Epigenetic remodeling under oxidative stress: Mechanisms driving tumor metastasis
Peilan Peng, Siyuan Qin, and Lei Li contributed equally to this study.
Abstract
Tumor metastasis is a multistep, inefficient process orchestrated by diverse signaling pathways. Compared to primary tumor cells, disseminated tumor cells inevitably encounter higher oxidative stress in foreign environments. The levels of reactive oxygen species (ROS) fluctuate dynamically during different metastatic stages, adding complexity to the regulation of metastatic progression. Numerous studies suggest that epigenetic remodeling, a key reversible mechanism of gene regulation, plays a critical role in responding to oxidative stress and controlling gene expression profiles that drive metastasis. Despite extensive research, a comprehensive understanding of how oxidative stress impacts metastasis through epigenetic modifications remains elusive, such as DNA methylation, histone modification, ncRNAs, and m6A modification. Epigenetic therapeutic strategies, such as DNMT inhibitors, HDAC inhibitors (HDACis), and miRNA mimics, have shown promise, yet challenges related to immunogenicity, specificity, and delivery also exist. Furthermore, due to limited understanding, some drugs targeting m6A modification have yet to be explored. In this review, we provided an overview of how oxidative stress influences tumor metastatic behavior, summarized the epigenetic mechanisms involved in these processes, and reviewed the latest advancements in epigenetic-targeted therapies, which may pave the way to develop novel strategy for preventing or treating tumor metastasis.
Graphical Abstract
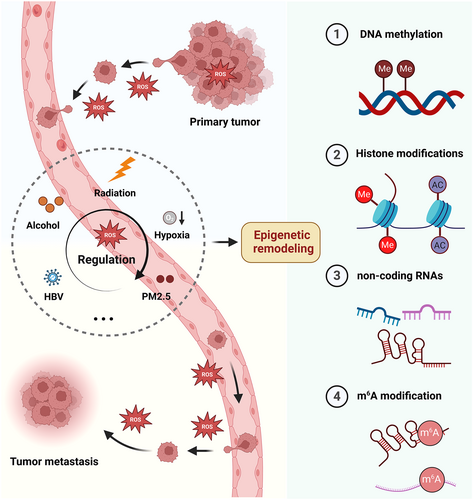
During the process of metastasis, tumor cells inevitably encounter the stimulation of various oxidative stressors, resulting in the fluctuation of intracellular reactive oxygen species (ROS) levels. Epigenetic reprogramming serves as a bridge between oxidative stress and tumor metastasis. In different stages of metastasis, ROS can modulate tumor behavior by reprogramming DNA methylation, histone modification, noncoding RNA, and N6-methyladenosine (m6A) modification.
1 INTRODUCTION
Cancer remains one of the most significant threats to human health due to the lack of effective treatments. In 2022, nearly 20 million new cancer cases and approximately 9.7 million cancer-related deaths were reported.1 Despite advances in cancer therapies, including targeted treatments and immunotherapy, achieving sustained therapeutic success remains elusive, particularly in the context of metastasis, the primary cause of cancer mortality.2 Metastasis represents a disseminated, often incurable condition that is typically resistant to conventional treatments.3, 4 A comprehensive understanding of the cellular and molecular mechanisms underlying this complex process is essential to develop methods for preventing or treating metastasis.
Metastasis is a multifaceted process that includes angiogenesis, epithelial-mesenchymal transformation (EMT), invasion and immune escape, metabolic reprogramming, and cancer stem cell maintenance.5, 6 During these processes, cancer cells face a variety of challenges, among which oxidative stress is inevitable. Oxidative stress refers to the imbalance between oxidative and antioxidant systems in the body under multiple pressures,7 mainly characterized by the overproduction of peroxide substances, such as reactive oxygen free radicals (ROS).8 Oxidative stress accompanies the entire process of tumor metastasis, encompassing the local invasion of the primary tumor,9, 10 stromal detachment,11, 12 dissemination of circulating tumor cells (CTCs) within the circulatory system,13, 14 and subsequent infiltration and colonization of distal sites.15, 16 It is worth noting that the effects of ROS on metastasis vary in different forms of tumors.17 For example, excessive ROS generally limits metastasis by inducing apoptosis,18-20 while high ROS levels abnormally promote metastasis to the lung and other organs in pancreatic cancer models.21 Therefore, understanding the dual role of ROS is conducive to developing novel therapeutic strategies to overcome tumor metastasis.
Epigenetic reprogramming provides a crucial link between oxidative stress and tumor metastasis. It has been demonstrated that prolonged exposure to oxidative stress can disrupt the original epigenetic regulatory machinery, mainly manifested by changes in DNA methylation, histone modification, noncoding RNA (ncRNA), and N6-methyladenosine (m6A) modification.22, 23 ROS gradually remodel epigenetic patterns with tumor progression by recruiting epigenetic modifiers and regulating their activity, thus silencing or activating metastasis-related gene expression.24-26 For instance, prolonged exposure to ROS leads to hypomethylation of oncogenes and hypermethylation of tumor suppressors, subsequently promoting tumor progression.27-29 ROS's alteration in histones also serves as a switch controlling gene expression.30 Since histones and DNA form nucleosomes together, epigenetic alterations in DNA generally occur with histone modifications under oxidative stress.26, 31 Moreover, multiple ncRNAs, including microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs), circular RNAs (circRNAs), and long noncoding RNAs (lncRNAs), are also responsible for the regulation of ROS in tumor metastasis.32-36 Interestingly, some changes in ncRNAs induced by ROS impact tumor metastasis by altering DNA methylation or histone modification.37, 38 Likewise, m6A methylation has also been recently reported to be sensitive to oxidative stress and then facilitate tumor metastasis partly by regulating the function of ncRNAs.39, 40 In recent years, therapeutic strategies targeting epigenetic modifiers have been widely studied, and many are currently in clinical trials to treat various cancers.41, 42 Nevertheless, small molecule inhibitors targeting epigenetic factors still need to fulfill their anticipated potential, underscoring the persistent challenges in developing epigenetic drugs. A more profound understanding of these mechanisms is imperative for formulating effective treatment strategies.
Here, we provided an overview of the intricate interplay between oxidative stress and metastasis, with particular focus on ROS-induced epigenetic modifications, mainly including DNA methylation, histone modifications, ncRNA alteration, and m6A modifications, and reviewed recent therapeutic advances targeting epigenetic reprogramming in metastatic cancer.
2 THE REGULATORY ROLE OF OXIDATIVE STRESS IN TUMOR METASTASIS
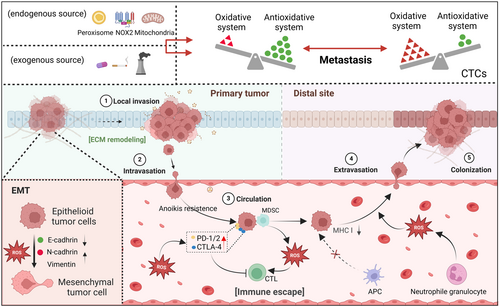
Oxidative stress is indispensable for cancer cell survival.43, 44 Normally, cancer cells exhibit markedly altered redox homeostasis,45, 46 characterized by increased ROS production and enhanced expression of antioxidant genes.47 Moderate ROS can promote cell proliferation by regulating redox signaling.48 Meanwhile, to avoid excessive ROS-induced damage, cancer cells enhance their antioxidant ability,49 such as increased glutathione (GSH) and nicotinamide adenine dinucleotide phosphate (NADPH) generation and antioxidant gene expression.50 Oxidative stress is involved in the whole process of metastasis, from EMT in the early stage of metastasis, local invasion, circulation, extravasation, to finally distal colonization.51-53 It is worth noting that ROS levels vary in different stages of metastasis, so the effect of ROS on tumor metastasis is complex (Figure 1).
