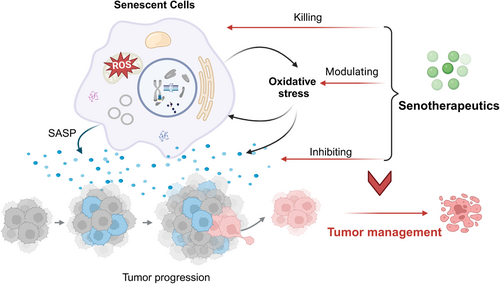
Oxidative stress and cellular senescence: Roles in tumor progression and therapeutic opportunities
Ping Jin and Xu-Dong Feng contributed equally to this study.
Abstract
Oxidative stress results from an imbalance between the production and neutralization of reactive oxygen species. It induces oxidative damage to cellular components including proteins, lipids, nucleic acids, and membranes, therefore intrinsically linking to aging-related diseases such as cancer, cardiovascular disease, and neurological disorders. Emerging evidence suggests that oxidative stress may promote tumor development by influencing various aspects of cellular senescence, such as its onset, pro-inflammatory secretion, and alteration of cellular function and structure. Modulating oxidative stress to target cellular senescence offers a novel strategy for cancer prevention and treatment. However, a thorough grasp of the specific mechanisms at play is lacking. This review will present the association between oxidative stress and cellular senescence and their regulatory role in tumor progression and treatment, with emphasis on senescence-associated secretory phenotype, immunosenescence and therapy-induced senescence. Current agents and strategies that remove side effects of cellular senescence via killing senescent cancer cells or modulating oxidative stress to improve antitumor efficacy will be summarized. This review will help readers better understand the complex relationship between oxidative stress and senescence in cancer, and will also provide a basis for further research in this area.
Graphical Abstract
1 INTRODUCTION
Organisms have developed redox systems to dynamically balance the production and elimination of reactive oxygen species (ROS).1 Preserving the intracellular redox balance is crucial for proper cellular and organismal function.2 Oxidative stress arises when ROS exceed the cell's antioxidant capacity, disrupting the intracellular redox balance.3 Various exogenous stimuli, such as environmental pollutants, chemicals, chemotherapy, radiation, and unhealthy diet and lifestyle, along with endogenous factors like DNA damage, antioxidant system defects, and mitochondrial dysfunction, can induce it.4 Oxidative stress damages cellular components like nucleic acids, proteins, and lipids, adversely affecting cellular structure and function. Disruptions in redox balance are linked to the initiation and progression of various age-related diseases, including cancer, diabetes, neurodegenerative disorders, and cardiovascular disease.5 Understanding the molecular basis of redox homeostasis is essential for developing strategies to prevent and treat diseases linked to oxidative stress.
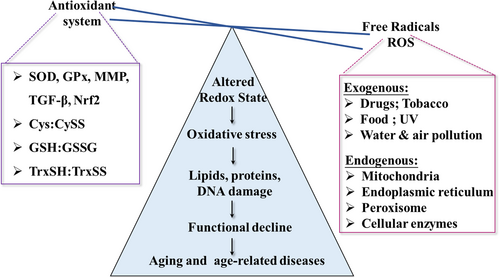
Aging is a natural biological process in which cellular damage accumulates as a person ages. It is characterized by a gradual decline in physiological functions and cellular machinery, which eventually leads to the development of age-related diseases.6 Its progression can be influenced by a variety of elements, including environmental, genetic, and lifestyle variables, and its complex process can be described by a number of complementary theories.7 One of the most prevalent correlative hypotheses at the moment is the oxidative stress theory.8 According to Leyane et al., aging and its associated functional impairments are attributed to oxidative damage affecting macromolecules, organelles, proteostasis, barrier structures, immune function, metabolic regulation, and regenerative capacity (Figure 1). In this context, oxidative damage is intrinsically correlated with age-associated pathologies or should even determines the lifespan. Cellular senescence, a fundamental aspect of aging, is characterized by irreversible cell growth arrest, often triggered by stressors like DNA damage and oxidative stress.9 They exhibit alteration in morphology, gene expression, and metabolism.10 The accumulation of senescent cells with age adversely affects tissues and contributes to age-related diseases like cancer. Research indicates that eliminating senescent cells in mice enhances health and postpones age-related diseases, suggesting a causal link between cellular senescence and aging-related conditions.11
Oncogenic signaling and metabolism can cause excessive ROS accumulation, resulting in DNA damage, genomic instability, altered cell signaling pathways, and immunosuppression.12 These cellular events can promote the survival, proliferation, and malignancy of cancer cells. Oxidative stress impacts the tumor microenvironment, influencing cancer cell invasion, angiogenesis, and metastasis.13, 14 Cellular senescence has a dual role in cancer development and progression, with oxidative stress potentially acting as a connecting factor, as noted in various studies.15 Cellular senescence is primarily defined as a barrier against cellular transformation and neoplastic development due to making cells less susceptible to pro-carcinogenic stimuli, clearance of premalignant cells by stimulating immune-mediated surveillance, and promotion of the transcriptional repression of genes that inhibit proliferation.16-18 The detrimental impact of accumulated senescent cells on tumors has also been extensively documented. Specifically, oxidative stress can lead to DNA damage, mutations and protein modifications, which in turn can lead to dysregulated intracellular signaling pathways and imbalance in redox homeostasis to accelerate the development of cancer.19 In addition, the secretion of pro-inflammatory and tumor-promoting factors by senescent cells contributes to chronic inflammation and leads to the promotion of tumorigenesis.20 Oxidative stress may undermine tumor suppressor functions, resulting in unchecked cell growth and DNA damage.21 Targeting oxidative stress and managing cellular senescence could lead to novel cancer prevention and treatment strategies.
This paper explores the regulatory impact of oxidative stress on cellular senescence from molecular, cellular, and systemic perspectives. The potential connection between oxidative stress, cellular senescence, and tumor development will be explored. The role of therapy-induced senescence (TIS) and its management in tumor treatment is given particular attention. Finally, we will explore the potential of targeting oxidative stress and cellular senescence in cancer prevention and treatment.
