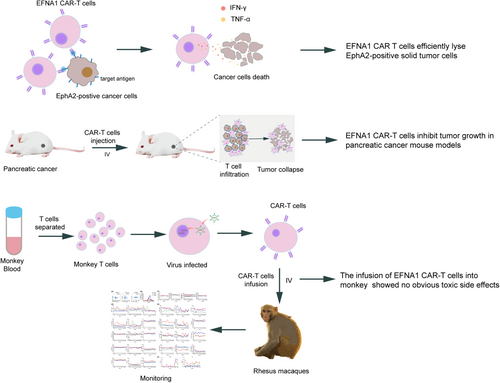
Ephrin A1 ligand-based CAR-T cells for immunotherapy of EphA2-positive cancer
Nan Liu, Wenwen Wei, and Kexing Ren contributed equally.
Abstract
Chimeric antigen receptor (CAR) T cells have demonstrated promising results in hematological malignancies; however, challenges remain in treating solid tumors. New CARs with more effectiveness and lower side effects are needed. Ephrin type-A receptor 2 (EphA2) belongs to the Ephrin family of receptor tyrosine kinases, which is overexpressed in several solid malignancies. Compared with some single-chain variable fragment (ScFv) CARs that exhibit excessively high affinity for their targets, natural receptor/ligand-based CARs maintain inherent affinity for their binding partners, potentially balancing cytotoxicity and side effects to better meet clinical needs. Here, we designed a CAR targeting EphA2-positive cancer cells by exploiting the extracellular domain of its natural ligand Ephrin A1 (EFNA1). EFNA1 CAR-T cells exhibited specific cytotoxicity against various cancer cells and cancer stem-like cells in vitro, and significantly suppressed tumor growth in a pancreatic cancer xenograft mouse model. Moreover, although these CAR-T cells specifically targeted mouse EphA2 and killed mouse tumor cell lines in vitro, they did not induce obvious side effects in mice. Additionally, it also showed good safety in rhesus macaques. Collectively, these results validate the therapeutic effectiveness and safety of EFNA1 CAR-T cells for treating solid tumors.
Graphical Abstract
1 INTRODUCTION
Immunotherapy opens a new era for cancer treatment.1 Chimeric antigen receptor (CAR) T cell therapy, a groundbreaking immunotherapy, has revolutionized the treatment of hematological malignancies.2-4 CAR-T cells targeting EGFR, HER2, Mesothelin, MUC1, EpCAM, AFP, and CEA have shown promising therapeutic efficacy against solid tumors in preclinical studies, but exhibited unencouraging therapeutic efficacy and potential side effects in clinical studies for a long time.5, 6 There are many hurdles, including the absence of a specific antigen, possible serious side effects, the difficulty of infiltrating into the tumor have impeded the process of CAR-T cell therapy for solid tumors.7 However, recently, CAR-T cells targeting CD70 for clear cell renal cell carcinoma, claudin 18.2 and EpCAM for gastric cancer, MUC1 for metastatic breast cancer, and GCC for metastatic colorectal carcinoma showed remarkable efficacy and controllable side effects in clinical studies.8-11 These encouraging results motivate us to design a new CAR that shows better efficacy and lower side effects for solid tumors.
Ephrin receptors (Ephs) constitute the largest group within the family of receptor tyrosine kinases (RTKs). Ephrin type-A receptor 2 (EphA2), a member of the Ephs family,12 is notably overexpressed in several cancers, including glioma, lung cancer, gastric cancer, and pancreatic cancer. Its expression, however, remains low in healthy tissues.13-18 Additionally, overexpression of EphA2 has been demonstrated to promote several cancer-related outcomes, including enhanced carcinogenesis, metastasis, angiogenesis, as well as increased motility and invasion of tumor cells.19-21 As a well-known therapeutic target, several ScFv-based CAR-T cells targeting EphA2 have been reported to efficiently eliminate multiple types of cancer cells in mouse models.14, 15, 22, 23 However, there is only one clinical study of CAR-T therapy targeting EphA2, which was focused on treating glioblastoma but showed limited effects and exhibited significant side effects.24 Thus, more efforts are needed to construct a more effective CAR targeting EphA2.
At present, conventional CAR structures typically utilize specific antibody ScFvs that bind to target antigens with high specificity and affinity.25, 26 However, employing ScFvs as the antigen recognition domain poses challenges due to immunogenicity, structural instability, and aggregation, leading to limited persistence and compromised efficacy.27, 28 In addition, in solid tumor, most tumor-associated antigens are expressed at low levels in certain normal tissues, and the high affinity of some ScFv-based CAR for their targets may cause systemic toxic side effects.26 Novel approaches are emerging to address or mitigate these limitations. Nanobodies-based CAR currently has been widely developed due to their stability, specificity, and high affinity, demonstrating promising therapeutic effects in both preclinical and clinical trials.29, 30 Receptor/ligand-based CAR-T cells represent another alternative solution,28 which are likely to offer the potential for more balanced affinity and reduced risk of off-target effects, carry a lower risk of dimerization and immunogenicity, while maintain effective tumor targeting. Research on natural receptor/ligand-based CAR-T cell therapies is growing, showing promising results.27 For instance, CAR-T cells targeting IL13Rα2 for glioma, NKp30 targeting B7H6 for lymphoma tumors, the NKG2D receptor for myeloma and lymphoma tumors have shown promising results in several studies.27, 31-33
Ephrin A1 (EFNA1) is a natural ligand of EphA2.34 In this study, we constructed an EFNA1-based CAR targeting EphA2, which exhibited specific cytotoxicity against various EphA2-positive solid tumor cells and pancreatic tumor cell-derived cancer stem cells (CSCs), as well as significant antitumor activity in a pancreatic xenograft mouse model. In addition, EFNA1 CAR-T cells did not induce any obvious adverse effects in mice, even in nonhuman primates. Our study thus confirmed the potential of EFNA1 CAR-T cells for treating EphA2-positive solid tumors. A clinical study needs to be designed to validate its application in patients with EphA2-positive solid tumors.