2.1 Production and elimination of ROS
ROS, as byproducts of oxygen consumption and cellular metabolism, are formed by the partial reduction of molecular oxygen and exist in both cancer cells and normal cells.54 It mainly comprising superoxide anions (O2−), hydroxyl radicals (•OH), and hydrogen peroxide (H2O2).55 Cancer cells exhibit an altered redox state compared to normal cells,56 which depends on ROS production efficiency and scavenging capacity.57 ROS accumulation is attributed to the exogenous environment and endogenous metabolism.58 Exogenous ROS can be generated secondary to exposure to many risk factors, including radiation, cigarettes, metals, asbestos, and air pollutants.59-61 Endogenous ROS mainly originate from the mitochondrial respiratory chain and NADPH oxidases (NOXs).62 Discrete subcellular organelles, including the endoplasmic reticulum, peroxisomes,63 and nuclei, also generate ROS.62, 64, 65 When excessive accumulation of ROS occurs, the antioxidant system functions as a ROS eliminator to maintain redox homeostasis.66 This system includes endogenous antioxidant enzymes,67 including superoxide dismutase,68 catalase,69 glutathione peroxidase,70 glutathione reductase,71 and peroxiredoxins,72 and antioxidant molecules such as glutathione, coenzyme Q, ferritin, and bilirubin.73-75 However, the balance between the oxidant and antioxidant system is generally broken in cancer cells.
2.2 ROS participate in tumor metastasis
In normal cells, the well-balanced function of the antioxidant system enables intracellular ROS concentration in a dynamic equilibrium.76 However, this balance is overturned in cancer cells,2, 77, 78 promoting tumor progression or cell death, due to the imbalance between oxidant and antioxidant systems. High ROS can cause mitochondrial membrane potential depolarization and damage the respiratory chain, leading to more O2− production by mitochondria,79 which initiates a vicious cycle of ROS production in cancer cells. ROS can regulate critical metabolic pathways by activating or inhibiting ROS-sensitive transcription factors and modifying central metabolic enzymes. Nuclear factor erythroid 2-related factor (NRF2) and BTB and CNC homology 1 (BACH1) are key transcription factors that regulate intracellular redox activity through interconnected but distinct mechanisms.80 As a central regulator of the cellular antioxidant response, NRF2 is negatively regulated by Kelch-like ECH-associated protein 1 (KEAP1).81 BACH1 participates in heme homeostasis by heterodimerizing with MAF proteins and binding to NF-E2 gene regulatory sites, suppressing NRF2-dependent oxidative stress response.82 NRF2 and BACH1 are often mutated in cancer cells,83 resulting in transcriptional repression of other genes, such as nuclear factor kappa-B (NF-кB) and FOXO.84, 85 For tumor cells undergoing metastasis, remodeling of their oxidant and antioxidant systems usually exerts their biological effect accumulatively over time.18, 86 Finding the underlying cellular or molecular mechanisms is essential for understanding the role of ROS in metastasis.
2.2.1 ROS-driven EMT regulation in metastasis
Tumor metastasis is a stepwise process that mainly includes angiogenesis in primary tumors, local invasion, dissociation from situ, and migration into circulating systems, which begins with EMT.87 This can be initialized through protein degradation.88 It is correlated with loss of cell-to-cell adhesion, loss of interaction with the extracellular matrix (ECM), and migration toward circulating systems.89, 90 ROS promote EMT by inducing cytoskeletal rearrangement, promoting ECM protein degradation, and accelerating angiogenesis.91, 92 Loss of cell polarity and detachment from primary tumors secondary to cytoskeletal rearrangement result from the coordinated function of small Rho family GTPases, mainly Rac1, RhoA, and CDC22.93 Interestingly, a potential relationship exists between Rho family GTPases, cytoskeletal dynamics, and ROS generation.94, 95 An early study found that RAC1-induced actin cytoskeleton reorganization in human endothelial cells required superoxide production.96 Consistently, in the case of tumor cells losing cell-cell adhesion, activated RAC1 can influence ROS generation and actin reorganization, thus promoting migration.97 RhoA, another small GTPase protein, can regulate the actin cytoskeleton to form stress fibers.98 Attenuated RhoA activity elicits cytoskeletal rearrangements by promoting microtubule elongation and expediting actin kinetics while contemporaneously suppressing focal adhesion assembly, enabling augmented cell migration in vitro.99 Notably, the activity of RhoA is redox dependent, which involves the oxidative modification of low-molecular weight protein tyrosine phosphatase.100 Collectively, both Rac1 and RhoA are critical regulators of actin cytoskeleton remodeling during EMT.101
The significance of ROS on EMT is also reflected in their involvement in several pathways directly linked to many critical EMT-inducing pathways. TGF-β1, well established as one of the most prominent players in EMT,102 can be mediated by Rac1-NOXs-ROS-NFκB, inducing the expression of urokinase-type plasminogen activator and matrix metalloproteinase 9 (MMP9) and thus promoting cellular migration and infiltration.103, 104 Additionally, ROS also manipulate the process of EMT by regulating Notch signaling,105 p53/AKT/mTOR signaling,106 JNK/ZEB1 signaling,107 and HIF-1α-SERPINE1 signaling.108
2.2.2 Role of ROS in tumor ECM regulation
The ECM in tumors and stroma is highly remodeled,109 predominantly characterized by ECM degradation and changes in its glycosaminoglycan/proteoglycan components.110 Enzymes secreted by cancer cells are responsible for ECM degradation, namely, MMPs, cathepsins, and glycolytic enzymes.111-113 Significantly, the secretion of these enzymes is partly regulated by ROS. For example, increasing ROS generation induced by polyamine oxidase can increase the expression of MMP-1 in fibroblasts.114 Similarly, Kim et al. found that superoxide dismutase 3 could suppress MMP-1 expression by reducing intracellular ROS levels and thus lowering the phosphorylation of NF-κB, p38 MAPK, ERK, and JNK.115 Moreover, ROS-regulated matrix hydrolase can directly influence EMT and, therefore, impact metastasis. Yang et al. revealed that coenzyme Q downregulated the PI3K/AKT/NF-κB/MMP-9 signaling pathways via ROS-mediated apoptosis, resulting in EMT inhibition.116 In another study, 4′-geranyloxyferulic acid (GOFA) exhibited the potential to inhibit metastasis by effectively suppressing LPS-induced MMP-9 enzymatic activity and expression by inhibiting the ROS/ERK pathway.117
2.2.3 CTCs face the challenges and opportunities of ROS in circulation