2 OXIDATIVE STRESS AND CELLULAR SENESCENCE
2.1 Cellular sources of oxidative stress
Aerobic cells generate reactive oxygen and nitrogen species (ROS and RNS), crucial components of oxidative stress that significantly influence aging and related diseases. ROS in mammalian cells are generated by four primary enzyme systems: the mitochondrial electron transport chain (ETC), xanthine oxidase (XOR), uncoupled nitric oxide synthase (NOS), and NADPH oxidases (NOX).22, 23 The two most common sources of these are the NADPH oxidases and the ETC. The NOX protein family, comprising seven members (NOX1–5 and DUOX1–2), facilitates the electron transfer from NADPH to molecular oxygen via NADPH oxidases, resulting in the production of superoxide (O₂·−) and hydrogen peroxide (H₂O₂).24, 25 These isoforms, which produce various ROS profiles, occur in the internal membranes of the nucleus, mitochondria, and endoplasmic reticulum (ER) in addition to the plasma membrane. They also show distinct regulation mechanisms and specialized subcellular localizations. Enzymes like glutathione peroxidase (GPx) in the mitochondria and catalase in peroxisomes can detoxify the generated H₂O₂ into water (H2O) and oxygen (O₂).26, 27 Alternatively, H₂O₂ could act as a precursor to more reactive species like hydroxyl radicals (HO·).28 Various enzymes contribute to oxidative stress across different cell types and tissues, including ER mixed-function oxidases, cytosolic enzymes such as lipoxygenases and cyclooxygenases, and peroxisomal enzymes like glycolate oxidase, d-amino acid oxidase, urate oxidase, and fatty acyl CoA oxidase.29, 30 Oxidative processes involve even enzymes that methylate DNA as well as those involved in the creation of hormones and neurotransmitters.31, 32
2.2 Signals leading to cellular senescence
DNA damage primarily induces senescent stress by activating the p53–p21 pathway and the DNA damage response (DDR). Together, tumor suppressor pathways like p53/ARF and RB/p16 prevent the cell cycle from progressing and encourage cellular senescence.33-35 The p53 pathway reacts to DNA damage and is associated with premature senescence, whereas the RB/p16 pathway is often active after protracted cell cycle arrest and correlates with replicative senescence.36 The activation of these pathways depends on the type of stress signal, the tissue involved, and the species. For example, p53 overexpression following RB loss functions as a defense against senescence escape and malignant transformation.33 According to research, p16 is essential for preserving the senescent state, whereas p21 is mostly activated early in the development of senescence. Since telomerase may restore telomere function and cellular proliferation, potentially leading to cell immortalization, telomere shortening in human fibroblasts is a hallmark of replicative senescence.37 Telomeres shorten with each cell division due to the end-replication problem. When they reach a certain length, however, cells go into irreversible growth block, telomere attrition being a defining characteristic of senescence. Furthermore, several oncogenes, including as BRAF and Ras, can trigger signaling cascades that cause senescence.38 And these oncogenes are also implicated in early senescence triggered by acute cellular stressors such as mitochondrial dysfunction and metabolic reprogramming.
2.3 Interaction between oxidative stress and cellular senescence
Oxidative stress can randomly damage DNA and telomeres and activate oncogenes, which speeds up DNA dysfunction and telomere shortening.39-41 In addition to producing oxidative damage to important biomolecules and encouraging cellular senescence, oxidative stress can impact signaling pathways and molecular mechanisms.42 Age-related illnesses and aging are greatly accelerated by oxidative stress-induced damage to cellular organelles, especially mitochondria.43-45 Systemic alterations such as inflammation, immunological dysfunction, and metabolic dysregulation can result from oxidative stress and are known to be important causes of comorbidities associated with aging.46
2.3.1 Transcriptional and posttranscriptional perspective
The senescence transcriptional program is highly variable and heterogeneous depending on the cell subtype, the kind of stress-induced, and the time after induction. Cell cycle arrest is a significant characteristic of cellular senescence, accompanying with the downregulation of genes involved in mitotic and cell cycle progression regulation, DNA replication, spindle assembly and chromosome segregation.47 Oxidative stress significantly influences the activity and expression of these genes, contributing to the regulation of cellular senescence.
Oxidative stress is involved in the transcriptional regulation mediated by cell cycle regulators, ultimately resulting in cell cycle arrest.48 p53, known as the genome's guardian, can trigger cell cycle arrest upon activation in response to oxidative damage, thereby preventing the spread of damaged cells. Similarly, p21, a downstream target of p53, can also inhibit cyclin-CDK complexes, further contributing to the halt in cell division. Thrombospondin 1 and its receptor CD47 have been shown to activate Nox1, increasing ROS production. This process enhances the p53-mediated damage response, resulting in cellular senescence. Further, elevated main ROS generator Nox2 and subsequent p53-p21/Rb-p16 signaling have been reported to mediate polystyrene nanoplastics (PS-NPs) induced spermatogenic cell senescence.34 Senescent cells undergo oxidative stress-induced upregulation of genes related to membrane trafficking and intercellular signaling (such as cell adhesion molecules and surface receptors).49 During senescence, the expression of antiapoptotic proteins from the BCL-2 family, including BCL-2, BCL-Xl, and BCL-w, is upregulated, whereas apoptotic effectors like caspase-3 are downregulated.
Senescent cells also exhibit a complex and dynamic secretory phenotype (termed senescence-associated secretory phenotype, SASP) that is regulated by various transcription factors and associated regulators. C/EBP and NF-κB are key regulators that collaboratively modulate the induction of SASP factors across various senescence contexts.50 The ATM kinase, JNK, and p38 MAPK pathways, along with the transcription factor GATA4, act as upstream and/or synergistic modulators.33, 51-54 Oxidative stress is tightly involved in their transcriptionally regulating of senescence and SASP phenotype. Carteolol, a commonly used medication for primary open-angle glaucoma, was reported to induce excess ROS via activation of the β-arrestin-ERK-NOX4-ROS pathway to trigger human corneal endothelial cells (HCEnC) senescence.55 In response to ROS, Akt phosphorylates Mdm2 on serine residue 183, which increases nuclear Mdm2 stability and decreases p53 levels, preventing senescence in primary cells.56 In addition, Tobacco smoke condensate induces senescence and a SASP phenotype via NF-κB and MAPKs p38 and ERK pathways activation, which can be ameliorated by colchicine treatment.57 Another study suggested that polystyrene microplastics (PS-MPs) induce testicular premature aging via Ca2+/ROS/NF-κB signaling axis, laying the foundation for further exploration of the effects of microplastics on testicular toxicology.58
In addition to transcriptional regulators, posttranscriptional modifications influence senescence by modifying mRNA translation or alternative splicing.59 RNA-binding proteins like human antigen R, AU-binding factor 1, and hnRNP A are involved in the direct or indirect regulation of mRNA translation for senescence-related proteins.33 MicroRNAs, short noncoding RNAs, exhibit differential expression during senescence and bind to mRNAs of senescence-regulatory proteins, inhibiting translation and modulating key senescence signaling pathways.60 Alternative splicing, exon exclusion, and intron retention can alter protein structure, localization, regulation, and function, disrupting normal splicing patterns.61 Alterations in genes like nuclear lamin A, S-endoglin, TP53, and the glutamate transporter EAAT2 can affect age-related phenotypes.62 Moreover, oxidative stress modulates posttranslational modifications of proteins that can impact important signaling pathways, such as the p53 pathway and the mTOR pathway, thus participating in cellular senescence.63 Understanding how oxidative stress influences transcriptional and posttranscriptional modulators can offer new insights into developing interventions for healthy aging and age-related diseases.