2 RESULTS
2.1 EFNA1 CAR showed promising transduction efficiency without affecting the phenotype of T cells
To obtain a natural ligand-based CAR targeting EphA2, we constructed a lentiviral vector containing a CD8 signal peptide, hinge and transmembrane domain, the 19–182 amino acid segment of EFNA1 as the recognition domain of EphA2, 4-1BB, and CD3ζ costimulatory signaling domains. To assess transduction efficiency, the red fluorescent protein mKate2 was linked to the CAR's C-terminus using a T2A peptide (Figure 1A). And the same lentiviral vector without the EFNA1 residues was used as a control CAR (MOCK) (Figure 1A). Human T cells were isolated from the peripheral blood of healthy donors, and their purity was validated by CD3 expression (Supporting Information S2: Figure S1A). T cells were activated with CD3/CD28 dynabeads and then transduced with MOCK and EFNA1 CAR lentiviruses. The transduction efficiency was measured by flow cytometry; non-transduced (NTD) T cells were used as a negative control. The percentage of positive T cells was 48.4%–51.3% for EFNA1 CAR-T cells and 45.3%–55.2% for MOCK T cells at Day 3 after transduction (Supporting Information S2: Figure S1B). Representative data are shown in Figure 1B, showing no significant difference between two groups (Figure 1B). Moreover, the expression of these CARs and the proliferation of MOCK and EFNA1 CAR-T cells did not change significantly after long-term culture (Supporting Information S2: Figure S1C,D). In addition, we detected the proportions of CD4+, CD8+, and memory phenotypes in NTD, MOCK, and EFNA1 CAR-T cells. The data showed that EFNA1 CAR transduction did not affect the phenotypes of T cells (Figure 1C,D). Thus, we designed a new EFNA1 CAR targeting EphA2, utilizing its nature ligand as the recognition domain, which can obtain sufficient CAR-T cells without phenotype changes.
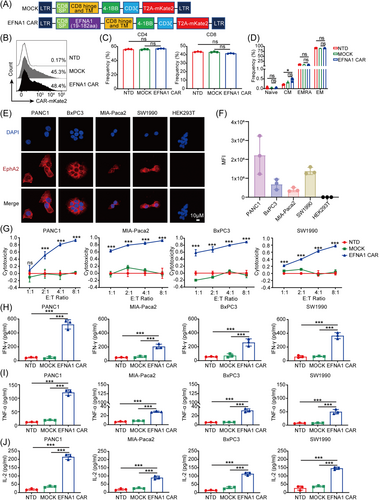
2.2 EFNA1 CAR-T cells efficiently lysed solid tumor cells in vitro
EphA2 is notably expressed in numerous malignancies and tumor cell lines.35 First, we validated the expression of EphA2 in various tumor cell lines by immunofluorescence staining, including pancreatic (PANC1, MIA-PaCa2, BxPC3, SW1990), colorectal (HCT116, DLD1), prostate (PC3, LNCap FGC), non-small cell lung (NCI H1299, A549), bladder (5637, RT4), glioma (U87), esophageal (TE-1), and the human renal epithelial cell line HEK293T. The results showed that EphA2 was expressed on the cell membrane of most tumor cell lines, except LNCap FGC, and HEK293T did not express EphA2 as a negative control (Figure 1E,F and Supporting Information S2: Figure S2A,B). Meanwhile, we further confirmed the expression of EphA2 in pancreatic cancer cell lines at both mRNA and protein levels using real-time quantitative PCR (RT-qPCR) and western blot analysis, respectively. The results were consistent with those from immunofluorescent staining (Supporting Information S2: Figure S3A–C). In addition, we analyzed the TCGA and Genotype-Tissue Expression (GTEx) datasets about the expression of EphA2 in human pancreatic cancer. The results revealed that EphA2 expression was much higher in cancer tissues than that in para-cancerous tissues (Supporting Information S2: Figure S3D).