Once tumor cells enter circulating systems and become CTCs, there are many barriers that CTCs need to overcome, namely anoikis, oxidative stress, immune system “cytotoxicity,” and fluid pressure of the blood.118-120 Acquiring anoikis resistance enables circulating cancer cells to survive and proliferate independently of integrin-mediated ECM contacts.121-123 Numerous studies have shown ROS as a critical instigator of anoikis sensitivity.124-127 The metastasis-facilitating protein angiopoietin-like 4 (ANGPTL4) stimulates ROS production through an outside-in signaling mechanism involving integrin engagement. This ROS subsequently activates the PI3K/Akt and ERK signaling cascades to confer anoikis resistance to malignant cells.121 In another study, enhanced generation of ROS by NOX4 was implicated in inducing anoikis resistance in gastric cancer cells.128 NOX4-mediated elevations in ROS upregulate epidermal growth factor receptor (EGFR), a prosurvival growth factor that inhibits anoikis.129 Indeed, intracellular ROS play an integral role in modulating growth factor signaling to elicit anoikis resistance in metastasizing cancer cells.130, 131 In addition, fluid shear stress (FSS) can “reshape” CTCs to some extent by generating ROS. FSS-caused oxidative stress facilitates the transition to the HER2-negative phenotype in CTCs.132 Upon exposed to FSS, certain CTCs that are resistant to anoikis aggregate into stable cell clusters in an E-cadherin-dependent manner.133 These clusters help shield the CTCs from excessive oxidative stress in the extracellular environment by promoting intercellular connections. Compared to individual cells, CTCs within these clusters reside in a relatively moderate hypoxic microenvironment. Hypoxia has been reported to enhance cell proliferation and promote mitophagy in cell clusters, which limits ROS production from damaged mitochondria in detached tumor cells.134 Meanwhile, the augmented interactions between tumor cells and their associations with platelets or immunosuppressive cells further enhance the stemness, proliferation, and immune evasion capabilities of CTCs within this stable cluster.135 FSS-treated breast cancer cells from stage III patients exhibited an increased expression of antioxidant enzyme genes, including superoxide dismutase, catalase, and glutathione peroxidase,136 which may enhance the survival of CTCs. Ma et al. found that FSS exposure upregulated ROS levels and enhanced the migration of tumor cells by activating the ERK1/2 pathway.137 FSS upregulates atonal bHLH transcription factor 8 (ATOH8) by activating the VEGFR2/AKT pathway, transcriptionally triggering HK2-mediated glycolysis and promoting the intravascular survival of CTCs.138 Pyruvate, a key glycolytic metabolite, functions as an extracellular antioxidant whereas lactate-driven pentose phosphate pathway and fatty acid oxidation can generate NADPH, reduce mitochondrial ROS production.139 In addition, other byproducts of glycolysis that enter the mitochondria undergo tricarboxylic acid (TCA) cycle and oxidative phosphorylation, generating more energy to support tumor metastasis. Contrary to the traditional view that cancer cells primarily rely on the glycolytic pathway to obtain energy for rapid proliferation, growing evidence suggests that the TCA cycle plays an equally important role in tumors. For instance, AMPK regulates the pyruvate dehydrogenase complex to adjust the TCA cycle, helping tumor cells adapt to distant tissue environments and promoting metastasis.140 Therefore, the balance between glycolysis and mitochondrial synthesis may contribute to tumor survival and metastasis under oxidative stress.
Additionally, CTCs in circulating systems have to evade immune surveillance.141 On one hand, malignant cells circumvent detection from antigen-presenting constituents by downregulating the expression of antigens, producing factors that help tumor cells to be recognized as “normal.”142, 143 Variations in immunosuppressive cell subset counts that suppress systemic immunologic reactions represent an additional mechanism behind malignant immune circumvention.144 These cells facilitate the endurance of cancerous corpuscles during dissemination, in part by subduing the cytotoxic functionalities of CD8+ T lymphocytes and natural killer constituents by secreting cytokines, including EGF, TNF-α, CXCL12, IL-1, and IL-6.145, 146 Some immunosuppressive cells rely on ROS to exert their functions. For example, Sprouse et al. revealed that myeloid-derived suppressor cells (MDSCs) promoted ROS production in MDSCs and induced NOTCH1 in CTCs by direct interaction with CTCs, thereby promoting metastasis.147 In vitro analyses indicate a role for ROS in the neutrophil-mediated enhancement of metastasis in multiple cancer cell types, which probably facilitates immune escape.148 A study on endometrial carcinoma demonstrated that ROS accumulation secondary to the dysfunction of IL-6 induced mitochondrial DNA and thus activated cGAS-STING signaling, forming an immunosuppressive tumor microenvironment and promoting tumor immune escape.149 As mentioned previously, CTCs can also evade immune surveillance by upregulating the expression of factors that disguise themselves as “normal” cells while downregulating antigens, such as class I major histocompatibility complex (MHC Ⅰ), or directly destroying immune cells.150, 151 Malladi et al. demonstrated that disseminated tumor cells impose a dormant state on themselves by inhibiting Wnt signaling in an autocrine manner and downregulating natural killer (NK) lymphocyte ligands, thereby impeding NK-mediated detection and lysis to achieve immune escape.151
2.2.4 ROS mediate CTCs colonization at distal sites
Before establishing metastatic niches, CTCs need to extravasate and colonize secondary tumor sites. The mesenchymal-epithelial transition (MET) of CTCs during their extravasation into the colonization site enables their proliferation as epithelial metastatic deposits.152 The loss of E-cadherin enhances the invasion of tumor cells, but it also reduces CTCs number and inhibits the colonization of CTCs.153 Indeed, the expression of E-cadherin is largely regulated by oxidative stress. It has been reported that FSS exposure decreased the expression of E-cadherin,154 while vitamin C treatment effectively upregulated E-cadherin.155 ROS involvement is an essential and inseparable part of successful colonization.137, 156, 157 Zhao et al. found that transient hypoxia-inducible factor-1 (HIF-1) activation induced metabolic reprogramming by reducing ROS levels, facilitating the survival of metastatic cancer cells during their colonization in the lungs.158 Similarly, ARHGAP15, a Rho GTPase activating protein, could enhance metastatic colonization by inhibiting the RAC1-ROS pathway in gastric cancer.159 However, an in vivo animal study indicated that NADPH oxidase 2 (NOX2)-derived ROS in neutrophils effectively drive tumor colonization through an IL-1β-dependent pathway.160 Decreased intracellular ROS levels resulting from hypermethylation of mitochondrial DNA markedly suppressed bone colonization in renal cell carcinoma.161 It has also been reported that oxidative stress significantly inhibits the distant metastasis of human melanoma cells,162 while administration of N-acetylcysteine effectively increases melanoma metastasis by activating RHOA.163 Therefore, antioxidants might promote CTCs survival and colonization by downregulating ROS levels.
ROS play a pivotal role in influencing the self-renewal and stemness of CTCs, thereby enhancing their antioxidant capacity and drug resistance. Notably, a minor subset (~1%) of CTCs exhibit characteristics akin to stem-like cells and is responsible for initiating metastases.164 Elevated ROS levels have been shown to contribute to the self-renewal and chemoresistance of cancer stem cells.165, 166 Dissociation of CTC clusters into individual cells induces a notable increase in the DNA methylation levels of stemness-related transcription factors, including OCT4, SOX2, NANOG, and SIN3A. Consequently, fluctuations in ROS levels may significantly impact the stem cell-like phenotype of CTCs. Indeed, it has been reported that FSS markedly enhances the cancer stem cell-like phenotype of CTCs without inducing an EMT phenotype.167 Furthermore, evidence suggests that FSS can promote the stemness of CTCs by upregulating NANOG, which is implicated in the chemoresistance of CTCs.168 Additionally, ROS generated by FSS upregulates the expression of manganese superoxide dismutase in CTCs, bolstering the antioxidant defenses and metastatic potential of CTCs.137, 169 Collectively, moderate ROS levels are needed to satisfy the activation of the ROS-regulated pathway, but excessive ROS exposure impedes tumor colonization.