2.3.2 Alteration in cell morphology, structure and function in senescent cells
Senescent cells exhibit flattened and enlarged cell shapes, redundant amounts and decreased activity of membranous organelles, and increased focal adhesions due to the activation of ezrin (a cytoskeleton protein)64-66 (Figure 2). While senescent organelles such as lysosomes, mitochondria, and ER always display functional defects upon damage from oxidative stress, senescent cells are prone to generate more organelles to compensate for their compromised function.67, 68 However, the newly produced organelles may cause excessive burden when exposed to oxidative stress, which accelerates the senescence phenotype.8 The increased activity of lysosomal β-galactosidase, due to accumulated lysosomal mass, is a widely recognized senescence biomarker detectable at pH 6. 0.69 Senescent cells exhibit diminished lysosomal activity, leading to lipofuscin buildup and lysosomal aggregates containing nondegradable oxidized lipids, proteins, and metals.70 Mitochondria also undergo pronounced changes, including mtDNA damage, disruption in dynamics, superfluous biogenesis and decreased degradation during senescence.71-74 Notably, ROS generation by dysfunctional mitochondria is the essential transmitter that contributes to the development of the senescent phenotype, even though the underlying mechanism is still not completely understood.75 Moreover, the high synthesis of organelles and secretion factors urges ER expansion and biogenesis in senescent cells, which leads to an aberrant ER capacity for proper protein synthesis.76 Increased ROS levels caused by ER failure can further impede proper protein folding and the production of disulfide linkages in many SASP proteins. Age-related oxidative stress or decreased expression of chaperones and folding enzymes impairs the ER's protein folding capacity.77-79
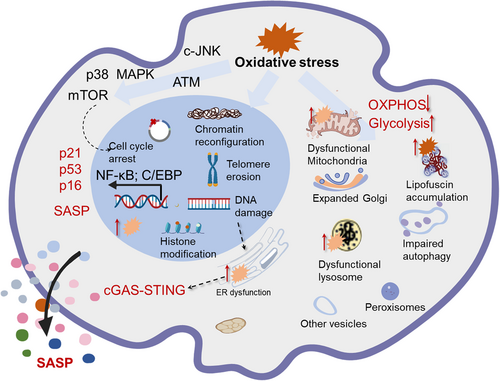
Apart from these well-established organelles, others, such as peroxisomes, cytoskeleton and nuclei, also display obvious alterations. Among them, peroxisomes enclose numerous enzymes and interact with mitochondrial ROS signaling to ensure ROS homeostasis.80 Its defects elicit oxidative stress and contribute to the initiation of cellular senescence as well as aging-associated diseases. In support of this, the reset of the oxidative balance by modulating peroxisomes is linked to a reduction in cellular senescence in several experimental models.81 A deeper knowledge exploring the alterations of organelles will facilitate a full understanding of cellular senescence and provide a novel strategy for the management of cellular senescence and age-associated diseases.
2.3.3 Oxidative stress elicits metabolic alterations in senescent cells
Senescent cells experience significant metabolic demands due to increased mass and size, along with heightened SASP expression, oxidative stress, and ER stress.82, 83 Accordingly, senescent cells always exhibit elevated aerobic glycolysis and mTOR activity.84-87 The increased glycolysis functions to compensate for the insufficient energy levels caused by mitochondrial dysfunction. ATM kinase activates the Akt/mTORC1 phosphorylation cascade to enhance mitochondrial biogenesis, compensating for diminished oxidative phosphorylation capacity and reduced inner membrane potential.88 During oncogene-induced senescence, fatty acid metabolism changes, characterized by elevated glucose consumption and pyruvate use in the tricarboxylic acid cycle, alongside reduced nucleotide levels.89 Restoring glucose and glutamine utilization can address nucleotide deficiency-induced DNA replication errors and senescence, demonstrating a causal link between metabolic alterations and the onset of senescence.90 Dysfunction in mitochondria also leads to loss of the precursor pools for biosynthesis and the disruption in redox balance that also affects senescence through a number of signaling pathways. An elevated AMP-to-ATP ratio or decreased NAD+/NADH levels activate AMPK, facilitating cellular signaling and enabling senescent cells to adapt to metabolic stress.91 Indeed, AMPK can inhibit the cytoplasmic translocation of HuR, a factor involved in mRNA stabilization. This leads to the stabilization of p21 and p16 mRNA, enhanced activity of Rb, activated p53, and eventually cell cycle arrest and cellular senescence.92
3 IMPLICATIONS OF OXIDATIVE STRESS-INDUCED SENESCENCE IN CANCER
Cellular senescence plays significant yet opposing roles in various stages of tumorigenesis and progression. On the one hand, it serves as a significant barrier to tumorigenesis by inhibiting cancer cell proliferation under certain conditions since irreversible cell cycle arrest.93 However, senescent cells may promote tumor progression in other situations. The buildup of senescent cells and their secretions can lead to inflammation and tissue dysfunction, creating an environment that supports tumor progression.94 Additionally, the decline in immune function that accompanies cellular senescence can impair immunosurveillance for detecting and eliminating cancer cells.95 Accordingly, while excessive ROS can damage DNA and other cellular components, triggering senescence or genomic instability that can lead to cancer; moderate levels of ROS function as cellular signals to activate repair mechanisms and antitumor immune responses.
