Lomitapide: Targeting METTL3 to Overcome Osimertinib Resistance in NSCLC Through Autophagy Activation
ABSTRACT
Osimertinib resistance remains a significant challenge in the treatment of non-small cell lung cancer (NSCLC). N6-methyladenosine (m6A) modifications are closely linked to various mechanisms of anticancer resistance and autophagy, offering new avenues for targeted therapies. However, the role of m6A-mediated autophagy in osimertinib-resistant NSCLC is still unclear. In this study, we utilized multi-omics sequencing analysis and found that overexpression of the m6A methyltransferase METTL3 contributes to osimertinib resistance in NSCLC. Importantly, we identified that METTL3 positively regulates the expression of the autophagy-related gene ubiquinone-cytochrome C reductase complex assembly factor 2 (UQCC2) through an m6A-dependent mechanism. Further, we confirmed that METTL3 knockdown leads to UQCC2 downregulation and triggers autophagy activation. Interestingly, lomitapide, a cholesterol-lowering drug, was repurposed to enhance the sensitivity of cancer cells to therapy by inhibiting METTL3, which in turn activated autophagy-associated cell death pathways, reversing osimertinib resistance. This study emphasizes the critical role of the METTL3/UQCC2 axis in autophagy-mediated drug resistance and positions lomitapide as a promising METTL3 inhibitor and autophagy inducer with potential therapeutic effects, either alone or in combination with other anticancer agents, in patients with osimertinib-resistant NSCLC.
Graphical Abstract
1 Introduction
Non-small cell lung cancer (NSCLC), the primary subtype of lung cancer, is one of the most common malignant tumors, with an extremely high incidence and mortality worldwide [1]. In patients with NSCLC, genetic mutations and alterations, especially the L858R and T790M mutations in epidermal growth factor receptor (EGFR), are the main pathogenic causes [2, 3]. Therefore, targeted therapy with EGFR tyrosine kinase inhibitors (TKI), such as gefitinib and erlotinib, has become a standard first-line therapy for the treatment of patients with NSCLC having EGFR mutations [2]. Osimertinib (AZD9291), a third-generation EGFR-TKI for the treatment of NSCLC patients with gefitinib or erlotinib tolerance, has made significant progress [4]; however, osimertinib resistance invariably develops, resulting in treatment failure and patient death during the clinical treatment [5, 6]. The advent of osimertinib resistance is a major challenge to the successful long-lasting treatment of EGFR-mutated lung cancer patients. Emerging clinical data suggest the etiology of triggering osimertinib resistance is complex and not fully understood, including EGFR-dependent and EGFR-independent pathways [7]. Hence, elucidating the underlying mechanisms of osimertinib resistance and discovering new medications to overcome drug resistance are urgently required.
Autophagy is critical for eliminating misfolded or aggregated proteins to maintain cellular energy metabolism, homeostasis, and genomic integrity in eukaryotic cells [8, 9]. Its dysfunction is closely associated with cancer development [10], invasion, migration [11-13], and resistance to cancer therapy [14, 15]. Accumulating evidence has revealed that autophagy plays a dynamic tumor-suppressive or -promoting role in different contexts and stages of cancer development [16], indicating it could act as a “double-edged sword” in tumor cells. Autophagy may serve as a protective mechanism to help tumor cells survive under drug pressure and may lead to the development of drug resistance. In addition, studies have explored the possibility of overcoming TKI resistance by regulating autophagy. Several drugs targeting the autophagy process were used or reused to enhance the antitumor effect of TKIs or overcome drug resistance by inhibiting or promoting autophagy. Therefore, autophagy-related inhibitors or stimulators have been developed in clinical trials to evaluate their potential in monotherapy or in combination with existing anticancer therapies [17-19]. In liver cancer, palmitoyl-protein thioesterase 1 (PPT1), an enzyme responsible for protein depalmitoylation and autophagy regulation, can be inhibited by GNS561. It has been reported that GNS561, combined with atezolizumab and bevacizumab, was assessed in a phase II clinical trial, providing novel insights into anticancer autophagy inhibitors acting as attractive therapeutic targets in liver cancer [18, 20]. In NSCLC treated with EGFR-TIK, autophagy is contributing to both cell survival and death. Osimertinib induces autophagy in NSCLC cells, inhibition of autophagy enhanced osimertinib cytotoxicity in both osimertinib-resistant and sensitive cells. And autophagy acts as a negative regulator in osimertinib efficacy [21]. However, it is unknown whether autophagy plays a role in osimertinib resistance, and potential autophagy-related therapies for osimertinib-resistant NSCLC are yet to be explored.
Posttranscriptional modification N6-methyladenosine (m6A), as one of the most important modifications of mRNAs, regulates RNA fate by influencing alternative polyadenylation, pre-mRNA splicing, as well as RNA stability, translation efficiency, and mRNA decay [22, 23]. m6A modification is dynamically and reversibly modulated by m6A modification factors, such as “writers” (methyltransferases), “erasers” (demethylases), and “readers” (RNA-binding proteins) [24], which are reported to play pivotal roles in lung cancer growth [25], progression [26], and drug resistance [27]. Among these, METTL3, one of the most important m6A methyltransferases, has been reported to regulate autophagy-related drug resistance in a context-dependent manner. For instance, METTL3 inhibits autophagy by increasing the expression of Forkhead Box Class O3 (FOXO3A) to sensitize HCC (hepatocellular carcinoma) cells to sorafenib [28]. In TCam-2 seminoma cells, METTL3 promotes autophagy and increases cisplatin resistance [29]. In NSCLC cells, METTL3 promotes autophagy and confers gefitinib resistance [30]. However, the relationship between METTL3-mediated m6A methylation and autophagy-mediated osimertinib resistance in NSCLC has not yet been explored.