3 ROLE OF OXIDATIVE STRESS-INDUCED EPIGENETIC REMODELING IN TUMOR METASTASIS
To achieve successful metastasis, tumor cells need to strengthen their reducing power to adapt to oxidative stress.170, 171 For example, breast cancer cells upregulate NAD+ kinase to enable de novo NADPH production and upregulate NADPH storage, thus combating oxidative stress during metastasis.172 Likewise, supplementation with exogenous antioxidants attenuates excessive oxidative stress and stabilizes BACH1, finally driving lung cancer metastasis.173 It should be noted that despite preventing excessive oxidative stress, tumor cells also maintain a certain level of ROS to drive metastasis, which requires delicate and complex regulation of oxidative stress.174-177 Epigenetic regulation is one way that tumors achieve this dynamic regulation.162, 178, 179 For instance, under unstressed conditions, KEAP1 induces the degradation of Nrf2 and Facilitates Chromatin Transcription (FACT) complex. Once exposed to oxidative stress, the function of KEAP1 is suppressed, leading to FACT complex upregulation. Upregulated FACT complex transcriptionally facilitates the expression of antioxidant genes by opening the chromatin structure to support hepatocellular carcinoma growth and metastasis.180, 181 In addition to controlling chromosome structure, quite a few studies have indicated that oxidative stress mediates tumor metastasis by other epigenetic regulations, namely, DNA methylations, histone modifications, ncRNAs, and m6A modifications.
3.1 Epigenetic remodeling under oxidative stress
Epigenetics refers to multiple covalent modifications made to nucleic acids and histones, or the production of specific ncRNAs, which could control gene expression without altering the DNA sequence.182, 183 This field primarily involves mechanisms such as DNA methylation, histone modifications, ncRNAs, and m6A modifications. DNA methylation and histone modifications can modulate gene transcription by altering the compaction state of chromatin, while ncRNAs and m6A modifications mainly interact with mRNA, influencing its stability and translational efficiency. The regulation and functional activity of epigenetic modifications are based on multiple enzymes and proteins known as epigenetic “writers,” “erasers,” and “readers,” which are responsible for the initial attachment, subsequent movement, and real-time recognition of various modifications, respectively.184, 185 It is worth noting that oxidative stress can also serve as an essential modulator of epigenetic modifications, directly altering the level of epigenetic modification, or indirectly impacting on certain writers, erasers or readers.186-188
3.1.1 Oxidative stress modulates DNA methylation patterns
Most studies indicate that DNA methylation may enhance the interaction between DNA and histones,189-191 promoting leading to tighter nucleosome packing and more condensed chromatin. Furthermore, DNA methylation plays a crucial role in regulating the three-dimensional structure of chromatin.192, 193 By disrupting the interaction between chromatin-remodeling enzymes and their targets, DNA methylation also suppresses chromatin-remodeling activities, effectively maintaining transcriptional repression. Oxidative stress mainly remodels the state of DNA methylation by changing the activity and function of DNA methyltransferases (DNMTs, writer), DNA demethylase (eraser) and altering the expression of methyl-CpG binding proteins (reader) within tumors.194, 195 It has been shown that DNMT1 is augmented and binds to promoter regions of tumor suppressor genes such as RUNX3 under peroxide-mediated oxidative stress.196 Interestingly, the level of DNA methylation in the genome is partly determined by the state of oxidative stress.197, 198 Hydroxyl radicals can elicit global hypomethylation by interfering with the DNA binding capacity of DNMTs,196 while high levels of oxidative stress can alter the catalytic iron cycle, thereby inhibiting the DNA demethylases of the ten-eleven translocation (TET, eraser) family and heightening DNA methylation levels.199 Additionally, excessive ROS-induced 8-hydroxydeoxyguanosine (8-OHdG) upregulation in certain cancers transduces chromatin from an active into a repressive state.200 When tumor suppressor genes are located in these suppressed regions, 8-OHdG promotes tumorigenesis.201 Furthermore, 8-OHdG obstructs the DNA binding of DNMTs, culminating in genome-wide hypomethylation.202, 203
3.1.2 Oxidative stress influences multiple histone modifications
Histone modifications exert their effects via two primary mechanisms. The first mechanism involves direct alterations to the overall structure of chromatin, whether at short or long distances.204 For instance, histone acetylation and phosphorylation effectively reduce the positive charge of histones, which may potentially disrupt the electrostatic interactions between histones and DNA, resulting in a less compact chromatin structure.205, 206 The second mechanism pertains to the regulation of chromatin factor binding, either enhancing or inhibiting their interactions, thereby influencing chromatin accessibility. For example, the methylation mark on histone H3 at lysine 4 (H3K4me3) is recognized by the ING protein family (ING1-5),207 which subsequently recruits additional chromatin modifiers; conversely, H3K9me3 is recognized by heterochromatin protein 1 (HP1),208 promoting chromatin compaction. ROS-regulated histone modifications mainly include acetylation, methylation, and phosphorylation.209, 210 Dysfunction of histone modification is generally correlated with tumorigenesis and progression.211, 212 A study revealed that exposure to low dose and long-term oxidative stress caused transiently increased global H3K4me3 and H3K27me3 methylation levels and decreased global H3K9ac and H3K8ac acetylation levels.213 Meanwhile, under oxidative distress, the methylation of H3K4 can be catalyzed via the binding of Set7 to the NF-κB p65 promoter.214 Furthermore, histone acetyltransferases (HATs) and histone deacetylases (HDACs), which are responsible for histone modifications, are targets of ROS.23 Hepatitis C virus-induced ROS elevated the activity of HDACs, which suppressed the expression of hepcidin by inhibiting the binding of CCAAT/enhancer-binding protein α (C/EBPα) and signal transducer and activator of transcription 3 (STAT3) with its promotor.215 Similarly, curcumin-induced ROS involved histone hypoacetylation by inhibiting HAT activity.216 Additionally, ROS generation can influence the state of other histone modifications, such as histone ribosylation and phosphorylation.216-218
3.1.3 Oxidative stress regulates the level of ncRNAs
Oxidative stress can directly or indirectly regulate ncRNAs, namely, miRNAs, piRNAs, snoRNAs, circRNAs, and lncRNAs.219-221 Some miRNAs are regulated by transcription factors that are sensitive to ROS increase, namely, miR-27a/b by c-MYC,222 miR-200 and miR-506 by p53,223, 224 and miR-206 by NRF2.225, 226 ROS accumulation can inhibit the function of DiGeorge critical region 8 (DGCR8), thus suppressing miRNA maturation.227 Similar to miRNAs, piRNAs are susceptible to oxidative stress. Upregulation of piR-31470 helped prostate cancer cells resist oxidative stress by suppressing glutathione S-transferase pi 1 (GSTP1) expression via DNA methylation.228 SnoRNA alteration is another mechanism of adaptation to oxidative stress.229, 230 Michel et al. found that snoRNAs U32a, U33, and U35a are upregulated in response to lipid-induced oxidative stress, while the knockdown of these snoRNAs can protect cells against lipotoxicity.231 In another study, disruption of snoRNAs conferred cells with the capacity to resist death from lipotoxic and generalized oxidative stress stimuli.232 More recently, bioanalysis authenticated 330 upregulated and 533 downregulated circRNAs under oxidative stress in human dental pulp stromal cells.233
3.1.4 Oxidative stress impacts the patterns of m6A modification
The m6A modification refers to adding a methyl group at position N6 of adenosine, which is extensively located in mRNA, lncRNA, and circRNA.234, 235 The m6A modification is also inconstant and reversible and plays a biological role mainly regulated by m6A methyltransferase (writers), m6A demethylase (erasers), and m6A-binding proteins (readers).236, 237 Indeed, varying degrees of oxidative stress signals can dynamically remodel the patterns of m6A modifications.238 A high-performance liquid chromatography-tandem mass spectrometry study found that m6A-modified mRNA levels changed under oxidative stress.239 Qu et al. observed a significant decrease in m6A modifications in pancreatic β-cells undergoing oxidative damage. Further study found that Fat mass and obesity-associated protein (FTO, reader) and methyltransferase-like 3 (METTL3, writer) were also downregulated under oxidative stress.240 However, another study identified that ROS accumulation caused by Bmal1 deletion remarkedly increased METTL3-mediated m6A mRNA methylation.241 Oxidative stress is also involved in the deposition of m6A into stress granules by mediating METTL and YTHDF3.242 Furthermore, hypoxia, the inducer of ROS production,243-245 has been gradually identified to engage in reprogramming m6A modifications.246-249 For example, HIF-1α and HIF-2α could upregulate the expression of alkB homolog 5 (ALKBH5, eraser) under hypoxia.250 Hypoxia-induced HIF-1α interacts competitively with YTHDF2 to maintain the stability of lncRNA-STEAP3-AS1, thus promoting tumor progression.251 However, it has also been reported that ROS can suppress the enzymatic activity of ALKBH5 by triggering the ERK/JNK pathway.252 Dysregulation of m6A modifiers is often responsible for reprogramming m6A modification under hypoxia conditions. For instance, hypoxia can induce the SUMOylation of YTHDF1 at Lys571, subsequently improving its binding affinity for m6A-modified mRNAs and facilitating the degradation of transcriptome-wide mRNAs.253
Collectively, it is clear that, at least in part, oxidative stress functions by modifying epigenetic modifications. Therefore, oxidative stress-regulated epigenetic modifications might be crucial in tumor metastasis.