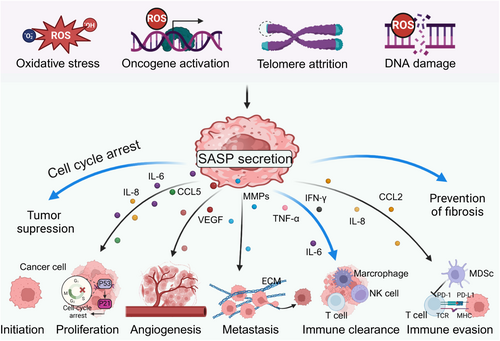
3.1 Senescence-associated secretory phenotype (SASP)
SASP factors enable senescent cells to effectively interact with and impact adjacent cells,96 eliciting positive or negative effects on tumors, which is dependent on senescent cell types, specific stimuli, and the diverse surrounding environment 97 (Figure 3). The SASP aids in eliminating preneoplastic cells and inhibiting early tumor development by activating both innate and adaptive immune responses. SASP factors, including TNF-α, IFN-γ, and IL-6, recruit immune cells like CD8+ T cells, NK cells, and macrophages to senescent cells or precancerous lesions, facilitating the elimination of damaged or transformed cells.98, 99 In oxidative stress conditions, NRF2 activates a specific subset of the SASP gene program, termed NRF2-induced secretory phenotype, which recruits CCR2-expressing monocytes to attract immune cells for the removal of damaged cells.100 Lao et al. found that glutaryl-CoA dehydrogenase impedes hepatocellular carcinoma progression by inducing senescence through crotonylation-mediated inhibition of the pentose phosphate pathway and glycolysis. Senescent cancer cells, through the SASP, promoted immune cell infiltration to create an antitumor immune microenvironment.101 Conversely, the SASP can enhance tumor progression and metastasis by promoting tumor cell proliferation, angiogenesis, invasion, and immune evasion. SASP factors, including interleukin-1-beta (IL-1β), matrix metalloproteinases (MMPs), and vascular endothelial growth factor (VEGF), promote cancer cell survival, motility, and angiogenesis, while altering the tumor microenvironment to support tumor growth and metastasis.102, 103 For example, osteocytes in established breast cancer bone metastases are enriched with senescence and SASP markers, leading to bone destruction in these metastases.104
3.2 Role of immunosenescence in tumor progression
Immunosenescence refers to the progressive decline in immune function that occurs with aging (Figure 4). It is thought to be driven by multiple factors, including chronic inflammation, thymic involution, exposure to environmental toxins, and genetic predisposition.105, 106 The distinguishing features of immunosenescence are receded lymphoid organs and decreased immune cell output, especially T cells.107 Furthermore, the cytotoxic function of immune cells diminishes with decreased expression of molecules associated with cytotoxic activity, including granzyme B, interferon-gamma (IFN-γ), interleukin (IL)-7, and perforin. As the immune system ages, metabolic changes occur, leading to increased ROS production, enhanced glycolysis, heightened SASP activity, and reduced mitochondrial synthesis.108, 109 Multiple immunosenescence biomarkers indicating the features of specific subtypes of aged immune cells have been proposed to date. Senescent T lymphocytes increase the expression of CD57 and KLRG-1 while reducing the expression of costimulatory molecules like CD27 and CD28. The increase in p16 and p21 expression is associated with the loss of CD27 and CD28.110 It is noteworthy that immunological checkpoint-related molecules such CTLA-4, Tight, and Tim-3 are present in higher amounts during senescence.111, 112 During immunosenescence, inhibitory receptors like KIR and NKG2C are upregulated, whereas NK-activating receptors such as NKP30 and NKP46 are downregulated.113, 114 Additionally, the expression of CD62L and TLR1/4 is reduced, whereas CD11b and TLR5 express more on monocytes and macrophages.115-117 Nonetheless, neutrophils display no significant alteration in the expression of CD11a and CD11b during senescence.118
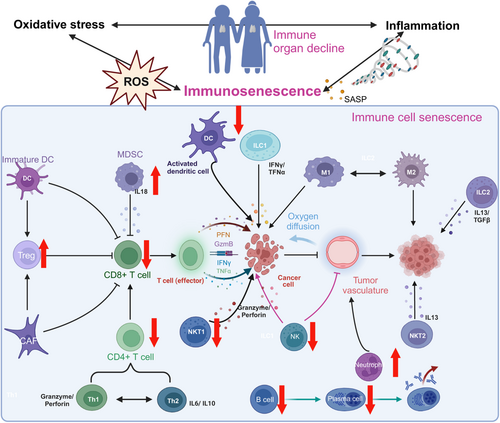
Generally, the likelihood of developing tumors rises as a person ages.119 The marked reduction in immune cell infiltration and T cell functionality in the aging immune system facilitates cancer cell evasion of immune surveillance, enhancing tumor invasion and metastasis.120, 121 A plethora of observations have demonstrated that immunosenescence, especially senescent CD8 + T cells, promotes pathogenesis and reduces treatment efficacy in patients with breast cancer. Consistently, CD8 + T cells were observed to have decreased IFN signaling in aged mice with cancer.122, 123 In addition, ECM rearrangement due to alterations in hyaluronan and proteoglycan link protein 1 can make melanoma cells more aggressive in older persons.124 Furthermore, activated Toll-like receptor 8 signaling can block the senescence of T cells and reverse their inhibitory effect, allowing the improvement of antitumor immunity.125 Despite the potential weakening of immune cell activity due to immunosenescence, aging immune cells can still identify and eliminate cancer cells in certain situations. For example, some aged cells have enhanced cytotoxic T-cell functionality, which can positively contribute to inhibiting tumor growth.126, 127 Immunosenescence can alter immune suppression balance, potentially increasing anti-inflammatory cells and factors that may help suppress tumor growth and spread.128 Evidence supporting this perspective includes the observed slower tumor proliferation in elderly mice across various models like B16, MC38, and 4T1, as well as the reduced growth and metastasis of bronchial cancer in older patients.129 Immunosenescence plays a role in the regulation of cancer development and progression. Additional studies are needed to fully understand the link between immunosenescence and tumors. This knowledge can contribute to insights into the roles of aging and the immune system in tumor development and provide new strategies and approaches for cancer prevention and treatment.
3.3 Therapy-induced senescence and their impacts on treatment outcomes
Cellular senescence is now recognized as a basic tumor cell response to treatment, known as TIS.130 Several therapies, such as chemotherapy or radiation can tiger senescence through the induction of DNA damage and a series of molecular and cellular changes.131 TIS cells display key senescence characteristics such as enlarged, flattened morphology, elevated lysosomal content, and a diverse TIS secretome. Oxidative stress occurs accompanied with these alterations. Excessive DNA damage can accumulate through a “vicious cycle” in which oxidative damage impairs DNA, leading to increased ROS production that intensifies oxidative stress. Bystander cells exposed to the senescent secretome consistently show increased oxidative stress.132 Research has shown that cytokines such as TNF-α, IL-1β, and IFN-γ can increase ROS levels in recipient cells within the cytokine-rich inflammatory SASP.133 The impact of senescence on cancer progression is influenced by the heterogeneity of SASP components and the dual role of ROS, varying with cell type, environment, and drug treatment duration.134, 135
3.3.1 Detrimental role
Chronic accumulation of senescent cells can promote aggressive tumor characteristics, including drug resistance, relapse, and metastasis.136 Cells can re-enter the cell cycle after an extended phase of senescence arrest. This can lead to the emergence of progeny with chromosomal instability or a cancer stem cell-like phenotype, providing them with a survival advantage.137, 138 Senescence allows tumor cells to evade the direct cytotoxic effects of treatment, enabling them to persist in a dormant state and regain their self-renewal capacity.139, 140 The concept of ‘senescence escape’ has emerged, suggesting that a subset of TIS cells may develop the capacity to evade senescence.141 It is a major factor in treatment failure and disease recurrence. This opinion is further proven by an investigation that model cell-state dynamics and TIS employing single-cell RNAseq and fluorescence reporters.142 They found that a range of possible senescence scenarios affected the survival of quiescent tumor programs created by phenotypic flipping during treatment. Additionally, these reactivated cells exhibited an overexpression of a stem-cell signature and elevated levels of the cyclin-dependent kinase Cdc2/Cdk1.143 In contrast to the pro-oxidant microenvironment and elevated ROS levels typical of senescence, TIS-escaped cancer cells exhibited reduced ROS levels and upregulated antioxidant systems, including glutathione peroxidase 1/2 (GPx1/2) and superoxide dismutase 1/2 (SOD1/2).144 This condition resembles that of cancer stem cells.145, 146 It's crucial to remember that these senescence-escaped cells have a greater capacity for malignant proliferation when they reenter the cell cycle than when they weren't senescent, which invariably results in the development of treatment resistance and an aggressive recurrence.