In this study, we successfully established osimertinib-resistant cells (NCI-H1975/OR) based on parental NSCLC cells (NCI-H1975). Further analysis of multi-omics sequencing, including RNA-seq, MeRIP-seq, and single-cell RNA sequencing (scRNA-seq), revealed that the m6A methyltransferase METTL3 is highly expressed to promote osimertinib resistance in NSCLC and the ubiquinone-cytochrome C reductase complex assembly factor 2 (UQCC2) serves as a novel downstream target of METTL3. The METTL3/UQCC2 axis is negatively correlated with autophagy. METTL3 or UQCC2 knockdown could activate autophagy pathways by upregulating the autophagy-related marker gene LC3. Furthermore, we also identified lomitapide as a potential METTL3 inhibitor that activated autophagy and reversed osimertinib resistance by inhibiting the METTL3/UQCC2 axis in NSCLC cells. This study elucidated the paradigm that m6A responds to drug resistance by controlling autophagy and provided a novel therapeutic target molecule and clinical treatment method for patients with NSCLC and osimertinib resistance.
2 Results
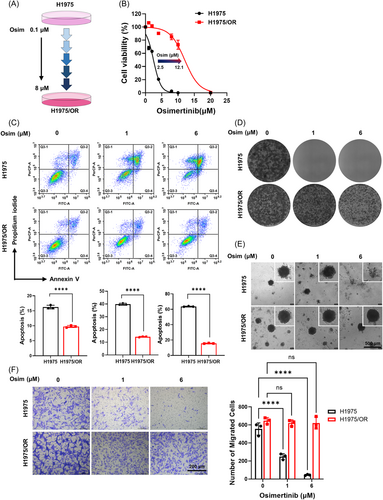
2.1 Construction and Characterization of Osimertinib-Resistant Cells in NSCLC
To study the molecular mechanism of osimertinib resistance in NSCLC, we successfully established NCI-H1975/OR cells by exposing NSCLC parental cells (NCI-H1975 with EGFR-L858R/T790M mutations) to dosage-escalation of osimertinib from 0.1 to 8 μM (Figure 1A). To further investigate the proliferative effect of osimertinib on NSCLC cells in vitro, a CCK-8 assay was conducted to detect cell viability in NSCLC parental and NCI-H1975/OR cells. As observed in Figure 1B, the half-maximum inhibitory concentration (IC50) of osimertinib in NCI-H1975/OR cells was approximately six times higher than that in the NSCLC parental cells, indicating that osimertinib resistance significantly promoted cell viability. Additionally, osimertinib failed to induce apoptosis in NCI-H1975/OR cells (Figure 1C), with normal cell clonogenicity (Figures 1D), 3D tumor spheroid formation (Figure 1E), and cell metastasis (Figure 1F) even the concentration of osimertinib was high. Collectively, the results illustrated that osimertinib resistance efficiently inhibited NCI-H1975/OR cell apoptosis but enhanced cell viability, proliferation, and metastasis in vitro.
2.2 Upregulated METTL3 Promoted Osimertinib Resistance in NSCLC by Inhibiting Autophagy-Mediated Cell Death
To further investigate gene fluctuations and signaling pathway changes in osimertinib-resistant cell lines, we conducted RNA-seq in NSCLC parental cells and NCI-H1975/OR cells. As shown in Figure 2A, osimertinib tolerance caused dramatic transcriptomic changes with 4744 differentially expressed genes (DEGs) upregulated and 6838 DEGs downregulated. Among the genes, METTL3, the major component of the m6A methyltransferase complex, was found to be significantly upregulated in NCI-H1975/OR cells. Further investigation of the GSE243564 database, which represents three different patient-derived tumor xenograft (PDX) models of EGFR mutations in lung cancer treated with three different concentrations of osimertinib until the appearance of drug tolerance, revealed that METTL3, as one of the 10 overlapping m6A modification factors, was upregulated in the osimertinib-resistant residual group (Supporting Information S1: Figure 1A–C). We next analyzed single-cell RNA sequencing (scRNA-seq) from 10696 cells following osimertinib treatment in the aforementioned PDX models (GSE243562). Unbiased clustering of cells identified 16 parallel clusters based on Uniform Manifold Approximation and Projection (UMAP) analyses (Figure 2B). Subsequently, three major cell populations were detected according to their gene profiles and canonical markers and were classified into the osimertinib-sensitive (residue_free), osimertinib-resistant (severe_residue), and moderate_residue groups (Figure 2C). The proportions of each cell type were distinct. In the osimertinib-treated group, the proportion of osimertinib-resistant types (severe_residue) was high. However, in the untreated group, the proportion of osimertinib-sensitive types (residue_free) was high (Figure 2D). Compared to that in the osimertinib-sensitive group (residue_free), METTL3 was highly overexpressed in the osimertinib-resistant group (severe_residue), confirming its positive role in promoting osimertinib tolerance (Figure 2E). Additionally, since previous reports had indicated that METTL3 could regulate autophagy-mediated drug resistance in a context-dependent manner, we hypothesized that METTL3 may also serve as a key factor in promoting osimertinib resistance by mediating autophagy in NSCLC.
