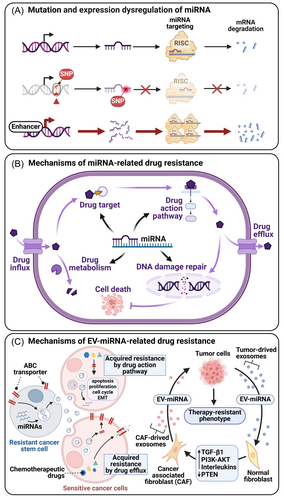
The TME plays a critical role in mediating cancer drug resistance.251 Factors such as tumor-TME communication, stress-induced selection pressure, and the acidic TME environment contribute to drug resistance.252 EV-miRNAs reflect the traits of parental tumor miRNAs257 and can transfer drug resistance traits to sensitive cells, enhancing drug resistance by promoting tumor progression and increasing drug efflux.251, 258 In the TME, EV-miRNAs play a regulatory role in chemotherapy resistance by modulating CAFs, macrophages, CSCs, EMT, and autophagy (Figure 5C).251 Autophagy, a metabolic process that eliminates damaged or redundant molecules and organelles, can also promote tumor resistance, particularly in hypoxic TMEs, where it delays cell apoptosis.259
CSCs, a subset of tumor cells with self-renewal capacity and multidirectional differentiation potential, often exhibit complex drug resistance mechanisms. Specific miRNAs (e.g., miR-34a, miR-21, and miR-155) regulate CSC properties and contribute to cancer drug resistance. The close relationship between EMT and cancer drug resistance has been established, with evidence showing that EMT enhances CSC chemotherapy resistance. EV-miRNAs play a key role in the interaction between CSCs and EMT.101 Additionally, CSCs communicate with the TME, further driving chemotherapy resistance.260 CAF-derived EV-miR-146a-5p promotes CSC proliferation in UBC and enhances drug resistance through tumor-stroma interactions.125 MDSCs also utilize miRNAs to increase the stemness of ovarian carcinoma cells by triggering miR-101 and regulating the co-repressor gene CtBP2, inducing stem cell traits.261
Paclitaxel (PTX) is a highly effective, low-toxic, broad-spectrum natural anticancer drug widely used in treating breast cancer, ovarian cancer, head and neck cancer, and lung cancer. PTX induces cell cycle arrest, inhibits mitosis, phosphorylates antiapoptotic proteins to inactivate B-cell lymphoma-2 (Bcl-2), activates immune responses to eliminate tumors, and regulates miRNAs associated with tumor progression. PTX's regulation of calcium signals leads to mitochondrial calcium ion release and programmed cell death.262 miRNAs are also involved in PTX resistance in various cancers. For instance, in breast cancer, miR-451 mediates PTX resistance by regulating YWHAZ, and PTX-induced exosomal miR-378a-3p activates the EZH2/STAT3 signaling pathway, promoting resistance.263 In lung cancer, miR-17-5p downregulation increases Beclin1 expression, a key autophagy regulator, enhancing PTX resistance. In ovarian cancer, circCELSR1 regulates PTX resistance through FOXR2 expression, while miR-106a and miR-591 also contribute to resistance. In cervical cancer, upregulation of miR-375 correlates with increased PTX resistance. Across various cancers, miR-21 overexpression inhibits PTEN, activates the PI3K/Akt pathway, and fosters PTX resistance.264
6.2 Strategies targeting miRNAs associated with drug resistance
The targeted regulation of therapeutic-related mRNAs by miRNAs constitutes the core mechanism of cancer cell resistance.254 Specifically, miRNAs can inhibit the expression of specific efflux transporters and enzymes, significantly enhancing drug accumulation in cells and improving therapeutic efficacy. Additionally, some miRNAs can directly target pharmacological targets, such as proto-oncogenes, to achieve therapeutic effects by inhibiting their protein production. miRNAs themselves can also serve as therapeutic targets. Their expression may be used as biomarkers to predict drug resistance.265 This unique mechanism provides a theoretical basis for developing miRNA therapeutic strategies to overcome drug resistance, including using miRNA mimics or antagonists, regulating key proteins that interact with anticancer drugs (such as MRP), and the synergistic effects of anticancer drugs.252, 254
Studying miRNA not only helps predict individual tumors' resistance to anticancer drugs but also guides oncologists in tailoring reasonable treatment plans for patients, providing a crucial basis for future drug resistance avoidance strategies.254 Experimental research has shown that applying ds-miR-634 mimics in vitro esophageal squamous cell carcinoma (ESCC) cells and xenograft mouse models significantly enhanced cisplatin's antitumor effect in ESCC, demonstrating the great potential of miRNA mimic drugs.96 Among tumor suppressor genes, PTEN is notable for its frequent inactivation in cancer, with its loss contributing to treatment resistance. Recent studies suggest that the ncRNAs/PTEN axis is pivotal in conferring resistance to various cancer therapies, presenting significant therapeutic potential in overcoming resistance.266
By targeting resistance-related miRNAs, relevant mRNAs can be regulated to reverse PTX resistance. miRNA-200c can reverse EMT-induced PTX resistance through cathepsin L (CTSL).267 Ectopic expression of miR‑335 or depletion of its target gene SETD8 increases cell sensitivity to PTX.268 Upregulation of miR-497-5p improves breast cancer patients' response to PTX by inhibiting MALAT1 and SHOC2.269 miR-497-5p binds to TRPM2-AS and reverses its promotion of PTX resistance.270 miR-522-3p reduces PTX resistance in paclitaxel-resistant ovarian cancer in vitro by downregulating E2F2.271
Despite the potential of miRNA therapy, miRNA-based therapies are still relatively rare in clinical trials, particularly in achieving anticancer efficacy by reversing drug resistance, which remains in the preclinical research stage. Future advancements in research and technology are expected to enhance miRNA therapy's role in cancer treatment.