Moreover, SASP significantly influences tumor therapy outcomes.147 For instance, SASP factors such as eotaxin, CXCL5 and CCL5 expressed on doxorubicin-induced senescent cells in a breast carcinoma mouse model can promote cancer cell invasion, angiogenesis and cancer relapse through multiple signaling pathways, such as CCR3-ERK-MMP3 and AKT/GSK3β/β-catenin signaling.148, 149 A cohort analysis of CRC tissues revealed that patients exhibiting higher p16 and p21 expression levels following chemotherapy and irradiation experienced reduced disease-free or progression-free survival. Senescent tumor cells released extracellular vesicles (EVs) enriched with SERPINE1, facilitating p65 nuclear translocation and activating the NF-κB signaling pathway. This process advanced the progression of recipient cancer cells, resulting in shorter disease-free survival or progression-free survival in patients.150 According to a different study, SASP increases the expression of LCN2 in human breast tumors that remain after neoadjuvant chemotherapy treatment. This enhancement of cancer cell motility and attenuation of the cancer cells' sensitivity to chemotherapy are demonstrated in animal models.151
In addition to their short-term protumorigenic effects, cellular senescence mediated by therapy-induced stress can contribute to both immediate and long-term side effects of cancer treatments,152 such as side effects such as bone marrow suppression, bone loss, cardiac dysfunction the development of secondary cancers, spinal disorders, and pulmonary diseases.153, 154 Key components of the SASP, such as IL-1A, IL-6, and CXCL12, are overexpressed following anticancer therapies and have been correlated with symptoms such as fatigue, cardiovascular complications, decline in physical function, and loss of appetite.155 A recent study showed that paclitaxel treatment induces systemic senescence by activating the p38 MAPK signaling pathway, leading to SASP development. However, inhibition of the p38 MAPK pathway has been shown to improve trabecular bone volume and density in mice treated with paclitaxel.135 Furthermore, research by Yao indicated that SASP plays a role in chemotherapy-induced bone loss, which can be mitigated by p38 MAPK or MK2 inhibitors and the removal of senescent cells.134 Taken together, senescent cells may elicit many side effects that destroy the long-term health status of patients. Attention should be given to these side effects as well as to the effect of the drug on the tumor.
3.3.2 Favorable role
Contradictorily, senescence can contribute to antitumor effects and improve treatment outcomes under some circumstances130, 156 (Figure 3). For example, a recent study demonstrated that senescent cancer cells are exceptionally immunogenic, which elicits antitumor immune for eliminating senescent tumor cells.157 Senescence acts as a tumor suppressor by inducing irreversible growth arrest, potentially leading to cell death. This state of senescence facilitates therapy-induced cell death and contributes to the overall efficacy of cancer treatments. Additionally, senescent tumor and stromal cells can recruit immune cells from the tumor microenvironment (TME) to clear cancer cells through the secretion of pro-inflammatory SASP factors. In this context, activation of p53 leads to cellular senescence and tumor regression in a variety of tumor types, such as liver carcinomas, lymphomas, and sarcomas.158 In addition, coadministration of aurora kinase and MDM2 inhibitor resulted in the induction of senescence and immune clearance of cancer cells by cytotoxic T cells.159 Research indicates that combining MEK and CDK4/6 inhibitors effectively suppresses pancreatic ductal adenocarcinoma (PDAC) proliferation. The induction of senescence and SASP secretion, mediated by the retinoblastoma (RB) protein, promotes CD8 + T cell accumulation in the tumor microenvironment and improves chemotherapy drug uptake.160 In preclinical mouse models of PDAC, combining MEK and CDK4/6 inhibitors effectively suppressed PDAC proliferation by inducing retinoblastoma (RB) protein-mediated senescence. Their findings indicated that TIS enhances the delivery and effectiveness of the cytotoxic drug gemcitabine by inducing proangiogenic factors that promote tumor vascularization. SASP-induced activation of endothelial cells promotes CD8 + T cell infiltration into the TME, transforming immunologically “cold” tumors into “hot” ones and enhancing their sensitivity to PD-1 checkpoint blockade.161 A more in-depth analysis of the effect of senescent cell clearance will help to increase treatment effectiveness by employing antitumor immunity.
4 THERAPEUTIC IMPLICATIONS
Therapy-induced senescent cells persisting after cancer treatment may harmfully alter the tissue environment, facilitating tumor recurrence and metastasis. This highlights the need for a strategic approach focused on eliminating these senescent cells to promote long-term patient outcomes and reduce the risk of adverse effects, termed senotherapeutic approaches.162, 163 Existing senotherapeutic approaches included drugs that selectively eliminate senescent cells (termed senolytics), drugs that inhibit the SASP (termed senomorphics).164 Several strategies for effectively targeting senescent cells including prodrugs, proteolysis-targeting chimeras (PROTACs), nanotechnology will accelerate the flourishing of this field.165 Nowadays, a new avenue in cancer treatment has been opened up by the combination of senotherapeutics and pro-senescence therapy. Besides directly eradicating the cancer cells, this “one-two punch” strategy a new avenue in cancer treatment has been opened up by the combination of senotherapeutics and pro-senescence therapy (Table 1).166, 167
| NCT number | Study status | Conditions | Interventions | Phases |
|---|---|---|---|---|
| NCT04733534 | RECRUITING | Childhood Cancer | Dasatinib plus Quercetin | Fisetin | PHASE2 |
| NCT05583175 | RECRUITING | Leukemia, Myeloid | Myelodysplastic syndrome | Hematopoietic Stem Cell Transplantation | Myeloid Malignancy | Venetoclax plus reduced intensity conditioning | PHASE2 |
| NCT03567876 | ACTIVE_NOT_RECRUITING | Lymphoma, Mantle-Cell | Venetoclax | PHASE2 |
| NCT06571825 | RECRUITING | Acute Myeloid Leukemia | Venetoclax | Allogeneic transplant | PHASE4 |
| NCT05909293 | RECRUITING | Acute Myeloid Leukemia | Venclexta 100 MG Oral Tablet | NA |