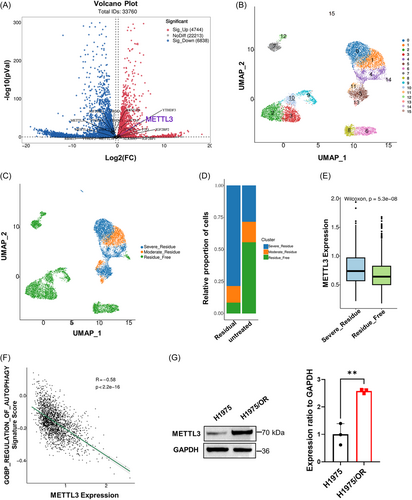
Then, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that genes regulated by METTL3 were mainly enriched in the autophagy-related electron transport chain (Supporting Information S1: Figure 1D) but had a negative correlation with autophagy (Figure 2F). Moreover, we also validated the upregulation of METTL3 in NCI-H1975/OR cells compared to that in NSCLC parental cells by western blot analysis (Figure 2G). Overall, these results confirmed our hypothesis that METTL3 promoted osimertinib resistance by inhibiting autophagy and may be utilized as a potential drug target and prognostic marker in patients with NSCLC.
2.3 Lomitapide, a Novel Potential METTL3 Inhibitor, Could Reverse Osimertinib Resistance by Activating Autophagy in NSCLC Cells
To acquire a novel targeted therapy anti-agent that could target METTL3 to inhibit osimertinib resistance and be further used clinically for the treatment of patients with NSCLC, we performed in-silico structure-based screens followed by antitumor activity evaluation (Figure 3A). An FDA-approved drug library of more than 1900 compounds was screened in silico against the crystal structure of METTL3, which represents the dominant conformation of the active site. Compounds with favorable docking scores were selected for antitumor activity in combination with osimertinib. Finally, lomitapide emerged as the top candidate from both screens, since it binds to METTL3 and has synergistic effects with osimertinib in killing osimertinib-resistant cells. Computational modeling predicted that lomitapide is located in the METTL3-active S-adenosylmethionine (SAM)-binding site with a binding energy of −7.88 kcal/mol. The co-substrate SAM-binding site is part of the METTL3 methyltransferase activity domain [31], which is bound to the catalytic pocket, whereas the methyl group is transferred from the SAM to the adenosine N6-atom of the substrate RNA during catalysis [32-34]. The binding pose of lomitapide in METTL3 (PDB code 8PWA) forms a hydrogen bond with the Arg379 residue (Figure 3B,C), which may act as a blocker for SAM binding. The catalytic inhibitor of METTL3 would require further investigation.

The inhibitory effects of lomitapide on NCI-H1975/OR cells were evaluated by CCK-8 assay (Supporting Information S1: Figure 2A). Then, we found the IC50 of NCI-H1975/OR cells sensitive to osimertinib with lomitapide addition is 2.5 μM (Figure 3D). To further determine the synergistic effects of lomitapide and osimertinib, we treated NCI-H1975/OR cells with osimertinib alone (Osim), lomitapide alone (Lomi) or a combination of osimertinib and lomitapide (Comb). The addition of lomitapide significantly promoted apoptosis (Supporting Information S1: Figure 2B). The 3D tumor spheroids showed that NCI-H1975/OR cells were more sensitive to Comb than Osim, hence indicating that osimertinib resistance in NCI-H1975/OR cells could be reversed by the addition of lomitapide (Supporting Information S1: Figure 2C).
Previous studies had shown that lomitapide, an effective cholesterol-lowering drug for the treatment of homozygous familial hypercholesterolemia (HoFH), has been repurposed to suppress drug resistance through the activation of autophagy to treat cancers such as colorectal cancer [35] and multiple myeloma [36]. To further validate whether lomitapide triggered the autophagy machinery to reverse osimertinib resistance in NSCLC, we also collected NCI-H1975/OR cells treated with Comb for RNA sequencing. Gene set enrichment analysis (GSEA) analysis showed that the autophagy pathway was mainly enriched in the lomitapide-treated group than in the control group (Figure 3E). To evaluate the impact of osimertinib and lomitapide on autophagy flux, we employed a tandem mRFP-GFP-LC3-expressing adenovirus in NCI-H1975/OR cells. Our results revealed a notable increase in yellow particles (represent the autophagosomes before fusion with acidic lysosomes) within the cells treated with Comb, as compared to the other treatments (Figure 3F). Besides, the heatmap showed that most genes involved in the autophagy pathway were significantly upregulated upon lomitapide treatment (Supporting Information S1: Figure 2D), as well as the expression of the autophagy-related classical marker genes LC3B-Ⅱ and p62 detected by western blot analysis (Figure 3G). Furthermore, transmission electron microscopy (TEM) data also confirmed that lomitapide inevitably increased autophagosome and autolysosome formation (Figure 3H). The autophagy inhibitor chloroquine (CQ) could not affect LC3B-Ⅱ protein levels after lomitapide treatment, indicating a strong complementary effect (Figure 3I). Additionally, CQ remarkably reduced osimertinib sensitivity (Supporting Information S1: Figure 2E) and apoptosis (Supporting Information S1: Figure 2F) in lomitapide-treated NCI-H1975/OR cells. Collectively, the results suggested that lomitapide, a novel METTL3 blocker, reversed osimertinib tolerance in NSCLC by activating autophagy.