3.2 ROS-regulated DNA methylation reprogramming modulates metastasis
ROS-induced DNA methylation alterations show multifaceted metastasis effects (Figure 2). Generally, cancer cells enhance their antioxidant ability during metastasis to defend against excessive ROS,254-256 whereby DNA methylation plays an irreplaceable role. For example, a study focusing on the state of DNA methylation in non‑small cell lung cancer (NSCLC) revealed that 79 of 121 NSCLC patients exhibited demethylation in the peroxiredoxin‑5 promoter region. Notably, hypomethylation of peroxiredoxin‑5 activated the Nrf2 signaling pathway and thus significantly decreased E‑cadherin and increased vimentin.257 Several earlier studies found that decreased catalase expression is also associated with tumor metastasis. For instance, ROS-induced hypermethylation in the catalase promotor is responsible for downregulating catalase during cancer development,258 while DNMT inhibitor treatment successfully upregulates catalase.259 Interestingly, ROS can facilitate their accumulation during metastasis by regulating DNA methylation, rather than upregulating the antioxidant system to circumvent oxidative damage. A study in 2021 revealed that ROS upregulate the expression of FOXC1 by activating ROS-ERK1/2-p-ELK1 signaling, leading to DNA hypermethylation of the cystathionine γ-lyase promoter by increasing DNMT3B expression, finally leading to a decrease in cysteine levels and an increase in ROS levels. This positive feedback loop induces further ROS accumulation, ultimately promoting metastasis.260
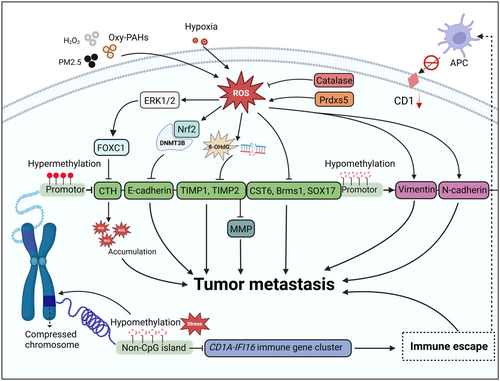
Additionally, the silencing of tumor suppressors is partly caused by DNA methylation under oxidative stress. ROS-induced DNA damage products, including 8-OHdG and DNA double-strand breaks, indirectly induce DNA hypermethylation-related transcriptional silencing of tumor suppressor genes by recruiting chromodomain helicase DNA-binding protein 4 and thus recruiting DNMTs, promoting the proliferation, invasion, and metastases of colorectal cancer cells.261 Moreover, it is imperative to recognize that ROS are also capable of conducting epigenetic remodeling on metastasis-related factors, including E-cadherin, N-cadherin, Vimentin, Snail, Slug, ZEB1, and ZEB2, to control metastasis.262-267 A comparative study in hepatocellular carcinoma demonstrated that ROS trigger hypermethylation of the E-cadherin promoter by snail-regulated DNMT1, leading to silencing of E-cadherin and thus promoting metastasis.268 A sublethal dosage of hydrogen peroxide treatment also decreased E-cadherin in breast cancer by enriching DNMT1, H3K9me3, and H3K27me3 in the E-cadherin promoter.269 Consistently, exposure to oxygenated polycyclic aromatic hydrocarbons (Oxy-PAHs) can prompt the downregulation of E-cadherin by DNA methylation, consequently facilitating lung cancer metastasis.270 Interestingly, it has been reported that ROS is indispensable to (NH4)2SO4-modulated lung cancer metastasis.271 Prolonged exposure to (NH4)2SO4 induced ROS accumulation and activated HIF-1α, resulting in hypermethylation of E-cadherin promoter regions and facilitating EMT.
Living in a high-intensity oxidative stress environment, CTCs are more susceptible to ROS.272, 273 The results of single-cell sequencing of isolated CTCs in 2017 showed that DNA methylation patterns in CTCs resembled those of epithelial-like cells. Notably, methylation at the promoter of the microRNA-200 family was remarkably higher in prostate CTCs, indicating that the distribution of methylation manifests spatial specificity, which is probably related to the organotropism of CTCs colonization.274 Another study concerning the DNA methylation pattern of CTCs in peripheral blood from patients with breast cancer revealed that, compared with healthy individuals, the promoter methylation of tumor suppressor and metastasis suppressor genes, including CST6, BRMS1, and SOX17, was at a higher level.275 Notably, surviving CTCs are characterized by immune escape, which can be partly attributed to DNA methylation remodeling. Recently, Guo et al. found by high-resolution genome-wide single-cell DNA methylation sequencing that 40 core domains are uniformly hypomethylated from early prostate malignancy to CTCs.276 Interestingly, hypomethylation of these regions silenced gene transcription, which contradicts the conventional view that hypermethylation of DNA suppresses transcription. One explanation is that the sites of DNA methylation determine transcriptional outcome, which means that hypomethylation in CpG islands of promotors promotes gene expression. In contrast, hypomethylation in long-range hypomethylation regions inhibits transcription. Subsequent analysis demonstrated that these transcriptionally silenced genes are mainly immune-related, including five CD1 genes that present lipid antigens to NKT cells.276 Therefore, hypomethylation-induced transcription inhibition contributes to the immune escape of CTCs, where oxidative stress might serve as an initiating factor. Collectively, DNA methylation in the promoter regions of key tumor genes responds to the regulation of oxidative stress, which is an important biological event during tumor metastasis.