Next, we evaluated the cytotoxicity of EFNA1 CAR-T cells against EphA2-positive cancer cell lines in vitro. We cocultured NTD, MOCK, and EFNA1 CAR-T cells with luciferase-expressing pancreatic cancer cells PANC1, MIA-PaCa2, BxPC3, and SW1990at various effector-to-target (E:T) ratios of 1:1, 2:1, 4:1, and 8:1. Cytotoxicity was measured using a luciferase cytotoxicity assay. The results demonstrated that EFNA1 CAR-T cells efficiently eradicated pancreatic cancer cells in a dose-dependent manner compared to NTD and MOCK T cells, the cytotoxicity reached 81%, 85%, 80%, and 57% at an E:T ratio of 4:1, respectively (Figure 1G). And EFNA1 CAR-T cells had no effect on the EphA2-negative HEK293T cells (Supporting Information S2: Figure S4). Meanwhile, enhanced green fluorescence protein (eGFP)-expressed pancreatic cancer cells were co-incubated with MOCK and EFNA1 CAR-T cells (E:T ratio = 4:1). The fluorescence images were obtained at 0, 6, 12, and 24 h. Over time, the EFNA1 CAR-T cells demonstrated a significant, time-dependent lysis of tumor cells, whereas the MOCK T cells did not obviously affect tumor cell viability (Supporting Information S2: Figure S5A–D). These findings initially demonstrated that EFNA1 CAR-T cells effectively eliminated pancreatic cancer cells in a dose- and time-dependent manner. To confirm the activation of T cells and the specificity of EFNA1 CAR-T cells, the release of the cytokines interferon gamma (IFN-γ), tumor necrosis factor-α (TNF-α), and interlelukin-2 (IL2) in the cocultured supernatants (E:T ratio = 2:1) was measured by enzyme-linked immunosorbent assay (ELISA). Consistent with the cytotoxic activity, the concentrations of IFN-γ (Figure 1H), TNF-α (Figure 1I), and IL2 (Figure 1J) in the cocultured supernatant of EFNA1 CAR-T cells were much higher than those of NTD and MOCK T cells. The results above showed that EFNA1 CAR-T cells specifically eliminated EphA2-positive pancreatic cancer cells in vitro.
Furthermore, we assessed the cytotoxic efficacy of EFNA1 CAR-T cells against other solid tumor cell lines. EphA2-positive and negative tumor cell lines shown in Supporting Information S2: Figure S2 were cocultured with NTD, MOCK, and EFNA1 CAR-T cells at various E:T ratios. The results revealed that EFNA1 CAR-T cells efficiently lysed EphA2-positive cancer cells (Supporting Information S2: Figure S6A), with the release of IFN-γ (Supporting Information S2: Figure S6B) and TNF-α (Supporting Information S2: Figure S6C). In contrast, EphA2-negative LNCap FGC cells were not be affected (Supporting Information S2: Figure S6). In summary, EFNA1 CAR-T cells selectively targeted and lysed EphA2-positive solid tumor cell lines in a dose-dependent manner in vitro.
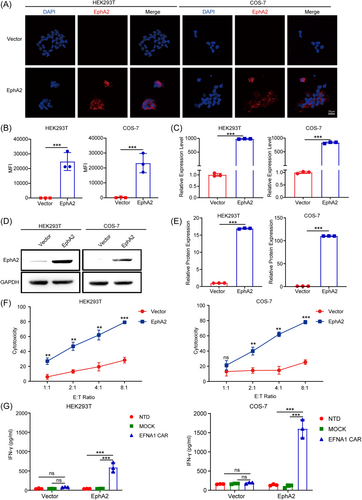
2.3 EFNA1 CAR-T cells killed target cells in a target-dependent manner
To further demonstrate that the cytotoxic specificity of EFNA1 CAR-T cells. We constructed the lentiviral particles pTomo-pCMV-EphA2-IRES-EGFP that induced EphA2 ectopic expression based on EphA2-negative cell lines HEK293T and COS-7 cell lines. The overexpression of EphA2 was confirmed by immunofluorescence staining. The data indicated that EphA2 is expressed on the membranes of EphA2-overexpressing HEK293T and COS-7 cells (Figure 2), and this expression further confirmed at both mRNA (Figure 2B,C) and protein (Figure 2D,E) levels. Correspondingly, the cytotoxicity of EFNA1 CAR-T cells against EphA2-overexpressing HEK293T and COS-7 cells was significantly increased at various E:T ratios compared to that against HEK293T and COS-7 cells at the same E:T ratio (Figure 2F). Additionally, IFN-γ was significantly induced by EphA2-overexpression cells other than respective control cells (Figure 2G). These findings suggest that EphA2 is necessary for the cytotoxicity of EFNA1 CAR-T cells.
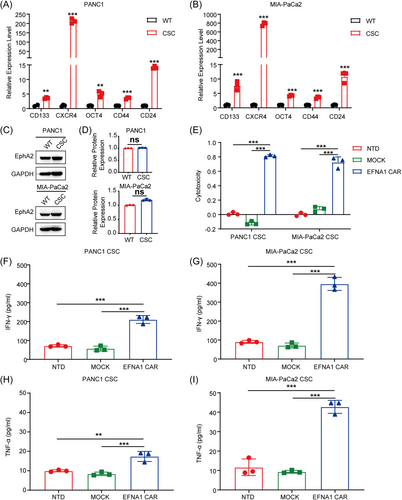
2.4 EFNA1 CAR-T cells exhibited potent cytotoxicity against cancer stem-like cells
Cancer stem-like cells (CSCs) are one of the main causes of tumor growth, invasion, metastasis, and recurrence and are prone to chemotherapy and radiotherapy resistance.36, 37 To evaluate the effects of EFNA1 CAR-T cells on CSCs, we obtained sphere cultures from PANC1 and MIA-PaCa2 by culturing in stem cell medium (PANC1-CSCs and MIA-PaCa2-CSCs). Stemness was validated by assessing the expression of CSC markers, including CD133, CXCR4, OCT4, CD44, and CD24.38, 39 The data showed that these CSC markers were significantly upregulated at the mRNA level in CSCs compared to the parental cell lines (Figure 3A,B). Moreover, western blot analysis analysis revealed no obvious difference in EphA2 expression level between CSCs and parental cells (Figure 3C,D). Then, NTD, MOCK, and EFNA1 CAR-T cells co-incubated with PANC1-CSCs and MIA-PaCa2-CSCs at an E:T ratio of 4:1. The cytotoxicity of EFNA1 CAR-T cells against PANC1-CSCs and MIA-PaCa2-CSCs reached approximately 81% and 72% respectively, and neither NTD nor MOCK T cells exhibited significant cytotoxicity (Figure 3E). Meanwhile, IFN-γ (Figure 3F,G) and TNF-α (Figure 3H,I) were released by EFNA1 CAR-T cells. These data demonstrated that pancreatic CSCs can be significantly killed by EFNA1 CAR-T cells in vitro, which may be a promising strategy for pancreatic cancer.