2.4 UQCC2, as a Novelty Downstream Factor of Lomitapide-Mediated Osimertinib Resistance, Was Negatively Related to Autophagy-Mediated Cell Death
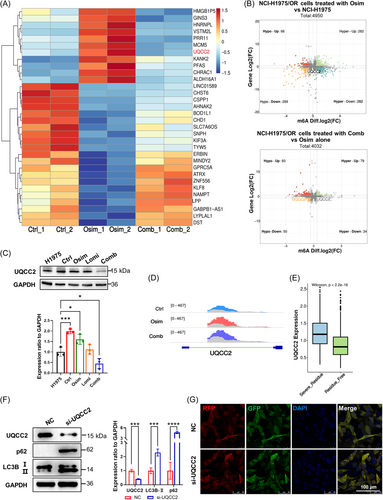
To further investigate the changes in the extent of m6A modifications involving in the process of osimertinib resistance and lomitapide reversion, we performed MeRIP-seq in NCI-H1975 cells (control), and NCI-H1975/OR cells treated with either Osim alone or Comb. Combined with RNA-seq, we identified 32 genes for which the m6A peak and gene expression fluctuated and exhibited an opposite trend after lomitapide treatment (Figure 4A). Among the genes, UQCC2 exhibited a substantial change, with hypermethylated m6A peaks and higher mRNA expression in NCI-H1975/OR cells while decreased upon lomitapide treatment (Figure 4B), which was further confirmed by western blot analysis (Figure 4C). IGV analysis demonstrated that UQCC2 m6A modifications mainly altered the intron regions during osimertinib resistance and lomitapide-induced reversion (Figure 4D). Strikingly, further scRNA-seq analysis (GSE243562) also revealed that UQCC2 was highly expressed in the osimertinib-resistant group (severe_residue) (Figure 4E). Then, we knocked UQCC2 down via small interfering RNA (si-UQCC2) and found the expression of LC3B-Ⅱ and p62 were upregulated (Figure 4F) as well as the autophagy-mediated cell apoptosis (Supporting Information S1: Figure 3A). Consistently, we also discovered that UQCC2 negatively regulated autophagy with autophagy flux activation in si-UQCC2 cells (Figure 4G). This regulatory role of UQCC2 was supported by scRNA-seq analysis (GSE243562) of the autophagy pathway (Supporting Information S1: Figure 3B) and the autophagy-related electron transport chain pathway (Supporting Information S1: Figure 3C). Taken together, these results suggested that inhibition of UQCC2, a key target of lomitapide, could reverse osimertinib resistance via autophagy activation.
2.5 Lomitapide Reverses Osimertinib Resistance Through METTL3/UQCC2 Axis-Mediated Autophagy
According to our western blot analysis, MeRIP-seq and RNA-seq analysis, we found METTL3 increased in NCI-H1975/OR cells but decreased after lomitapide addition (Figure 5A; Supporting Information S1: 4A), with the same trend expression of UQCC2. Therefore, we urgently wanted to answer the relationship between METTL3 and UQCC2. Based on the scRNA-seq analysis (GSE243562), we found there was a positive correlation between them (Supporting Information S1: Figure 4B). Therefore, we knocked METTL3 down (si-METTL3) and found UQCC2 was downregulated with LC3B-Ⅱ and p62 upregulation, whereas decreased UQCC2 (si-UQCC2) expression did not affect METTL3 expression in turn. These results indicated that UQCC2 could act as a possible novel downstream target of METTL3 (Figures 5B,C).
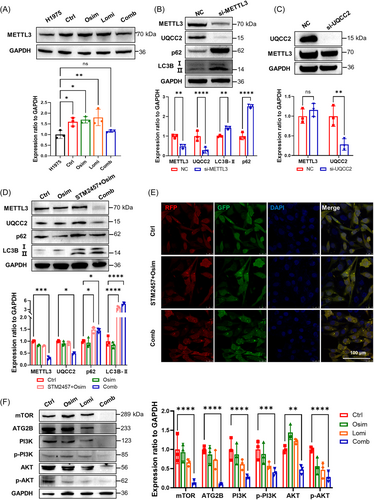
Then, we explored the mechanism of lomitapide reversing osimertinib resistance and found the IC50 of NCI-H1975/OR cells to osimertinib with the classical METTL3 inhibitor STM2457 [37] addition is 7.6 μM, which was triple fold higher than lomitapide (IC50 = 2.5 μM), indicating lomitapide could be one of superior METTL3 inhibitors to promote the drug sensitivity to osimertinib in NCI-H1975/OR cells (Supporting Information S1: Figure 4C). Western blot analysis and fluorescence signaling showed that lomitapide combined with osimertinib (Comb) had more effective effects on METTL3/UQCC2 inhibition and LC3B-Ⅱ/p62 activation than STM2457 combined with osimertinib (Figure 5D,E). Besides, autophagy-related pathways, including mTOR and PI3K, also involved in lomitapide-mediated osimertinib resistance (Figure 5F). These data demonstrate that lomitapide, as a potential METTL3 inhibitor, reversed osimertinib resistance through METTL3/UQCC2 axis, which could be suitable for in vivo investigations.
2.6 In Vivo Efficacy of Lomitapide
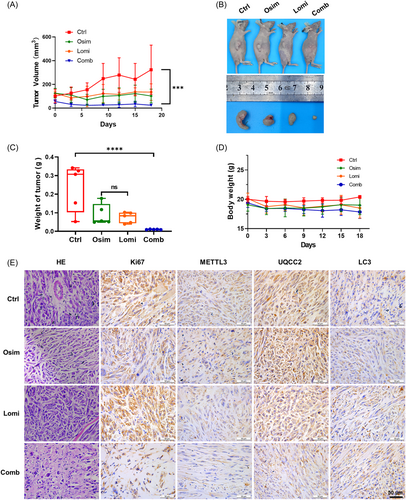
To further study the therapeutic potential of lomitapide in vivo, we established the NCI-H1975/OR xenograft models in BALB/c nude mice, which were divided into four groups (five mice per group), namely those administered 100 μL saline solution intraperitoneally with equal concentration of DMSO (2%) as control, those treated with Osim alone (10 mg/kg), Lomi (20 mg/kg) alone, or Comb (10 mg/kg Osim and 20 mg/kg Lomi). As shown in Figure 6A, the growth curve of tumors was remarkably inhibited by Comb than with Lomi or Osim treatment alone. The tumor size and weight in the drug-treated group were significantly smaller than those in the control group, particularly in the Comb group (Figure 6B,C). Lomitapide treatment did not influence the body weight, indicating no toxic effect on the animals (Figure 6D). Hematoxylin and eosin staining (H&E) and Ki-67 assays were performed to assess cell proliferation (Figure 6E). The results showed that xenografts from lomitapide- and osimertinib-treated mice had a decreased cell proliferation rate, which was consistent with the in-vitro results. NCI-H1975/OR cells were sensitive to the combination of lomitapide and osimertinib, which suppressed cell viability and proliferation. IHC showed that lomitapide downregulated the METTL3/UQCC2 axis, but activated autophagy with decreased METTL3 and UQCC2 expression and increased LC3 expression (Figure 6E). Taken together, the results illustrated that lomitapide could act as a potential METTL3 inhibitor as well as an autophagy stimulator to sensitize cells to drug resistance via METTL3/UQCC2 axis-mediated autophagy, providing novel therapeutic targets in patients with osimertinib-resistant NSCLC.