3.3 Histone modification remodeling caused by ROS affects metastasis
Histone modifications, akin to DNA methylation, are both subjected to modulation by ROS and serve as epigenetic switches for gene transcription,277, 278 which also regulate metastasis (Figure 3). ROS induced by Oxy-PAHs simultaneously promoted DNA methylation of E-cadherin and histone acetylation of snail by separately activating DNMT3a and suppressing HDAC1. Consequently, E-cadherin inhibition and snail activation facilitate lung cancer metastasis.270 Consistently, H2O2 treatment also augmented DNA methylation and histone deacetylation by activating the ERK pathway and thus stimulating DNMT and HDAC1, leading to transcriptional silencing of E-cadherin.269 However, ROS can also play a regulatory role in some cases, dependent on histone acetylation but not DNA methylation. For instance, exposure to hexavalent chromium, a common human carcinogen that can generate ROS, strengthened the binding of HDAC1 to the E-cadherin gene promoter but did not change DNA methylation levels.279
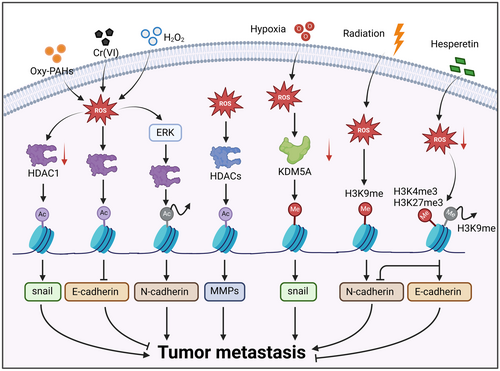
Notably, histone methylation remodeling is essential for tumor adaptation to oxidative stress. Hypoxia in pancreatic cancer facilitated ROS generation in a HIF-1-dependent manner and thus limited the activation of lysine-specific demethylase 5A (KDM5A). Subsequently, increasing methylation of histone H3 facilitated the transcription of snail and tumor metastasis.280 Another study revealed that H3K9 methylation by radiation-induced ROS downregulated E-cadherin expression and upregulated N-cadherin expression, thus promoting metastasis.281, 282 Conversely, it has been reported that hesperetin administration resulted in the accumulation of H3K4me3 and H3K27me3 but a decrease in H3K9me3, finally increasing E-cadherin expression and decreasing N-cadherin expression. In this regard, the methylation sites determine whether histone methylations function as transcription promotors or inhibitors. Specifically, H3K4me3 and H3K36me3 are associated with active transcription, whereas H3K27me3, H3K9me2/3, and H4K20me3 are correlated with repressed gene expression.283, 284 Moreover, oxidative stress-induced ECM remodeling is tightly associated with tumor metastasis.285-287 It has been reported that oxidative stress stimulates HDACs and thus upregulates the expression and activity of MMPs,288-290 facilitating the degradation of the endothelial glycocalyx.110 Notably, ROS-induced fragmentation of the glycocalyx favors entry into the circulating system for tumor cells.291 Moreover, loss of endothelial glycocalyx is indispensable for CTCs colonization. Circulating lung cancer cells facilitate their adhesion to the brain endothelium by secreting factors to destroy the endothelial glycocalyx.292 Taken together, ROS-mediated histone modification remodeling regulates multiple biological events associated with tumor metastasis, including EMT, ECM remodeling, and distal colonization.
3.4 Metastasis involves different ncRNA regulation patterns under oxidative stress
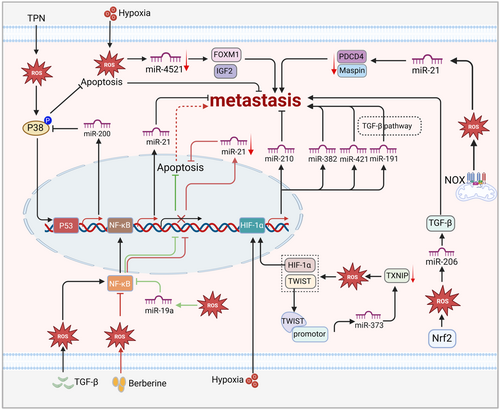
MiRNAs have been observed to participate in cancer metastasis under oxidative stress (Figure 4). Generally, various miRNAs, including miR-99a,293 miR-373,294 miR-372,295 miR-142,296 and miR-212,297 are involved in metastasis by directly or indirectly regulating ROS generation. More importantly, ROS can also regulate the transcription and biogenesis of miRNAs and thus influence metastasis. It has been reported that the antioxidant molecule Nrf2 modulates miR-206 to promote tumorigenesis.226 Meanwhile, miR-206 also participated in metastasis, which suppressed TGF-β signaling and thus controlled EMT, migration, and invasion.298, 299 Similarly, downregulated miR-4521 suppresses gastric carcinoma metastasis under hypoxia conditions by regulating IGF2 and FOXM1.300 Nonetheless, another study showed that ROS play a carcinogenic role by upregulating miR-21 and thus downregulating programmed cell death 4 protein (PDCD4) in patients with gastric cancer.301 Noticeably, some ROS-sensitive transcription factors, including P53, NF-κB, and HIF-1, are also involved in tumor metastasis by regulating miRNAs. ROS resulting from total parenteral nutrition indirectly activate p53 by phosphorylating p38 and thus drive the transcription of miR200, which mediates EMT.223, 302 Likewise, the function of miRNAs in metastasis partly depends on NF-кB. For example, TGF-β-activated NF-кB is involved in the binding of Smad and miR21.303 ROS-inhibited NF-κB dampened miR-21, thus suppressing apoptosis and indirectly driving metastasis.304 Consistently, miR-19a accumulation secondary to ROS also dampened apoptosis by inactivating NF-κB.305 In addition, it has been reported that, under hypoxia conditions, HIF-1α indirectly influences tumor metastasis by regulating miR210, miR382, miR-421, and miR191.306-309 Overall, miRNAs can respond to oxidative stress to directly or indirectly mediate the process of tumor metastasis.
In addition to miRNAs, other ncRNAs exhibit ROS response properties during tumor metastasis (Figure 5). PiRNA is a kind of ROS sensor that plays a dual role in tumor progression.310, 311 For example, piR-823 showed tumor oncogenic activity in esophageal squamous cell carcinoma by upregulating DNMT3B.312 However, another study indicated that piR-2158 suppressed IL11 expression and IL-11 secretion by competing with the AP-1 transcription factor subunit FOSL1, thus blocking JAK-STAT3 signaling to inhibit tumorigenesis, invasion, and EMT in breast cancer.313 It should be noted that some snoRNAs, including SNORD32A, SNORD33, and SNORD35A, are found to be under the control of oxidative stress.229, 314 Studies have reported that these snoRNAs are closely correlated with tumor metastasis.315-318 Additionally, transient receptor potential melastatin 8 (TRPM8) supported hepatocellular carcinoma metastasis by upregulating SNORA55 to induce nuclear and mitochondrial dysfunction.319 It is essential that the activation of TRPM8 is under the control of oxidative stress in tumors.319, 320 Therefore, it is conceivable that ROS may facilitate tumor metastasis by modulating snoRNAs, although further investigations are warranted to support this postulation. In addition to the short ncRNAs, growing studies have suggested that circRNAs participate in ROS-regulated metastasis. In solid tumors, circRNAs mainly serve as oncogenic components in metastasis, the function of which is often dependent on miRNAs.321-324 Nevertheless, circRNAs can inhibit tumor metastasis in some specific metastatic tumors. For example, circLMO1 suppresses cervical cancer metastasis by competing with miR-4291 to drive ferroptosis.325 Consistently, circPTK2 inhibits TGF-β-induced EMT and metastasis by regulating transcriptional intermediary factor 1 γ (TIF1γ) in NSCLC.325 Similar to miRNAs, circRNAs are generally regarded as ROS regulators in ROS-related tumors.326 Interestingly, oxidative stress can also change cell fate by altering circRNA levels. It has been reported that oxidative stress-induced circHBEGF supports ECM production by sponging miR-646 and thus activating the downstream EFGR/AKT/STAT3/ERK pathway.327 In addition, circFoxo3 promoted FOXO3 expression and pulled down high levels of mouse double minute 2 homolog protein, facilitating apoptosis under oxidative stress.328 However, the roles of ROS-induced circRNAs in tumor metastasis remain largely elusive. Therefore, further investigations are needed to explore the function of ROS-responsive piRNAs, snoRNAs, or circRNAs involved in tumor metastasis.