2.5 EFNA1 CAR-T cells showed effective antitumor activity against PANC1 xenografts mouse model
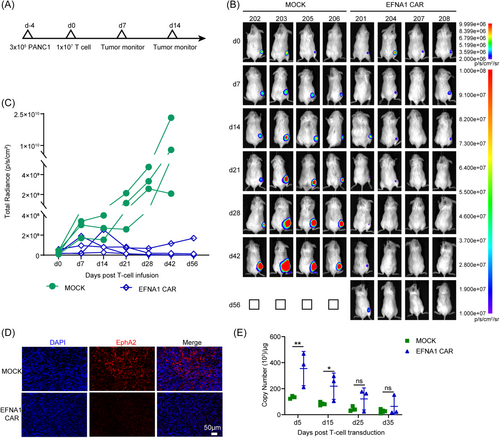
To evaluate the antitumor effect of EFNA1 CAR-T cells in vivo, PANC1 labeled with luciferase (PANC1-luc) was used to construct a subcutaneous xenograft mouse model in immunodeficient NCG mice. At Day 4 after cellular transplantation, the bioluminescence of the mice was measured, and these mice were categorized into two groups with similar tumor sizes and bioluminescence levels. Then, 1 × 107 MOCK or EFNA1 CAR-T cells were infused intravenously by the tail vein, and the tumor burden was monitored regularly (Figure 4A). Seven days post-infusion, the tumor growth was significantly suppressed by EFNA1 CAR-T cells compared to MOCK T cells (Figure 4B). In general, tumor of mice treated with EFNA1 CAR-T cells was progressively suppressed compared to the MOCK T cells group, EFNA1 CAR-T cells completely eliminated tumors in two of the mice until the end of the experiment at Day 56, whereas the other two mice had only minimal residual tumor remaining. In contrast, the mice in the MOCK group all died at Day 56 (Figure 4B,C). And immunofluorescence staining revealed that EphA2 expression was absent in residual tumors of mice treated with EFNA1 CAR-T cells (Figure 4D). The content of CAR-T cells in peripheral blood is correlated with tumor clearance. We also measured the copy number of CAR-T cells in peripheral blood. The data revealed that EFNA1 CAR-T cells were significantly expanded in vivo, and were more significant than MOCK T cells (Figure 4E). The data demonstrated that EFNA1 CAR-T cells target and eliminate EphA2-positive pancreatic cancer cells and successfully inhibit the growth of tumor in the pancreatic cancer mouse model.
2.6 EFNA1 CAR-T cells showed good safety in mice and nonhuman primates
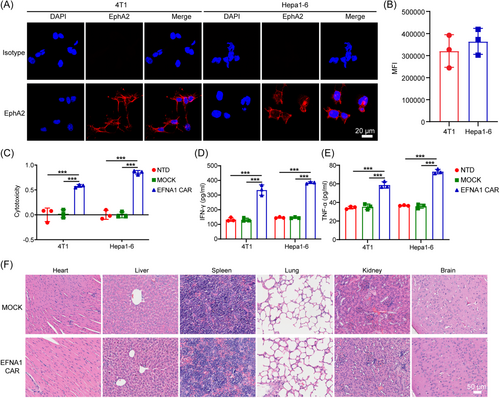
The presence of tumor-associated antigens (TAAs) on normal tissues often leads to toxicity to healthy tissues, raising safety concerns and restricting the clinical application of CAR-T cells.40, 41 Owing to the high conservation of receptor–ligand systems across species and the minimal difference of 2–3 amino acids between human and mouse Eph family proteins, we guess that human EFNA1 is likely to recognize the mouse EphA2 protein.42 We detected the EphA2 expression in mouse breast cancer cell line 4T-1 and hepatocellular carcinoma cell line Hepa1-6 by immunofluorescence staining. The results showed that EphA2 is positive in 4T-1 and Hepa1-6, which is expressed on the cell membrane (Figure 5A,B). Importantly, EFNA1 CAR-T cells exhibited potent cytotoxicity against murine tumor cell lines (Figure 5C) and induced strong release of IFN-γ (Figure 5D) and TNF-α (Figure 5E). Hematoxylin and eosin (H&E) staining of tissues from major organs such as the heart, liver, spleen, lung, kidney, and brain in PANC1-bearing mice receiving EFNA1 CAR-T cells showed no obvious pathological lesions compared to those receiving MOCK T cells (Figure 5F). These results suggested that EFNA1 CAR-T cells exhibit minimal on-target off-tumor effects in mice.