3 Discussion
Patients with NSCLC harboring EGFR mutations are at a heightened risk of an unfavorable prognosis. Despite advancements in treatment with EGFR TKIs, particularly the third-generation TKI osimertinib, which has demonstrated a significant improvement in the overall survival of patients with EGFR TKI progression and T790M positive mutation, the development of drug resistance remains a major obstacle to cancer treatment. It is mainly due to the complexity and diversity of resistance mechanism of osimertinib, which could be divided into two categories: One is EGFR signaling pathway associated, such as T790M deletion, C797S mutation, and L718Q/V, G724S, G796R/D, L792 to affect the interaction between Axitinib and the ATP binding site of the EGFR kinase domain, or alter the structure and function of EGFR; another is other parallel molecular pathways beyond EGFR signaling pathway, such as MET/HER2 amplification, activation of the RAS-mitogen-activated protein kinase (MAPK) or RAS-phosphatidylinositol 3-kinase (PI3K) pathways, novel fusion events, as well as histological/phenotypic transformation [38, 39]. Therefore, further research is still warranted to elucidate the mechanisms underlying osimertinib resistance and identify novel therapeutic strategies to combat drug resistance in NSCLC.
Drug repurposing or repositioning is a promising strategy for cancer therapy. Compared to de-novo drug discovery, drug repurposing as an ideal replacement significantly shortens the time, reduces investment, and improves the success rate of preclinical drug discovery. Since the safety of FDA-approved drugs has been well-validated, the risk of adverse effects has been largely reduced. Thus, there is a growing interest in identifying repurposed drugs with the potential to activate antitumor effects, thereby providing novel strategies to enhance cancer treatment outcomes. These repositories not only amplified the therapeutic potential of repurposed drugs across various diseases [40, 41] but also revealed their potential for combination therapies, including targeted treatments, immunotherapy, and chemotherapy, providing novel strategies to reduce the development of resistance and tailor treatments to maximize patient-specific outcomes [42, 43]. Although only a few repurposed drugs have been recognized for their direct anticancer effects, their multifaceted therapeutic targets are noteworthy. Our results indicated that the effective and well-tolerated cholesterol-lowering drug [44-46], lomitapide, can potentially be repurposed for the treatment of cancer.
Lomitapide is a first-in-class microsomal triglyceride transfer protein (MTTP) inhibitor for the treatment of HoFH [47]. By inhibiting the MTTP, lomitapide impedes lipoprotein production and decreases low-density lipoprotein (LDL) cholesterol levels [48, 49]. Emerging studies have suggested its additional antitumor potential in colorectal [35, 50], pancreatic [51], and glioma cancers [52]. Zuo et al. discovered that lomitapide demonstrates potential against colorectal cancer by directly targeting and inhibiting PP2A, thereby activating an AMPK-mediated autophagic process [35]. Lee reported that lomitapide targets mTOR to suppress tumor growth by regulating autophagy without adverse effects on major organs or mouse body weight, demonstrating its good safety profile and tolerability [50]. In this study, lomitapide was repurposed to reverse osimertinib resistance in NCI-H1975/OR cells through autophagy activation, further highlighting its versatility and potential in cancer therapy.
Autophagy, a process of intracellular self-degradation, plays a “double-edged sword” role in the development of drug resistance. It may maintain cellular homeostasis by degrading damaged or excess organelles and proteins, thus enhancing cell resistance to osimertinib. While, excessive autophagy may lead to cell death, resulting in tumor growth inhibition. Besides, the mechanism of cell death also plays an important role in drug resistance. When cells could not go to death normally through the apoptotic pathway, they may evade the killing effect of osimertinib through other pathways such as necrosis. In addition, osimertinib resistance is associated with the transformation of tumor cells into more invasive and resistant phenotypes, which may involve changes in cell death mechanisms. Therefore, some studies suggested that targeting autophagy-related genes or pathways could affect the resistance and antitumor efficacy of osimertinib. The use of autophagy inhibitors or stimulators has gained increasing attention as a promising therapeutic interventions. Numerous clinical trials have been conducted to elucidate the complex effects of autophagy manipulation on treatment outcomes (https://clinicaltrials.gov/). For instance, the autophagy stimulator FYARRO, approved by the FDA, has been used to treat adult patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumors [17]. Other modulators, such as hydroxychloroquine, CQ, and the PPT1 inhibitor GNS561, have been extensively studied and have shown promising results [18, 19]. Therefore, autophagy modulation may be a prospective strategy to overcome or reverse osimertinib resistance in patients with NSCLC harboring EGFR mutations. In our study, METTL3 served as a key factor in driving osimertinib resistance by mediating autophagy in the H1975/OR cell line, and lomitapide as a novel METTL3 blocker, reversed osimertinib tolerance by activating autophagy via METLLT3/UQCC2 axis, which could be suitable for further clinical study to overcome the resistance of EGFR-TIKs.