6.3 Emerging technologies for studying miRNA-Related drug resistance
miRNA holds great potential and offers new strategies for treating cancer resistance by regulating genes related to drug targets, drug pharmacokinetics, DNA damage repair, and key cell signaling pathways.2 However, traditional methods for synthesizing miRNA drugs often require complex artificial modification processes, increasing production costs and potentially introducing different physical, chemical, and biological properties, and even potential toxicity.272
The miRNome profile is closely related to tumor-specific histopathology and molecular characteristics, such as stromal cell infiltration and tumor driver mutations.273 miRNA mutations play an important role in human cancers' occurrence and development, as shown in Figure 5A. Therefore, the miRNA expression profile in human cancers is closely related to cancer detection, staging, progression, and response to treatment.97 More importantly, as a class of epigenetic drugs, miRNA provides a new direction for cancer treatment at the gene regulation level. The epigenetic and genomic properties of miRNA drugs reveal differences between individuals, offering new insights into personalized drug treatment and opening new avenues for developing more effective treatments.274
Ferroptosis, distinct from other forms of cell death, is triggered by the accumulation of intracellular iron and subsequent lipid peroxidation.275 Increasing evidence supports ferroptosis as a target for overcoming chemotherapy resistance, as some chemotherapeutic drugs synergize with ferroptosis by promoting iron metabolism or inhibiting antioxidant mechanisms such as glutathione synthesis.276, 277 miRNAs play a pivotal role in this process; for example, miR-522 secreted by CAFs blocks lipid-ROS accumulation, inhibiting ferroptosis and promoting drug resistance in gastric cancer.278
In the realm of cancer treatment, drug repurposing strategies are also opening new possibilities. Several existing drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs), metformin, dichloroacetate, enalapril, ivermectin, bazedoxifene, melatonin, and S-adenosylmethionine, are being explored for new applications in cancer therapy.279 miRNAs play a central role in these strategies by modulating tumor suppressor and oncogenic miRNAs. For instance, dichloroacetate (DCA) and let-7a have been shown to work together to induce apoptosis in triple-negative breast cancer cells, while reducing reactive oxygen species and stimulating mitochondrial biogenesis.280 Metformin has demonstrated anticancer effects through miRNAs such as let-7, miR-26, and miR-200, which regulate tumor metabolic pathways.281 Similarly, aspirin can improve lung cancer outcomes by targeting the miR-98/WNT1 axis.282 Other examples include simvastatin, which reduces let-7 levels to inhibit cell viability,283 and miR-192, which enhances osteosarcoma chemosensitivity to methotrexate.284
Multidrug combinations can be used to reverse resistance, PTX and miRNA co-delivery play a crucial role in breast cancer management. These strategies reduce the toxicity of free PTX by improving cellular uptake and bioavailability, achieving tumor site accumulation. Molecular docking studies showed the enhanced anticancer activity of nanoformulations like ANG1005 and Abraxane, which significantly interact with α-tubulin and Bcl-2 proteins. Therefore, ANG1005 and Abraxane may be more suitable for breast cancer therapy than free PTX.285
In recent years, the scientific community has focused on developing recombinant RNA technology to produce large quantities of miRNA preparations economically and efficiently. These miRNAs are naturally produced in cells, with structures consisting of unmodified or post-transcriptionally modified nucleobases, more accurately mimicking natural miRNAs' structure, function, and safety.286, 287 The tRNA/pre-miRNA-based method in recombinant RNA technology is particularly noteworthy for its power and efficiency, enabling continuous and efficient production of miRNA reagents.288 Additionally, a study successfully developed a recombinant miRNA vector that significantly increased recombinant miRNA expression in bacteria.289
RNA nanoparticles can also be used for specific cancer treatment. A novel multivalent RNA nanoparticle was designed to store three copies of hepatocyte targeting ligands, one copy of miR122, and 24 copies of PTX to overcome drug efflux and chemoresistance for synergistic treatment of HCC. These nanoparticles enhance tumor localization and effectively deliver miR122 and PTX to liver cancer cells, enhancing their EPR and receptor endocytosis mediated by hepatocyte targeting ligands. miR122 silences drug efflux proteins and oncoproteins, synergizing with PTX to enhance cytotoxicity.290
7 DISCUSSIONS
miRNAs play a critical role in the initiation, progression, and metastasis of cancer. Studies demonstrate that miRNAs regulate key cellular processes, including proliferation, apoptosis, migration, and invasion, by modulating the expression of specific genes. Abnormal miRNA expression has been widely observed in various cancers, and these dysregulated miRNAs have potential as diagnostic and prognostic biomarkers. Furthermore, miRNAs hold promise for therapeutic applications, as they can target and regulate cancer-related gene networks. A deeper understanding of miRNA mechanisms in cancer is crucial for developing novel diagnostic tools and therapeutic strategies.