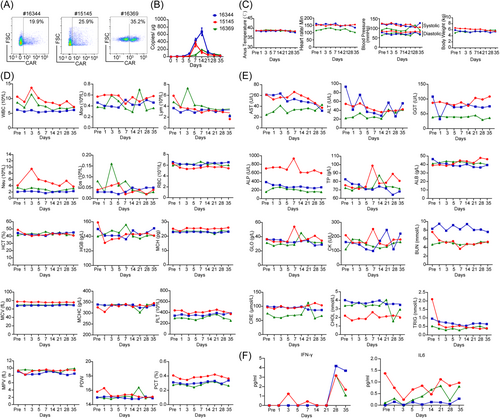
The nonhuman primate rhesus macaque is the experimental animals closest to humans in terms of gene homology and the immune system, and so forth.43 And the EphA2 protein (XP_028708593.1) of rhesus macaques shares 98.59% homology with human EphA2 (NP_004422.2), which means that rhesus macaques are better candidates for safety evaluation of EFNA1 CAR-T cell therapy. T cells from three adult rhesus macaques were isolated and subsequently activated and expanded with nonhuman primate CD3/CD28 dynabeads. Then T cells were infected with the EFNA1 CAR lentivirus, the transduction efficacy ranged from 19.9% to 35.2% (Figure 6A), which was detected by flow cytometry. Then these CAR-T cells were collected for autologous intravenous injection into monkeys. Blood was collected on pre (pre-infusion) -, and Days 1, 3, 5, 7, 14, 21, 28, and 35 post-T cells infusion. The DNA of whole blood cells at different time points was extracted to quantify the CAR-T cells by RT-qPCR. The data showed that EFNA1 CAR-T cells were expanded in the monkey with a peak at days from 7 to 14, and gradually decreased within 1 month (Figure 6B). These results indicated a moderate proliferation of CAR-T cells in vivo. Meanwhile, we monitored diastolic and systolic blood pressure, heart rate, anus temperature, and body weight after CAR-T cells infusion, there was no obvious changes compared with those before CAR-T cells infusion (pre) (Figure 6C). Immune cells in the peripheral blood, including white blood cells (WBCs), monocytes (Mons), lymphocytes (Lyms), red blood cells (RBCs), platelets (PLTs), and granulocytes (Grans) presented a minimal change within normal level (Figure 6D). The parameters used for serum chemistry analysis, such as glutamic oxalacetic transaminase (AST), glutamic-pyruvic transaminase (ALT), glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total protein (TP), albumin (ALB) and globulin (GLO) for liver function; creatine kinase (CK) for cardiovascular function; blood urea nitrogen (BUN) and serum creatinine (CRE) for renal function; and cholesterol (CHOL) and triglyceride (TRIG) for serum lipids, were not significantly changed compared to those in blood pre-infusion (Figure 6E). In addition, IFN-γ and IL6 levels in serum were detected by ELISA. IFN-γ peaked on day 28, followed by a decrease by Day 35, and IL6 exhibited only a minimal fluctuation. Both cytokines were present at very low levels (Figure 6F). These data suggested that EFNA1 CAR-T cells rarely target normal cells in vivo and do not induce obvious on-target off-tumor effects.
In summary, the natural ligand-based EFNA1 CAR-T cells efficiently eliminated EphA2-positive tumor cell lines and showed antitumor activity against the PANC1-bearing mouse model, and exhibited good safety in mice and nonhuman primate, which may provide a promising new strategy for cancer immunotherapy.
3 DISCUSSION
Ephrin receptors are the most significant RTKs. EphA2 is an RTK that triggers oncogenic signaling in response to ligands, as well as tumor suppressive signaling independent of ligands.44 EphA2 interacts with ephrin A-family ligands on adjacent cells and triggers various diverse signaling networks upon cell-to-cell contact, which regulates motility, viability, and proliferation of malignant tumor cells by inhibiting focal adhesion kinase (FAK), extracellular regulated protein kinases (ERK), and Akt phosphorylation.12 As the most commonly affected Eph receptor in human malignancies, EphA2 is frequently overexpressed in various tumors, including breast, prostate, hepatocellular, prostate, lung, and pancreatic cancers,45 while its expression remains relatively low levels in most normal tissues. Significantly, the expression of EphA2 is linked to poor prognosis, metastasis, and decreased survival of tumor patients.46-48 Overexpression and the functional relevance of EphA2 in malignant cells highlight its potential as a therapeutic target in cancer. Inhibiting EphA2 expression via siRNA reduces tumor growth in lung,49 breast,50 and ovarian cancer.51 In addition, soluble EFNA1 and EFNA1-Fc, and EphA2 monoclonal antibodies can suppress signaling by promoting internalization and degradation of EphA2.19 Compounds that bind to EphA2 or EFNA1 can suppress signaling by blocking EphA2 activation, such as dasatinib, which inhibits cancer growth by inhibiting the phosphorylation and kinase activity of EphA2.52 EphA2, an overexpressed membrane protein on tumor cells, serves as a novel antigen for tumor immunotherapy. EphA2-derived peptide-dendritic cell (DC) vaccines have been shown to inhibit tumor growth in EphA2-positive mouse colon carcinoma MC38-bearing xenografts.53 Moreover, CAR-T cells targeting EphA2 have demonstrated efficacy in mouse models of lung cancer22 or glioblastoma.15 In this study, we designed a new CAR that targets EphA2 based on the natural ligand EFNA1. We demonstrated that EFNA1 CAR has notable antitumor effects, including against various solid tumor cells, pancreatic CSCs in vitro, and pancreatic cancers in vivo. Importantly, CAR-T cell therapy targeting EphA2 in pancreatic cancer has not been investigated, and our study will provide a new strategy for CAR-T cell therapy in pancreatic cancer.