Accumulating evidence has shown that multiple transcription factors and histone modifiers are involved in regulating autophagy in response to drug treatment. However, little is known about the role of epigenetic modifications, particularly m6A, in autophagy regulation. Recently, m6A methyltransferase METTL3-mediated autophagy was shown to reverse gefitinib resistance in NSCLC cells by β-Elemene. However, no study has highlighted the role of METTL3 in autophagy regulation in response to osimertinib resistance or the corresponding drug reversion to date. METTL3, a primary methyltransferase-catalyzing RNA m6A modification, has been identified as an oncogene in several cancer types and is a potentially effective target for therapeutic inhibition [53]. Emerging evidence shows that METTL3 promotes drug resistance in many different tumor types, including lung cancer [54]. In a cisplatin-resistant lung cancer model, METTL3 upregulated YAP expression to promote drug resistance and metastasis via the YTHDF1/3, eIF3b, and MALAT1-miR-1914-3p-YAP axes [55]. In small-cell lung cancer (SCLC), METTL3 enhances chemotherapy resistance by manipulating DCP2, thus controlling mitophagy and mitochondrial injury via the Pink1-Parkin pathway [56]. Additionally, in NSCLC cells, METTL3 regulates the expression of autophagy-related genes, such as ATG5, ATG7, LC3, and SQSTM1, to promote cell survival and gefitinib resistance in an m6A-dependent manner [30].
Based on these studies, we discovered that METTL3 is upregulated in NSCLC and promotes osimertinib resistance. Several series of SAM-competitive and -selective inhibitors of METTL3 have been reported to date [37, 57-59]. The first METTL3 inhibitor (STM2457), which reversed the acute myeloid leukemia (AML) phenotype [37], reached phase I clinical trials in 2022 (ClinicalTrials.gov identifier NCT05584111) to evaluate its safety, pharmacokinetics, pharmacodynamics, and activity in patients with advanced malignancies. Therefore, the inhibition of METTL3 as a potential therapeutic strategy against osimertinib resistance could provide proof of concept that targeting RNA-modifying enzymes represents a promising avenue for anticancer therapy. However, due to the limitation of resistance model, the study was based on only one lung cancer resistance modle-H1975/OR, and the effectiveness of lomitapide to anti-EGFR TKI resistance should be evaluated on more models. As tumor exist heterogeneity, more treatment strategies will be needed to provide the most suitable treatment for NSCLC patients. For this purpose, the effects and safety assessment of novel strategy should be proved sufficiently in preclinical studies. We have found that the m6A methyltransferase METTL3 is highly expressed to promote osimertinib resistance and proved that METTL3 is a potential anti-resistance target, while the mechanism of how EGFR resistance inducing the upregulation of METTL3 is still unclear. The clarification of this process will help to understand the process of drug resistance. Several METTL3 inhibitors are now under different stages of preclinical and clinical evaluation, however, there are no FDA-approved drugs specifically targeting METTL3 for lung cancer treatment.
In this study, we conducted MeRIP-seq and RNA-seq to analyze alterations in m6A modification of autophagy-related specific genes and observed a global increase in m6A-methylated mRNA, implying that m6A methylation plays an important role in drug resistance and reversion. We found a novel METTL3 downstream target gene UQCC2 to be involved in regulating m6A modifications during autophagy in NSCLC cells. UQCC2 is a protein involved in the assembly of mitochondrial respiratory chain complex III. Its dysfunction results in defects in the synthesis or stability of cytochrome b [60]. However, whether UQCC2-mediated autophagy mediates drug resistance is still uncertain. Our results showed, for the first time, that UQCC2, a novel downstream effector of METTL3, has a negative relationship with autophagy and is involved in drug resistance. Moreover, we employed a structure-based screening assay to identify lomitapide as a potential METTL3 inhibitor that activates autophagy and reverses drug resistance by inhibiting METTL3, thus providing new insights into autophagy-mediated therapy to overcome osimertinib tolerance and improve clinical efficacy.
4 Materials and Methods
4.1 Cell Line and Culture
The human NSCLC cell line NCI-H1975 (parental cell line) was purchased from Wuhan Pricella Biotechnology Co. Ltd. (CL-0298). To establish a corresponding osimertinib-resistant cell line (NCI-H1975/OR), parental cells were treated with a drug dose escalation of osimertinib (Osim) from 0.1 to 8 μM. All cells were cultured in an RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin, and placed in a humidified atmosphere within a 5% CO2 incubator at 37°C.
4.2 Cell Viability Assay
Effects of the indicated agents on cell viability of NCI-H1975 and NCI-H1975/OR cells were studied using cell counting kit-8 (CCK-8, HY-K0301, MCE) assay. Exponentially growing cells were seeded in 96-well plates and cultured to approximately 70%–80% confluence. Cells were treated with the indicated concentrations of the test compounds. Then, 10 µL of CCK-8 reagent was added to each well and incubated for 2 h at 37°C. Absorbance was recorded at 450 nm using a microplate reader (SpectraMax 190, Molecular Devices).
4.3 Cell Apoptosis Analysis
The apoptosis assay was performed using an Annexin V-FITC Apoptosis Detection Kit (C1062, Beyotime) according to the manufacturer's instructions. Stained cells were analyzed using a NovoCyte Advanteon flow cytometer (Agilent NovoCyte Advanteon).