Research on miRNA interactions utilizes several methods. First, differential gene expression profiling reveals the collective impact of miRNA regulation but cannot distinguish direct from indirect targets. Second, bioinformatics analysis identifies potential miRNA targets in large-scale gene expression studies but requires experimental validation to confirm direct targets. High-throughput technologies like quantitative PCR, Western blot analysis, and reporter gene vectors help validate miRNA–mRNA interactions.
Recent technological advancements, including high-throughput sequencing and imaging, have significantly improved understanding of miRNA interactions in biological processes. High-throughput sequencing is used as a prognostic marker in cancer, though it has limitations in detecting core cellular and molecular functions.11 CRISPR/Cas9 technology enables precise validation of miRNA-responsive elements (MRE).291 Visualization techniques like single-molecule imaging and cryo-electron microscopy allow real-time monitoring of dynamic interactions,292 facilitating precision treatment.293
Bioinformatics and computational models predict miRNA functions by integrating experimental data, using methods such as conserved site matching (TargetScan), tissue mapping (mirSOM), manual curation and verification (DIANA-TarBase), and text mining (Context-MMIA).23 Combining experimental and analytical techniques enhances the accuracy of miRNA interaction predictions and uncovers new regulatory relationships, laying the foundation for miRNA's diagnostic and therapeutic applications.
miRNAs are promising biomarkers for early tumor detection, clinical diagnosis, and treatment monitoring due to their stability, specificity, and presence in body fluids. However, noninvasive miRNA-based diagnostics need further development to meet clinical standards.291 miRNA therapeutics offer targeted approaches in preclinical models, but a deeper understanding of miRNA changes is necessary. miRNAs also play a crucial role in drug resistance, aiding in the prediction of patient responses to specific drugs and guiding precision treatment.2, 253
8 FUTURE DIRECTIONS AND INNOVATIONS IN MIRNA RESEARCH
miRNAs have broad applications in cancer research. As biomarkers, their stability and specificity, combined with advanced detection technologies, support the development of sensitive biosensors for clinical use.161 miRNA's role in the TME supports its application in synergistic immunotherapy, enhancing treatment efficacy and overcoming immune rejection. Rapid advancements in nanotechnology for miRNA delivery improve drug bioavailability and effectiveness.123
Innovative miRNA therapeutics include ionic liquid-based ointments containing ds-miR-634 mimics, enhancing transdermal drug permeability without device assistance.294 miRNA therapy's ability to target multiple genes supports personalized medicine, with potential cross-cancer treatment strategies.
Challenges in miRNA delivery systems, such as controlling in vivo stability and precise tissue targeting, highlight the emerging field of combining miRNA with nanoparticles. This interdisciplinary approach enhances cancer-targeted therapies and early diagnosis through sensitive biosensors for detecting miRNAs in body fluids.
In summary, miRNA research spans molecular biology, immunology, pharmacology, bioinformatics, and materials science. From a technical standpoint, improving miRNA detection specificity and sensitivity is critical for their use as biomarkers. Likewise, as therapeutic molecules, miRNA delivery technologies still require advancements in stability and targeting specificity. Technological advancements are enhancing miRNA's role in clinical diagnosis and treatment, driving precision medicine initiatives in cancer care.
AUTHOR CONTRIBUTIONS
Zehua Wang: Conceptualization (equal); writing—original draft (equal); data curation (equal); visualization (equal). Hangxuan Wang: Writing—original draft (equal); visualization (equal); data curation (equal). Shuhan Zhou: Writing—original draft (equal); data curation (equal). Jiasheng Mao: Writing—original draft (equal); data curation (equal). Zhiqing Zhan: Writing—original draft (equal). Shiwei Duan: Conceptualization (equal); funding acquisition (equal); supervision (equal); writing—review and editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank PubMed and NCBI databases for the valuable information. We also thank BioRender (https://www.biorender.com/) for providing schematic plot elements. This work was supported by the Qiantang Scholars Fund in Hangzhou City University (No. 210000-581835).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.