| NCT03404193 | ACTIVE_NOT_RECRUITING | Acute Myeloid Leukemia | Acute Myeloid Leukemia Arising From Previous Myelodysplastic Syndrome | Blastic Plasmacytoid Dendritic Cell Neoplasm | Chronic Myelomonocytic Leukemia | Mixed Phenotype Acute Leukemia | Myelodysplastic Syndrome | Recurrent Acute Biphenotypic Leukemia | Recurrent Acute Myeloid Leukemia | Recurrent Blastic Plasmacytoid Dendritic Cell Neoplasm | Recurrent Chronic Myelomonocytic Leukemia | Recurrent Mixed Phenotype Acute Leukemia | Refractory Acute Myeloid Leukemia | Refractory Blastic Plasmacytoid Dendritic Cell Neoplasm | Refractory Chronic Myelomonocytic Leukemia | Refractory Mixed Phenotype Acute Leukemia | Decitabine | Laboratory Biomarker Analysis | Venetoclax | PHASE2 |
| NCT05264883 | RECRUITING | Acute Myeloid Leukemia | Venetoclax-Decitabine/Azacitidine-Aclarubicin Association | Venetoclax-Decitabine/Azacitidine Association | PHASE3 |
| NCT04476199 | COMPLETED | Acute Myeloid Leukemia | Venetoclax and Decitabine | PHASE2 |
| NCT05371054 | RECRUITING | Lymphoma | Non-Hodgkin Lymphoma | Non-Hodgkin lymphoma (NHL) | Hematologic Malignancies | Lymphoid Malignancies | Apart Rearrangement Probe Kit | venetoclax | VIP152 | prednisone | PHASE1 | PHASE2 |
| NCT05901974 | RECRUITING | Acute Leukemia of Ambiguous Lineage | Venetoclax | azactidine | PHASE2 |
| NCT04746235 | RECRUITING | Acute Myeloid Leukemia | Recurrent Acute Myeloid Leukemia | Refractory Acute Myeloid Leukemia | Decitabine and Cedazuridine | Venetoclax | PHASE2 |
| NCT04687761 | RECRUITING | Leukemia, Myeloid, Acute | De Novo | Age More 60 yr | Azacitidine | Venetoclax | Quizartinib | Cytarabine | Venetoclax | Quizartinib | PHASE1 | PHASE2 |
| NCT05053425 | UNKNOWN | Acute Myeloid Leukemia | Venetoclax | Azacitidine | Cladribine | Cytarabine | Idarubicin | NA |
| NCT04509622 | COMPLETED | Acute Myeloid Leukemia | Venetoclax | Cytarabine | PHASE3 |
| NCT01794520 | COMPLETED | Relapsed/Refractory Multiple Myeloma | Venetoclax | Dexamethasone | PHASE1 | PHASE2 |
| NCT05099471 | NOT_YET_RECRUITING | Waldenstrom Macroglobulinemia | Venetoclax; Rituximab | cyclophosphamide | PHASE2 |
| NCT05262465 | RECRUITING | Adult Acute Myeloid Leukemia | Microtransplantation, HLA-mismatched donor peripheral stem cell infusion | Azacitidine | Venetoclax | NA |
| NCT05211336 | ACTIVE_NOT_RECRUITING | Primary Diffuse Large B-cell Lymphoma of the Central Nervous System (CNS) | Aggressive B-cell Lymphoma With Secondary Involvement of the CNS | Obinutuzumab | Prednisone | Lenalidomide | Venetoclax | Ibrutinib | PHASE1 |
| NCT05554406 | RECRUITING | Acute Myeloid Leukemia | Acute Myeloid Leukemia Arising From Previous Myelodysplastic/Myeloproliferative Neoplasm | Acute Myeloid Leukemia Post Cytotoxic Therapy | Acute Myeloid Leukemia, Myelodysplasia-Related | Azacitidine | Biospecimen Collection | Bone Marrow Aspiration | Cytarabine | Daunorubicin Hydrochloride | Echocardiography | Liposome-encapsulated Daunorubicin-Cytarabine | Multigated Acquisition Scan | Venetoclax | PHASE2 |
| NCT05520567 | RECRUITING | Acute Myeloid Leukemia (AML) | FLT3-mutated Acute Myeloid Leukemia | Gilteritinib | Venetoclax | Azacitidine | PHASE1 | PHASE2 |
| NCT04824924 | UNKNOWN | Leukemia, Myeloid, Acute | The combination of HHT, venetoclax, AZA, G-CSF | PHASE2 |
| NCT03226418 | ACTIVE_NOT_RECRUITING | Adult Acute Myeloid Leukemia | Secondary Acute Myeloid Leukemia | Therapy-Related Acute Myeloid Leukemia | Cytarabine | Decitabine | Idarubicin | Laboratory Biomarker Analysis | Liposome-encapsulated Daunorubicin-Cytarabine | Quality-of-Life Assessment | Questionnaire Administration | Azacitidine | Venetoclax | glasdegib | PHASE2 |
| NCT03530683 | TERMINATED | Lymphoma | Multiple Myeloma | Acute Myeloid Leukemia | Diffuse Large B-Cell Lymphoma | Maplirpacept (PF-07901801) | Azacitidine | Venetoclax | Carfilzomib | Dexamethasone | Anti-CD20 Targeting agent | Isatuximab | PHASE1 |
| NCT00840346 | COMPLETED | Acute Myeloblastic Leukaemia | Panobinostat | PHASE1 | PHASE2 |
| NCT01463046 | COMPLETED | Acute Myeloid Leukemia | Advanced Myelodysplastic Syndrome | Panobinostat | Cytarabine | Daunorubicin | PHASE1 |
- Note: Data was obtained from https://clinicaltrials.gov/.
4.1 Senolytic
Senolytic emerges as a promising method to selectively target and remove these cells, thereby suppressing chronic inflammation and suggesting its viability as an effective anticancer therapy.168, 169 Their mechanisms of action mainly target critical survival pathways in senescent cells. The dasatinib and quercetin combination, a pioneering senolytic therapy, has demonstrated efficacy in treating diseases such as pulmonary fibrosis and tumors in both preclinical and clinical studies [NCT06355037] [NCT04733534].170, 171 D+Q significantly mitigated organ aging and damage caused by carboplatin or olaparib by reducing senescent adipose-derived stem cells in peritoneal adipose tissue, thereby inhibiting ovarian cancer cell metastasis to adipose tissue.172 A clinical trial [NCT05724329] is underway to assess the efficacy and safety of combining dasatinib and quercetin with Tislelizumab in patients with head and neck squamous cell carcinoma scheduled for surgery. Fisetin, a member of the flavonoid family, demonstrated greater efficacy than quercetin and has been extensively studied in various cancers, both as a standalone treatment and in combination with other anticancer agents. Senolytic therapy research has extensively focused on compounds targeting the antiapoptotic BCL-2 protein family due to their elevated levels in senescent cells. Navitoclax (ABT263), a selective inhibitor of BCL-2, BCL-XL, and BCL-W, is extensively studied for its senolytic properties in eliminating senescent cells by reactivating the apoptotic pathway.173 For example, it was observed that ABT-263 can destroy gemcitabine-induced senescent-like highly chemoresistant pancreatic cancer cells and eradicate xenografted tumors.174 Similarly, another study suggested that ABT-263 treatment could induce survived senescence cells upon chemotherapy treatment into apoptosis in the breast cancer model.175 A phase I clinical trial demonstrated that combining navitoclax with gemcitabine was generally well tolerated and showed a favorable safety profile in patients with advanced solid tumors176 [NCT00891605]. In addition, the effect of ABT-737, another BCL-2 family inhibitor, has also been evaluated in samples of ovarian tumors [NCT01440504].
Other senolytic compounds target p53, mTOR, Na+/K+ ATPase, and immune cell surface proteins. Intrathecal delivery of a p53-specific senolytic peptide has been shown to reverse spinal cord cellular senescence and improve lifespan.177 However, clinical study is needed to evaluate its therapeutic use. Another type of senolytics is mTOR inhibitors, since mTOR acts as an essential regulator of the SASP.178 Preclinical studies suggest that rapamycin, an mTOR inhibitor, may contextually reduce NF-κB activity, inhibit pro-inflammatory SASPs translation, and prevent senescent fibroblasts from promoting prostate tumor growth in mice.