4.4 Colony Formation Assay
Cells were plated in 6-well plates (2000 cells/well) and incubated with the compounds for 14 days, with the medium replaced every 3 days. Colonies were fixed with 4% paraformaldehyde and stained with crystal violet solution (0.1%). The stained colonies were photographed after washing with PBS.
4.5 Three-Dimensional (3D) Spheroid Assay
The 3D sphere culture was performed as described previously [61]. Briefly, cells were seeded on plates coated with Matrigel (356234, Corning) and 50% serum-free medium. The cells were grown in a complete culture medium with or without drug treatment. The medium was replaced every 3 days. The 3D structures were imaged using an inverted phase-contrast microscope (DMI8, Leica Microsystems).
4.6 Cell Migration Assay
The cultured cells were routinely processed. Six hundred microliters of 20% FBS-containing culture medium were added to a 24-well plate. The cells were resuspended in 10% FBS medium and then added into the Transwell chamber (3422, Corning, 1 × 104 cells per well) and incubated in a 37°C incubator for 24 h. The invading cells in the upper chamber were fixed and stained with 0.1% crystal violet. Finally, the chambers were imaged using an inverted microscope (DMI1, Leica).
4.7 Western blot Analysis
Cell lysates were prepared using RIPA buffer supplemented with protease/phosphatase inhibitors (B14001, B15001, Biomark). Western blot analysis was conducted as described previously [62]. The following antibodies were used: anti-LC3 (1:2000; #81004-1-RR; Proteintech), anti-p62 (1:5000, #80294-1-RR, Proteintech), anti-METTL3 (1:2000, #15073-1-AP, Proteintech), anti-UQCC2 (1:1000, #25781-1-AP, Proteintech), anti-mTOR (1:10000, #66888-1-Ig, Proteintech), anti-ATG2B (1:1000, #25155-1-AP, Proteintech), anti-PI3K (1:1000, #67071-1-Ig, Proteintech), anti-pPI3K (1:1000, abcam, #ab278545), anti-AKT(1:5000, #60203-2-Ig, Proteintech) and anti-p-AKT (1:5000, #80455-1-RR, Proteintech). Anti-GAPDH (1:50000, #60004-1-lg, Proteintech) was used as the loading control.
4.8 Tandem mRFP-GFP Fluorescence Microscopy
To detect tandem fluorescent LC3 puncta, a adenovirus (Genechem company) with an mRFP-GFP-LC3 fusion protein was transfected to NCI-H1975/OR cells as indicated previously [63]. After being treated with indicated drugs for 48 h, the cells were visualized by a Leica TCS SP8 laser scanning confocal microscope.
4.9 Computational Docking and Molecular Simulation
The crystal structure of METTL3 was obtained from Protein Data Bank (PDB code: 8PWA). Water molecules were removed from the crystal structure, followed by the addition of hydrogen atoms, assignment of bond orders, optimization of H-bonds, and restrained minimization of energy. The structure of lomitapide was obtained from the PubChem database, which generated possible ionization states and three-dimensional conformations. Molecular docking among the candidates and METTL3 was performed using Discovery Studio, and the docking results were visualized using the PyMOL viewer.
4.10 Transmission Electron Microscopy
TEM was used to monitor the autolysosome formation after treatment with or without drugs. Briefly, cells were harvested, pre-fixed in a 2.5% glutaraldehyde solution at room temperature, and fixed with 1% osmium tetroxide for 2 h. Subsequently, the samples were gradually dehydrated with increasing concentrations of ethanol and acetone, and embedded in araldite. Finally, a 50- to 60-nm section was prepared on an LKB-1 ultrathin microtome, transferred to a copper mesh, and photographed using a JEM-1400 plus transmission electron microscope (JEOL).
4.11 In-Vivo Subcutaneous Tumor Model
BALB/c nude mice (female, 6-week-old, weighing 20–25 g) were purchased from Liaoning Changsheng Biotechnology Co. Ltd. When the tumor volume reached approximately 100 mm3, the mice were divided randomly into four groups: a control group (Ctrl), an osimertinib treatment group (Osim), a lomitapide treatment group (Lomi), and a combination treatment group of osimertinib and lomitapide (Comb). For in-vivo experiments, osimertinib was administered at a dose of 10 mg/kg every 2 days by oral gavage, lomitapide was given at a dose of 20 mg/kg every 2 days by intraperitoneal administration, and mice in the Ctrl group were administered an equal saline with 2% DMSO. Body weight was recorded throughout the experimental period. Tumor volume was measured using calipers and calculated using the formula ([width]2 × [length]/2). At the end of the experiment, the mice were killed and their tumors were removed. Subsequently, tumor tissues were embedded for immunohistochemical evaluation.
4.12 Histochemical or Immunohistochemical Staining
The tumors were harvested for histopathological examination. Tissues were sliced into 5-µm-thick sections. Paraffin sections were dewaxed with xylene, rehydrated by passing through decreasing concentrations of ethanol, and stained with hematoxylin and eosin (H&E).
For immunohistochemistry, anti-Ki67 (1:10000, # 84192-4-RR, Proteintech), anti-UQCC2 (1:70, #25781-1-AP, Proteintech), anti-METTL3 (1:1000, #15073-1-AP, Proteintech), and anti-LC3 (1:300, #81004-1-RR, Proteintech) were used overnight at 4°C, followed by incubation with secondary antibodies. The sections were visualized using a DAB kit (P0202, Beyotime), and images of three randomly chosen fields on each slide were captured under a microscope.