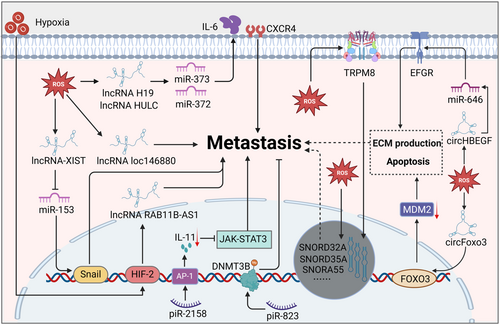
LncRNAs are also engaged in tumor metastasis, serving as competing endogenous RNAs (ceRNAs) of miRNAs.329, 330 Studies have reported that lncRNAs modulate tumor metastasis as effector molecules of oxidative stress. For example, ROS resulting from PM2.5 exposure upregulated lncRNA loc146880 expression, causing malignant metastasis of lung cancer cells.330 LncRNA-XIST drives osteosarcoma metastasis under oxidative stress. Mechanistically, oxidative stress induces osteosarcoma cell invasion, migration, and EMT by regulating the XIST-miR-153-SNAI1 axis, where XIST is caused by oxidative stress and miR-153 is inhibited by XIST.331 In addition, an analysis based on RNA-seq datasets identified aberrantly expressed lncRNAs.332 These results demonstrated that two lncRNAs, H19 and HULC, were increasingly expressed under the treatment of hypoxic or inflammatory factors. Further study identified that these lncRNAs, upregulated by oxidative stress, drive cell migration and invasion by targeting IL-6 and CXCR4 in ceRNA patterns of sponging let-7a/let-7b and miR-372/miR-373.332 Consistently, another study revealed that the HIF-2 activation response to hypoxia can induce lncRNA RAB11B-AS1 upregulation, which mediates angiogenesis and breast cancer metastasis.332 It can be seen that the mechanism by which ROS affect tumor metastasis through regulating ncRNA function is complex, and more in-depth exploration is needed to explore the cross-talk between different ncRNAs in the context of oxidative stress.
3.5 Metastasis relies on precise m6A reprogramming under oxidative stress
The remodeling of m6A methylation is an adaptive mechanism, which makes it possible for tumor cells to utilize oxidative stress signals while circumventing oxidative damage. For example, the downregulation of YTHDF1 in NSLCC under chemotherapy stress reduces the m6A level of KEAP1, thus upregulating Nrf2 to attenuate oxidative stress.333 Another study revealed lung adenocarcinoma cells upregulated METTL7B and enhanced antioxidant capacity, resulting in resistance to EGFR-tyrosine kinase inhibitors.334
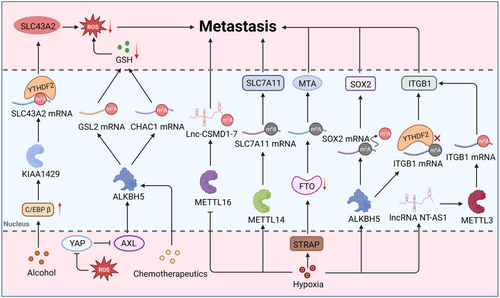
The reprogramming of m6A modifications under various oxidative stress might also serve as an “engine” driving tumor metastasis (Figure 6). Exposed to the stress of alcohol, C/EBP β boosts the expression of KIAA1429 (an RNA methyltransferase), thus accelerating pancreatic cancer metastasis.335 Another study indicated that WZ35-induced ROS imbalance attenuated the function of ALKBH5 by regulating the YAP1-AXL axis, subsequently upregulating the m6A modification levels of glutaminase 2 (GSL2) and then leading to GSH depletion.336 ALKBH5 suppressed the expression of γ-glutamylcyclo transferase 1 (CHAC1) to modulate intracellular GSH and ROS, which conferred adaption to cisplatin-induced oxidative stress onto gastric cancer cells.337 Within the hypoxic tumor microenvironment, serine/threonine kinase receptor-associated protein mediated the ubiquitination degradation of FTO, subsequently liberating metastasis-associated protein 1 (MTA1) and promoting metastasis.338 Notably, hypoxia can strengthen the migration ability of tumor cells by reducing the m6A level of target gene mRNA. Under normal oxygen levels, METTL14 promotes the degradation of SLC7A11 mRNA in a YTHDF2-dependent pathway, finally facilitating the ferroptosis of hepatocellular carcinoma. However, the function of METTL14 can be suppressed under hypoxia conditions, facilitating the expression of SLC7A11 and then supporting the survival and migration of hepatocellular carcinoma.339 Consistently, another study also found that hypoxia remarkedly remodeled the patterns of m6A modification in integrin subunit beta 1 (ITGB1) mRNA and attenuated its degradation mediated by the YTHDF2 protein, subsequently upregulating ITGB1 and resulting in the generation of proto-oncogene proteins, thus driving lymph node metastasis.39 Moreover, hypoxia can activate the expression of ALKBH5 in a manner dependent on HIF-1α and HIF-2, which subsequently facilitates SOX2 expression through demethylation of SOX2 mRNA.340 Upregulated SOX2 maintains the stem-like state of tumor stem cells, which is conducive to tumor metastasis.341 In addition to affecting mRNA's m6A methylation, hypoxia can greatly influence specific ncRNAs’ m6A methylation and then orchestrate tumor metastasis. Under a hypoxic microenvironment, HIF-1α-induced METTL16 directly binds to Lnc-CSMD1-7 and then decreases the RNA stability of Lnc-CSMD1-7 through m6A methylation, ultimately facilitating hepatocellular carcinoma metastasis.342 However, it has also been reported that hypoxia-modulated ncRNAs are capable of regulating the patterns of m6A methylation, contributing to tumor metastasis. For instance, HIF-1α upregulation transcriptionally promotes lncRNA NT-AS1 expression. Then it activates METTL3 to increase ITGB1 expression via m6A modification, thereby activating the MAPK/ERK/PD-L1 signaling pathway and ultimately promoting the immune escape of pancreatic cancer cells.343 Likewise, m6A modification-mediated macrophage reprogramming orchestrates an antitumor immune response.344 Specifically, METTL3 ablation suppresses SPRED2 mediated by YTHDF1, thus modulating cytokine responses, promoting Treg infiltration, and enhancing tumor-promoting macrophages. In short, oxidative stress impacts tumor metastasis, where m6A modification remodeling serves as an important bridge.