O-GlcNAcylation inhibitors were reported to promote SN38 or etoposide-induced senescent colon cancer cells turn into apoptosis, thereby reducing treatment side effects and preserving efficacy.179 Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide, inhibitor of glutaminase1, was reported to selectively remove low-dose gemcitabine-induced senescence through apoptosis induction, which improved the therapeutic effect of gemcitabine.180 Cardiac glycosides like digoxin and ouabain have been shown to eliminate senescent cells by inhibiting Na+/K+ ATPase pump activity in various cancer models.181, 182
4.2 Senomorphics
Senomorphics is another promising strategy to counteract the negative consequences of senescence by changing the characteristics of senescent cells (like SASP) without causing cell death. Senomorphics such as the NF-κB inhibitors apigenin and kaempferol, along with the mTOR inhibitor rapamycin, modify the SASP of senescent cells.183-187 Apigenin suppresses the SASP induced by persistent MAPK signaling, ionizing radiation, oncogenic RAS, or replicative exhaustion. This reduces the aggressive phenotype of human breast cancer cells through suppressing invasion of the extracellular matrix, cell proliferation, and the transition from epithelial to mesenchymal.188 Many other plants' phytochemical components, include rutin, have demonstrated exceptional ability to target senescent cells by reducing expression of the whole spectrum SASP by interfering with ATM's interactions with TRAF6 and HIF1α.189 Metformin, an antidiabetic drug, enhances the anticancer efficacy of CDK4/6 inhibitors by altering SASP profiles in head and neck squamous cell carcinoma.190 Recently, NAMPT-mediated NAD+ biosynthesis was found to act an important role in cisplatin-induced senescence-associated cancer stem cells (CSCs). These TIS-associated CSCs would be suppressed by FK866, a clinically applicable NAMPT inhibitor.191
4.3 Modulating oxidative stress
Given the crucial influence of oxidative stress on molecular signaling and aging, altering the redox balance may serve as an effective approach to counteract senescence and age-related cancer.131, 180, 192 While senescence induced by ROS can be beneficial under certain conditions, it is also linked to unfavorable pathological inflammation. Researchers are exploring various agents to promote ROS-induced senescent cell death and others to mitigate ROS and limit senescence-related pathologies.193 Resveratrol (RV) is a natural compound with antioxidant properties found in various plants. Research indicates that resveratrol can mitigate inflammation-induced senescence of nucleus pulposus cells in vitro.194 High doses of resveratrol may cause oxidative stress, DNA damage, and cancer cell senescence through pathways involving p38 MAPK, mitochondria, and the DLC1-DYRK1A-EGFR axis.Consequently, it inhibits the proliferation of various cancer cell types.195-197 A clinical trial (CTRI/2019/07/020289) demonstrated that resveratrol combined with copper acts as a pro-oxidant, significantly mitigating the non-hematological side effects of chemotherapy in patients with advanced stomach cancer.198 A Ginseng extract, Ginsenoside Rh2, holds promise to overcome doxorubicin-resistance and cancer progression through relieving oxidative stress and suppression NF-κB/IL-8 pathway. This function reduced the secretion of SASP and the growth ability of MCF-7- cells.199 Apigenin, a flavonoid from edible plants, induces sustained oxidative stress-mediated cellular senescence via p21 signaling pathways independent of p16-Rb and p53, offering a potential chemosensitive treatment strategy for human colorectal cancer cells.200 Several other promising natural products, including miliusanes, phloretin, silybin, genistein, sulforaphane, quercetin, and curcumin analogs, have been identified as senescence inducers and senotherapeutics for cancer treatment in light of recent findings.200 Importantly, some of these have already received preclinical trial validation and exhibited tremendous promise (Table 2).
| Natural agents | Effects on redox status | Evidence of senescence | Effects on tumor | Refs |
|---|---|---|---|---|
| Pentagamavunon-1 | Increasing intracellular reactive oxygen species (ROS) levels | Inducing M phase arrest and cell senescence | Inducing tumor cell apoptosis with few side effects and low risk of relapse | [201] |
| Curcumin | ROS generation | Inducing Cell senescence | Tumor-suppression | [202] |
| Melatonin | Reducing intracellular ROS | Suppressing doxorubicin-induced premature senescence | Preventing the side effects of anticancer drug | [203] |
| Resveratrol | Destroying antioxidant pool and increasing ROS production | Inducing senescence | Tumor suppression | [204] |
| Apigenin | Inducing oxidative stress | Inducing cellular senescence through p21 signaling pathways | Chemosensitizing in human colorectal cancer cells | [205] |
| Sulforaphane | Inducing ROS via disrupting the balance between glutathione and oxidized glutathione | Leading to DNA damage, autophagy and exosome-mediated paracrine senescence | Anticancer effect | [206] |
| Piperlongumine | Inducing ROS | Inducing apoptosis and senescence | Suppressing tumor growth | [207] |
| Vitamin D | Inhibiting oxidative stress | Suppressing cellular senescence and senescence-associated secretory phenotype | Preventing spontaneous tumor development | [208] |
| Carotenoid-enriched nanoemulsions | Scavenging ROS | Decreasing the expression of the senescence marker p16INK4 | Inducing Cell Death in a Novel Radioresistant Osteosarcoma Cell Line | [209] |
| Oridonin | Increased intracellular hydrogen peroxide levels and reduced the glutathione content | Inducing apoptosis and senescence | Suppressing colorectal cancer cells | [210] |
| Fisetin | Stimulating the dissipation of mitochondrial membrane potential and oxidative stress | Resulting in apoptosis | Eliminating drug-resistant senescent breast cancer cells | [211] |
| Rutin | Reducing oxidative stress | Restraining the acute stress-associated phenotype (ASAP) by interfering with the interactions of ATM with HIF1α | Improving chemotherapeutic efficacy in prostate cancer cells | [189] |
4.4 Immunotherapy
Importantly, the immune system can also be employed for senescent cell elimination via exploiting surface proteins on immune cells. Targeting senescent cells that overexpress urokinase-type plasminogen activator receptor (uPAR) with uPAR-specific chimeric antigen receptor (CAR) T cells can effectively eliminate senescent lung cancer cells induced by MEK and CDK4/6 inhibitors, thereby delaying tumor growth.212 The upregulation of the ligand for the NK cell activating receptor NKG2D may enhance NK cell-mediated cytotoxicity to eliminate senescent cells.213, 214 Given PD-L1's crucial role in the buildup of senescent cells and age-related inflammation, targeting PD-L1+ senescent cells with immune checkpoint inhibitors could offer a promising approach for cancer treatment.215, 216 Chibaya et al. found that EZH2-mediated epigenetic repression of pro-inflammatory SASP genes in the pancreatic tumor microenvironment diminishes NK and T cell surveillance during TIS. In mouse models of PDAC, inhibiting EZH2 enhanced the production of SASP chemokines CCL2 and CXCL9/10, leading to improved infiltration of NK and T cells and the eradication of PDAC. This suggests that EZH2 inhibition in conjunction with senescence-inducing therapy could be an effective way to achieve immune-mediated tumor removal in PDAC.217
4.5 Strategies