4.13 Data Sources
The datasets used in this study were derived from public repositories and encompassed both single-cell and bulk RNA-sequencing data. The GSE243562, GSE243564, and GSE253742 datasets were obtained from the Gene Expression Omnibus (GEO) database, providing insights into the molecular responses of LUAD samples to osimertinib treatment. GSE243562 included four samples with two pretreatment and two posttreatment residual samples, GSE243564 encompassed 15 samples with six pretreatment and nine posttreatment residual samples, and GSE253742 contained eight clinical LUAD samples with an equal distribution of pre- and posttreatment samples. Additionally, the m6A gene set was extracted from the RM2Target database, a curated resource for genes targeted by RNA-modification enzymes, for a total of 30 genes. The autophagy gene set, comprising 222 genes, was compiled from the MsigDB database and relevant literature, focusing on genes implicated in autophagy pathways.
4.14 RNA-Seq Analysis (GSE243564 and GSE253742)
Differential gene expression analysis was performed using the R package limma (version 3.58.1) on grouped samples from the GEO data (GSE243564 and GSE253742). Genes with |log2FC | > 0.5 and a p-value < 0.05 were considered to be differentially expressed. Intersections of these differentially expressed genes with the genes of interest yielded the relevant differentially expressed genes. Volcano and box plots were generated using the R package ggpubr, heat maps were constructed using the ComplexHeatmap package, and Venn diagrams were created using the Venn diagram package. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was performed using the ClusterProfiler package. Enrichments with a p-value < 0.05 were considered significant, and the top 30 pathways were plotted (if fewer than 30 were significant, all the pathways were plotted).
4.15 Single-Cell Analysis
GSE243562 single-cell data were aligned to the human genome (GRCh38) using Cell Ranger V6.1.2. Single-cell data were processed using Seurat V4.1.1, and cells with mitochondrial content > 20%, hemoglobin content > 5%, and expressed genes fewer than 200 were excluded. Normalization, cell clustering, and dimensionality reduction were performed using the Seurat functions. The “FindVariableFeatures” function was applied to identify 2000 highly variable genes from the normalized expression matrix, followed by principal component analysis (PCA) using the “RunPCA” function, retaining the top 20 principal components for further analysis. Batch effects were corrected using the “RunHarmony” function from the R package harmony. Cellular clustering was conducted using the “FindClusters” function with a resolution parameter set at 0.6, and nonlinear dimensionality reduction was performed using “RunUMAP.” Cells were annotated into Moderate_Residue, Residue_Free, and Severe_Residue populations based on the residual proportions of 40% and 60% within the cell clusters. Differential gene analysis was conducted using the “FindMarkers” function.
4.16 RNA-Seq and MeRIP-Seq
RNA-seq and MeRIP-seq were performed by LC Biotechnology Co. Ltd. Briefly, after extracting total RNA from H1975 parental cells, H1975/OR cells were treated with osimertinib alone or with osimertinib and lomitapide. Total RNA was isolated using TRIzol, quantified using a NanoDrop ND-1000 and assessed for integrity using a Bioanalyzer (Agilent). Poly(A) RNA was purified using Dynabeads Oligo(dT) 25-61005 (Thermo Fisher) and fragmented into small pieces. Then a part of the RNA incubated with m6A-specific antibody (No. 202003, Synaptic Systems) was separated. IP RNA was reverse-transcribed using SuperScript II Reverse Transcriptase (1896649, Invitrogen), followed by U-labeled second-stranded DNA synthesis, and the ligated products were amplified by PCR. Finally, we performed paired-end sequencing (PE150) of the input and IP RNA libraries using an Illumina NovaSeq. 6000 platform (LC-Bio Technology Co. Ltd. China).
4.17 GSEA Analysis
We performed GSEA using software GSEA (v4.1.0) and MSigDB to identify whether a set of genes in specific GO Terms, KEGG pathways shows significant differences in two groups. Briefly, we input gene expression matrix and rank genes by Signal2Noise normalization method. Enrichment scores and p value was calculated in default parameters. GO Terms, KEGG pathways meeting this condition with |NES | > 1, NOM p-val < 0.05, FDR q-val < 0.25 were considered to be different in two groups.
4.18 Statistical Analysis
Significance was calculated using the GraphPad Prism 9.0 software. All statistical analyses were performed using data from at least three independent experiments. Multiple comparisons were compared using two- or one-way ANOVA with Bonferroni correction, and two groups were compared using the unpaired Student's t-test; p < 0.05 was considered significant.
Author Contributions
Xiaohui Du: conceptualization (equal), funding acquisition (equal), investigation (equal), project administration (equal), writing–original draft (equal). Congcong Zhang: data curation (equal), investigation (equal), validation (equal). Ying Li: data curation (equal), investigation (equal), validation (equal). Peipei He: investigation (equal), validation (equal). Jian Wang: methodology (equal). Xuena Chen: conceptualization (equal), funding acquisition (equal), investigation (equal), writing–review and editing (equal). Han Wang: conceptualization (equal), funding acquisition (equal), methodology (equal), writing–review and editing (equal). Qi Wang: funding acquisition (equal), project administration (equal). All authors have read and approved the final manuscript.
Acknowledgments
We would like to thank Elsevier's Webshop for their help in polishing our paper. Additionally, we acknowledge the utilization of Figdraw 2.0 for the development of the Graphical Abstract. This work was funded in part by the Natural Science Foundation of China (NSFC, 81903185, 82027805 and 81703540), the Talent Innovation Support Plan of Dalian (2021RQ008), Project of Education Department of Liaoning Province (JYTZD2023045), Shandong Provincial Natural Science Foundation (ZR2023QC170).
Ethics Statement
All experiments were approved by the Animal Care Committee of Dalian Medical University (AEE23117) and were conducted in accordance with the institutional guidelines for animal care and handling.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data are available from the corresponding authors upon reasonable request. The RNA sequencing and m6A sequencing raw data have been deposited in NCBI GEO with accession number GSE274308.