The scarcity of tumor-exclusive antigens with high expression levels poses a major challenge for CAR-T cell therapy, increasing safety concerns and making solid tumors potentially more challenging than hematological malignancies.54, 55 In solid tumors, the nonspecific targeting of ScFv-based CARs has caused serious safety concerns due to indiscriminate attack on both tumor and normal tissues expressing the targets. The affinity between natural ligands and receptors is typically more moderate than that between antigens and antibodies. Natural ligand-based CARs are likely to efficiently target tumor cells with higher target expression while sparing normal cells with lower expression.56 In our study, EFNA1 CAR-T cells demonstrated minimal cytotoxicity towards nonmalignant HEK293T and COS-7 cells, while efficiently destroying EphA2-positive tumor cells in vitro. Moreover, EFNA1 CAR-T cells were able to recognize EphA2 in mice. Despite the low expression level of EphA2 was reported in some normal tissues, no apparent side effects were observed in the vital organs of the mice, indicating the safety of EFNA1 CAR. Strategies using ScFv to target EphA2 CARs did not cause obvious side effects in mice.18, 23 However, in a first-in-human trial patients with recurrent glioblastoma, EphA2-redirected ScFv-CAR-T cells induced lung adverse effect, specifically pulmonary edema, and showed limited therapeutic effects.24 EphA2 is a tumor-associated antigen also present in normal lung tissues. Consequently, the pulmonary edema observed in these patients may result from the “on-target, off-tumor” effect. Due to the homology of EphA2 between humans and rhesus macaque, the natural ligand-based CAR could be conducted for safety evaluation in nonhuman primates. In our study, safety testing was performed in rhesus monkeys, and no obvious adverse effects were observed. The safety data obtained in this study provided the basis for further clinical applications.
Cancer stem-like cells with strong self-renewal, continuous proliferation and differentiation abilities are directly linked to tumor development, recurrence, and, in some cases, resistance to conventional therapies, which ultimately leading to cancer progress and death57, 58 Previous research has shown that CAR-T cells targeting HER2 and CD133 can efficiently eliminate CSCs both in vitro and in vivo.59 The findings indicate that CAR-T cell-mediated lysis of CSCs holds promise for therapeutic applications. EphA2 expression in CSCs promotes self-renewal and carcinogenicity.60 In previous studies, downregulating EphA2 expression in CSCs by RNA interference (RNAi) has been shown to inhibit tumorigenicity in vivo.61 These results suggest that EphA2 is crucial for CSCs. In our study, we observed comparable EphA2 expression in CSCs derived from pancreatic cancer cells and their parental cells. Our research also showed that EFNA1 CAR-T cells, which target EphA2, have specific cytotoxic effects against CSCs and lead to increased cytokine release. Therefore, EFNA1 CAR-T cells may be capable of eliminating CSCs and prevent tumor recurrence and metastasis.
The risk of developing second tumors after CAR-T cell therapy, primarily in the form of T-cell neoplasms associated with viral vector integration, has raised increasing concern. One patient suffering from diffuse large B-cell lymphoma who received CAR19 therapy was found to have a lethal T-cell lymphoma.62, 63 Second tumors are a long-term risk of CAR-T cell therapy; the safety evaluation of EFNA1 CAR needs long-term toxicity assessments in further study. Meanwhile, EphA2 predominantly interacts with EFNA1 but also binds to any of the eight different ephrin A-family ligands. Targeting EphA2 with CAR based on natural ligands may involve the use of other ephrin-family ligands. In addition, the efficacy and safety of the natural ligand need to be further compared with the ScFv-based CAR in future studies.
4 MATERIALS AND METHODS
4.1 Animals
Six-week-old female NOD‒Prkdc-enriched IL2rgtm1/Bcgen (B-NCG) mice were sourced from GemPhamotech. These mice were housed in SPF-level clean conditions and fed a standard diet at the Laboratory Animal Center, West China Hospital, Sichuan University (Chengdu, China). Rhesus macaques (4–5 years old) were sourced from the Kunming Primate Research Center, Kunming Institute of Zoology, Chinese Academy of Sciences. The relevant animal ethics committees approved all animal care and experimental procedures.