In addition to conventional senotherapeutics and their combination use, growing interest focuses on developing novel senotherapeutics via employing prodrugs, protein degrading systems and nanocarriers to improve the targeting efficacy and mitigate side effect.218 The ubiquitin‒proteasome system is explored to induce the degradation of a protein of interest (POI), termed PROTAC. ARV825 is a documented example of a PROTAC drug. The compound consists of pomalidomide, an E3 ligase-binding agent, and a potent inhibitor targeting the bromodomain and extraterminal domain (BET) proteins BRD2, BRD3, or BRD4. ARV825 inhibited nonhomologous end-joining (NHEJ), the main DNA double-strand break repair mechanism, by promoting BRD4 degradation. Additionally, it stimulated autophagy, which in turn caused senolysis through two distinct routes.219 Employing a BCL-XL-specific PROTAC drug that utilizes the cereblon E3 ubiquitin ligase to degrade BCL-XL proteins has reduced the platelet toxicity associated with navitoclax. Targeting BCL-2 antiapoptotic proteins enhances senolytic specificity in senescent cancer cells while reducing toxicities such as thrombocytopenia, due to the minimal expression of Cereblon in platelets compared to its presence in all human cancer cells.220
A frequently employed strategy is the use of prodrugs, which are initially pharmacologically inactive but convert into an active form upon metabolism. For instance, β-galactosidase, which is overexpressed in senescent cells, may selectively activate a galacto-conjugated navitoclax (nav-Gal). When palbociclib and nav-Gal are used together in vivo, senescent triple-negative breast cancer cells are eliminated, which slows the growth of tumors and lessens navitoclax's cytotoxicity.221 Nanoparticle delivery systems enable the controlled delivery and release of diverse payloads.216, 222 A lipid-based nanoparticle coencapsulation system was designed to deliver STING and TLR4 innate immune agonists alongside senescence-inducing RAS-targeted therapies. This regimen triggers STING and TLR4-mediated type I interferon signaling, activating innate and adaptive immune responses to enhance T cell control of pancreatic cancer.223 In a different study, inhibitors targeting CD47 and CDC7 are coloaded into a nanosystem to induce senescence and immunotherapy after the development of chemoresistance. Results suggested this nanosystem effectively inhibited tumor growth in in vivo liver cancer mouse model and a chemotherapy-resistant mouse model.224
5 FUTURE DIRECTIONS AND CHALLENGES
Senescence is generally considered an unfavorable outcome of cancer therapy due to its harmful effects; however, senolytic agents offer a promising complement to conventional and targeted cancer treatments that induce tumor cell senescence. Sequential pro-senescence followed by senolytic therapy may enhance traditional chemotherapy efficacy and improve patients' quality of life. Senolytics offer a significant benefit by specifically targeting and removing senescent cells, which are linked to inflammation and tumor growth. By reducing these harmful cells, the efficacy of chemotherapy may be significantly boosted, leading to a decrease in tumor burden and a reduction in the side effects commonly experienced during treatment.
However, many challenges remain before the successful translation of these findings into clinical interventions since cellular senescence is clearly more complex and nuanced than initially thought. First, senescence is a dynamic process over time, and the senescent state exhibits very high heterogeneity between different cell types and depends on the stimuli that induce senescence. In this regard, a single biomarker cannot represent cellular senescence, and multiple markers are still needed for characterization. Second, despite the great therapeutic advances achieved by senotherapeutics, their application revolves around nondeterminacy on the time, dosage, and means of administration. The unascertainability of the properties, subtypes, and abundance of senescent cells makes it difficult to precisely use specific senotherapeutics, which may cause tolerability and side effects. Third, oxidative stress exerts a double-edged sword and complex role on cellular senescence, which together with the different roles of natural agents with different dosages on cellular senescence make it difficult to decide which dosage should be used. Fourth, while the long-standing notions that redox stress postulates a causal role in cellular senescence and aging-associated diseases such as cancer, a number of studies suggest that cellular oxidative stress does not unequivocally translate into organismal aging, and not all cellular senescence causes tumorigenesis. Currently, there are no definitive biomarkers to accurately identify the senescent state, and no individual marker can uniquely distinguish senescence from other types of growth arrest.
Future studies could establish single-cell multiomics technology and spatiotemporal clustering approaches to identify more feasible senescent biomarkers, investigate the types and mechanisms of senescent cells that are dying, and monitor the safety of a given senolytic. Furthermore, it is essential to develop alternative methods for quantifying senescent cells beyond traditional tissue staining. Techniques like liquid biopsy could serve as valuable biomarkers to assess the efficacy of senolytic drugs. Future research should delve into the optimization of drug combinations, seeking to identify the most effective pairings and dosages that maximize benefits while minimizing harm. Moreover, understanding patient-specific profiles will be crucial for tailoring treatments that consider individual genetic, environmental, and biological factors. Investigating novel senolytic agents could also lead to advancements in the field, potentially unveiling compounds that are more selective and effective. Attention should be given to the influence of tumor microenvironment components, including microorganisms. For example, Fusobacterium nucleatum promoted chemotherapy-induced SASP to drive esophageal squamous cell carcinoma (ESCC) progression and chemoresistance. Fusobacterium nucleatum infiltrated and persisted in senescent ESCC cells, escalating DNA damage and activating the DNA damage response pathway and SASP, thereby promoting ESCC cell migration and invasion.225 Overall, this multifaceted approach could revolutionize cancer therapy, paving the way for improved outcomes and a more personalized approach to treatment.
AUTHOR CONTRIBUTIONS
Ping Jin: Funding acquisition (lead); writing—original draft (lead); writing—review and editing (lead). Xu-Dong Feng: Writing—review and editing (equal). Cheng-Shuang Huang: Writing—review and editing (supporting). Jia Li: Writing—review and editing (supporting). Hui Wang: Software (equal); visualization (supporting). Xian-Mei Wang: Software (supporting); visualization (supporting). Lei Li: Writing—review and editing (supporting). Lan-Qing Ma: Conceptualization (lead); supervision (lead). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Figures were created by BioRender (BioRender.com). Data of clinical trials presented in Table 1 was obtained from https://clinicaltrials.gov/. This research was funded by the National Natural Science Foundation of China (82303506), the First-Class Discipline Team of Kunming Medical University (2024XKTDYS02), the Basic Research Program of Yunnan Province (202401CF070184), the independent research fund of Yunnan Characteristic Plant Extraction Laboratory (2022YKZY006), the Open Research Fund of Yunnan Characteristic Plant Extraction Laboratory (YKKF2023003), and the Natural Science Foundation of Sichuan Province (25QNJJ1079).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.