4.2 Plasmid construction and lentiviral package
The synthesized human Ephrin-A1 (EFNA1) domain (19–182 aa) was ligated into a previously validated CAR backbone. The backbone was described previously.64 The viral vector pTomo-pCMV-EphA2-IRES-EGFP was used for the overexpression of EphA2 in HEK293T and COS-7 cells. The sequences of all plasmids were confirmed by Sanger sequencing. The pertinent sequences are shown in Supporting Information S2: Table S1. Lentiviral packaging was performed as described previously.65
4.3 Cell lines and culture
The human cancer cell lines PANC1, MIA-PaCa2, HCT116, SW1990, NCI H1299, LNCap FGC, 5637, RT4, and TE-1, the mouse cancer cell lines 4T1 and Hepa1-6, and the monkey kidney fibroblast-like cell line COS-7 were obtained from the China Infrastructure of Cell Line Resources. BxPC3, A549, PC-3, U87, and DLD1 cells were cultured and stored in our laboratory. The human renal epithelial cell line HEK293T was obtained from A. Lasorella (The Institute for Cancer Genetics, Columbia University Medical Center). These cell lines were transduced with pTomo-CMV-luciferase-IRES-puro and then selected using puromycin to establish the corresponding luciferase-expressing cells. The PC-3, NCI H1299, LNCap FGC, TE-1,4T1, and Hepa1-6 cells were cultured in RPMI 1640 medium (Life Technologies), while other cells were maintained in DMEM (Life Technologies), both supplemented with 10% FBS and 100 U/mL penicillin‒streptomycin (Life Technologies). Cancer stem cells PANC1-CSCs and MIA-PaCa2-CSCs were established by using serum-free neural stem cell medium (DMEM/F12 from Gibco, EGF at 20 ng/mL, bFGF at 20 ng/mL, and B27 at 1×, all from Life Technologies based on PANC1-luc and MIA-PaCa2-luc, respectively. Cells were maintained in a 37°C humidified incubator with 5% CO2 and routinely confirmed to be mycoplasma-free by PCR. The human and mouse cells used were identified via short tandem repeat (STR) profiling; the COS-7 cells were identified by species-specific PCR amplification.
4.4 Generation of CAR-T cells
The primary T-cell isolation was performed as described previously.64 T-cell purity was evaluated using flow cytometry with a PE-conjugated mouse anti-human CD3 antibody (552127, BD Biosciences). T cells were cultured in RPMI 1640 (Gibco) with 10% FBS, 1× penicillin‒streptomycin, 200 U/mL IL2 (PeproTech), and 1× GlutaMAX (Gibco). To generate CAR-T cells, T cells were activated using CD3/CD28 beads (Thermo Fisher, 354234) at a ratio of 1:1 for 72 h, followed by transduction with lentiviral particles at a multiplicity of infection (MOI) of 10, using LentiBOOST (Sirion Biotech, SB-A-LF-901-01) at a ratio of 1:100. The expression of CAR-T cells was assayed via flow cytometry by detecting mKate2 autofluorescence 3 days post transduction.
4.5 In vitro cytotoxicity assays
Bioluminescence assays were used to assess the specific cytotoxicity of the CAR-T cells against various cell lines at defined effector-to-target (E/T) ratios. Luciferase-expressing tumor cells were cultured with RPMI 1640 supplemented with 10% FBS and 1× penicillin‒streptomycin (Life Technologies) in the presence of different T-cell ratios in a 96-well microplate. Cytotoxicity was assessed after 24 h of coculture using a Cell-Mediated Cytotoxicity Fluorometric Assay Kit (Promega, E1501) following the manufacturer's instructions.
4.6 Cytokine release assays
Effector T cells (NTDs, MOCK T cells, or EFNA1 CAR-T cells) were cocultured with tumor cells for 24 h, then the supernatant was collected, and the concentrations of IFN-γ (Invitrogen, KHC4021), TNF-α (Proteintech, KE00154), and IL2(DAKEWE, 1110202) were measured using specific ELISA kits according to corresponding manufacturer's instructions.
4.7 Cellular immunofluorescence staining
The expression of EphA2 in various cell lines was detected by immunofluorescence staining. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Cells were washed with 1× phosphate-buffered saline (PBS) and then permeabilized with 0.3% Triton X-100. The cells were subsequently blocked with 5% BSA for 1 h. Next, the cells were incubated at 37°C with an EphA2 primary antibody (Santa Cruz 1:200, SC-398832) for 2 h. After washing, the cells were incubated with a secondary antibody (Invitrogen, 1:1000, A-10521) for 1 h at room temperature. Nuclei were stained with 1 µg/mL DAPI (Sigma) for 5 min. Fluorescence images were obtained using a confocal microscope (Nikon). The mean cellular fluorescence was calculated using imageJ software.
4.8 RNA isolation and RT-PCR
The total RNA was extracted as previously described.66 EphA2 and CSC markers expression levels were measured using a SYBR Green qPCR Kit (Life Technologies) according to the manufacturer's guidelines. Supporting Information S2: Table S2 contains all the primers utilized in this study.
4.9 Western blot analysis
Western blot analysis was performed as described previously.64 Cells were lysed at 4°C for 30 min in RIPA buffer (Beyotime, P0013B), supplemented with protease and phosphatase inhibitor cocktails (Thermo Fisher) and 0.1 mg/mL phenylmethylsulfonyl fluoride (PMSF, Sigma). The protein concentrations were quantified by BCA protein assay kits (Beyotime). Then the protein samples were subjected to standard SDS-PAGE electrophoresis and immunoblotting. The study utilized mouse β-actin monoclonal antibody (Santa Cruz, 1:1000, SC-47778), rabbit GAPDH monoclonal antibody (abcam, 1:1000, ab9484), and recombinant mouse EphA2 monoclonal antibody (Santa Cruz 1:1000, SC-398832).
4.10 Flow cytometry
The expression of CAR-T cells was determined by directly detecting the autofluorescence of the mKate2 protein by flow cytometry. Genetically modified T cells were detected after staining according to the following steps. 1 × 106 cells were collected, washed with 1× PBS, and stained with specific antibodies for 1 h in the dark. These antibodies utilized for T-cell phenotypic analysis including FITC anti-human CD4 (BioLegend, Cat# 357406), APC/Cyanine7 anti-human CD8 (BioLegend, Cat# 344714), PE anti-human CD45RO (BioLegend, Cat# 304206), and APC anti-human CCR7 (BioLegend, Cat# 353214). Flow cytometry was performed on a FACSAria III flow cytometer, and the data were analyzed with FlowJo v10.8.1 software (FlowJo).
4.11 In vivo xenograft mouse model
To establish a human pancreatic cancer cells-derived mouse model, approximately 6-week-old female NCG mice were inoculated subcutaneously (s.c.) in the flank with 3 × 105 PANC1-luc cells. The tumor cells were suspended in saline containing 30% Matrigel (Corning), and then 100 μL of the cell suspension was subcutaneously injected into NCG mice. After 4 days, the mice were grouped according to the average bioluminescence signals, the mice were treated with 100 μL of MOCK T cells, or EFNA1 CAR-T cells (1 × 107) via intravenous injection, respectively. Subsequently, bioluminescence imaging was utilized to monitor tumor growth. Bioluminescent signals were quantified with Living Image software 4.1 (PerkinElmer).
4.12 Immunohistochemistry and H&E staining
The tumors and vital organs, such as hearts, livers, spleens, lungs, kidneys, and brains obtained from the MOCK and EFNA1 CAR groups. After fixation and dehydration, these samples were paraffin-embedded and sectioned into 4 μm slices. H&E staining was performed on tissue sections from the heart, liver, spleen, lung, kidney, and brain to observe their structure. The tissue tumor slides were dewaxed and dehydrated, then boiled in citrate buffer (pH 6.0) for antigen retrieval. After blocking with 5% normal goat serum at room temperature for 1 h, the slides were incubated with the EphA2 primary antibody (Santa Cruz, 1:200) overnight at 4°C. The next day, samples were incubated with the corresponding HRP-conjugated secondary antibodies for 1 h and TSA Plus Fluorescein Reagent (Akoya, 1:50, NEL744001KT) for 10 min at room temperature. The nuclei were ultimately stained with DAPI. Fluorescence images were captured using a confocal microscope (Nikon).
4.13 Safety validation of EFNA1 CAR-T cells in nonhuman primates
PBMCs were collected from three rhesus monkeys and activated using a nonhuman primate T-cell activation/expansion kit (Miltenyi Biotec, 130-092-919), followed by culture in RPMI-1640 medium supplemented with monkey-specific CD2/CD3/CD28 Dynabeads to selectively expand T cells. Monkey T cells were infected with CAR lentivirus at a MOI of 20, using LentiBOOST (Sirion Biotech, SB-A-LF-901-01) at a ratio of 1:100. 1 × 107 T cells in 0.5 mL of saline were intravenously injected into the respective monkeys. Approximately 0.5 mL blood samples were collected at days pre, 1, 3, 5, 7, 14, 21, 28, and 35 to analyze the complete blood count and serum chemistry at the animal facility. Cytokines levels including IFN-γ and IL6 in the plasma samples of monkeys were examined by Luminex (R&D).
4.14 Statistical analysis
Statistical analyses were conducted using GraphPad Prism 8.0 software. An unpaired t-test with Welch's correction was used to analyze statistical differences between the two groups. Comparisons among three or more groups were conducted using two-tailed one-way ANOVA with Sidak correction. Data are presented as the means ± SDs. Statistical significance was defined as *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001, and not significant (ns).
AUTHOR CONTRIBUTIONS
Xudong Zhao, Dan Cao, and Liqun Zou conceived the study and were responsible for study supervision. Wenwen Wei designed the lentiviral vectors encoding CARs. Nan Liu, Wenwen Wei, and Kexing Ren performed the in vitro experiments. Nan Liu and Wenwen Wei performed the in vivo experiments. Dong Yang, Bin Sun, Dandan Liang, Beibei Yang, Weishan Zhang, and Jincheng Zhao performed partial experiments. Xudong Zhao, Dan Cao, Liqun Zou, and Nan Liu analyzed the results and wrote the manuscript. All the authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of the Core Facility of West China Hospital (Li Chai, Yi Li, and Xing Xu). Additionally, we used Adobe Illustrator 2022 to possess all the figures and create a graphical abstract image. This work was supported by grants from the National Natural Science Foundation of China (82103107 to Bin Sun), the Sichuan Science and Technology Program (2024YFHZ0063), and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYYC20002 to Xudong Zhao).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The publicly available summary statistics for acute pancreatitis were obtained from the UK Biobank study (Neale Lab) and the FinnGen consortium, and no original data were involved in the current MR study. The indicated consortia described ethical approval and informed consent from every individual for the corresponding studies included in their research. All animal experimental protocols were approved by the Ethical Committee of West China Hospital Sichuan University, Chengdu, China (20230106003). Rhesus macaques care and experimental protocols were approved by the Animal Care and Use Committee of Kunming Institute of Zoology (hIACUC-PE-2021-05-002).
Open Research
DATA AVAILABILITY STATEMENT
The main data supporting this study's results are available in the paper and its Supplementary Information. Source data for the figures are provided in this paper. All the data generated in this study will be available from the corresponding author upon reasonable request.