The role of ROS as either a promoter or inhibitor during metastatic progression remains inconclusive, contingent upon the ROS levels, specific downstream targets, and cancer types involved. According to the available evidence, the influence of ROS-regulated epigenetic modifications on tumor metastasis is highly variable, with even identical modifications exerting divergent effects on different tumor types. Tumor metastasis is a complex, multi-stage cascade, and the oxidative stress levels within tumor cells fluctuate dynamically. Consequently, it is crucial to analyze epigenetic profiles at different stages of metastasis, particularly focusing on the regulatory patterns associated with tumor driver genes. However, obtaining epigenetic information from CTCs presents significant challenges due to their low abundance in the bloodstream and existing technical limitations. Additionally, the high heterogeneity of tumors leads to variability in the epigenetic regulation of the same gene across different tumors, and these effects can even be contradictory. Furthermore, within tumor tissue from the same patient, oxidative stress levels may differ significantly between sites, resulting in spatial variations in the epigenetic status of the same gene. Addressing these gaps requires in-depth investigations to fully elucidate the role of oxidative stress-induced epigenetic modifications in driving tumor metastasis.
4 THERAPEUTIC INTERVENTIONS TARGETING OXIDATIVE STRESS AND EPIGENETIC REMODELING AGAINST METASTASIS
In terms of ROS-induced epigenetic reprogramming regulation on tumor metastasis, interventions targeting these modifications could theoretically inhibit the malignant progression of tumors. Numerous investigations targeting epigenetic modification have recently been conducted and shown remarkable therapeutic efficacy in animals. Meanwhile, several natural antioxidants have also been evaluated in treating metastatic tumors, some of which effectively suppress metastasis through modulating epigenetic modification. In addition, the therapeutic strategy of combining oxidative stress modulators and epigenetic drugs has also shown certain advantages. Here, we mainly summarized different therapeutic strategies against tumor metastasis, including single epigenetic drugs, oxidants/antioxidants with epigenetic regulation, and nano-drugs combining ROS-inducer and epigenetic modulators.
4.1 Targeting DNMTs against metastatic tumors
Targeting DNA methylation is a promising cancer therapeutic strategy. Generally, inhibition of DNMTs results in either the upregulation or downregulation of essential genes involved in cancer metastasis.345 5-Azacitidine (5-aza) and 5-aza-2′-deoxycytidine (DAC) are two major DNMT inhibitors (DNMTis) approved by the US Food and Drug Administration and European Medicines Agency for the treatment of hematological malignancies.346, 347 Several phase I/II clinical trials have investigated the potential of 5-aza against metastatic cancer.347-350 It has been reported that 5-aza administration drives EMT and facilitates the CSC phenotype, finally promoting bone metastasis of prostate cancer.351 DAC can also combat tumor metastasis. The results of a phase I-II study in the 1990s indicated the possibility of using DAC against metastatic carcinoma, including lung cancer and prostate cancer.352, 353 In recent years, researchers have tried to elucidate the underlying DAC-related mechanism and exploring new strategies to widen their clinical usability. For example, it has been reported that DAC suppressed colorectal peritoneal metastasis by regulating macrophage-dependent T-cell activation in the premetastatic microenvironment.354 Moreover, the combination of DAC with other drugs, namely, everolimus, cisplatin, and 5-fluorouracil, has been extensively explored to combat tumor metastasis.355-357 Some other DNMTis, including zebularine,358 5-fluoro-2′-deoxycytidine,359, 360 SGI-110 (guadecitabine),361, 362 CP-4200,363 4′-thio-20-deoxycytidine,364, 365 and RX-3117,366 have been developed to improve therapeutic effects with fewer side effects. Due to the function of DNA methylation in immunogenic modulation, some DNMTis have exhibited potential in the tumor immunotherapy. In mouse models and clinical studies, the use of methyltransferase inhibitors can induce the reprogramming of DNA methylation in exhausted T cells, preventing their generation.367 For tumor cells, DNMT inhibitors can enhance the natural immune response of tumor cells from multiple dimensions.368
It has been reported that 5-aza attenuates H2O2-induced senescent phenotype in tumor cells by inducing autophagy, suggesting that oxidative stress may be beneficial for the antitumor effect of 5-aza.369 Consistently, 5-aza significantly attenuated the tumorigenic potential of HK-2 cells under chronic oxidative stress.370 Therefore, strategies combining DNMTis and ROS-generators might achieve better therapeutic effect. Ruthenium (Ru) complex-mediated apoptosis coupled with decitabine-regulated DFNA5 demethylation induced immunogenic cell death to suppress gastric tumor growth and metastasis.371 Several studies also indicated that the combination of DAC and photodynamic therapy (a treatment based on ROS generation) remarkedly potentiated antitumor immune responses.372-374 In addition, DAC combined with paracetamol effectively limited the progression of head and neck squamous cell carcinoma by induction of oxidative stress. DAC could also downregulate antioxidant responses to support ROS accumulation.375 Some natural products with excellent antioxidative or prooxidative properties can restrict tumor malignant progression by dampening DNMTs.376, 377 Resveratrol elevated ROS accumulation by inducing mitochondrial destruction and thus upregulated DLC1 (tumor suppressor gene) expression by inhibiting DNMT1, finally promoting cellular senescence in breast cancer.378 Another in vivo study indicated that resveratrol management effectively inhibited lung metastasis of MDA231 human breast cancer cells by attenuating TGF-β-induced EMT.379 Similarly, epigallocatechin-3-gallate (EGCG) downregulated ubiquitin-like containing PHD and Ring finger 1 (UHRF1) and DNMT1 in a ROS-dependent manner, which silenced tumor suppressors such as p16.380 In addition, high concentrations of curcumin triggered DNA damage and induced DNA demethylation by suppressing DNMT1 binding, partly responsible for the attenuated migration ability of human gastric cancer cells.381
Collectively, DNMT inhibitors have shown great potential against tumor metastasis. Given that oxidative stress drastically alters DNA methylation remodeling in tumors, DNMT inhibitors, to some extent, deprive tumor cells of the adaption to oxidative stress, thus disrupting redox homeostasis and limiting metastasis. Some antioxidants or oxidants target DNMTs by modulating ROS levels, remodeling tumor cell DNA methylation patterns, and inhibiting metastasis.
4.2 HDAC intervention relieves tumor metastasis
HDACis can enhance the acetylation of lysine residues on histone and nonhistone proteins. For many years, HADCis have been widely investigated in metastatic tumors. Studies have reported that monotherapy with vorinostat (suberoylanilide hydroxamic acid, SAHA) yielded good effectiveness against metastatic tumors, including invasive human NSCLC382 and metastatic breast cancer.383 Furthermore, SAHA is effective against some metastatic tumors in combination with other drugs, namely, brigatinib,384 carboplatin, paclitaxel,385 and pembrolizumab.386 Despite this, the results of several phase I/II trials indicated that SAHA with or without other drugs yielded only limited therapeutic effects in solid tumors, such as metastatic breast cancer, metastatic thyroid carcinoma, and metastatic transitional cell carcinoma.387-389 Similar to hydroxamic acid, entinostat in combination with chemotherapeutics or targeted drugs, including pembrolizumab, exemestane, enzalutamide, and capecitabine, has also been evaluated in various metastatic tumors.390-392 It is worth mentioning that entinostat, combined with entinostat, generally gained good tolerance and increased antitumor activity against metastatic breast cancer.390 Romidepsin is another HDACi based on cyclic peptides. Combined therapy with romidepsin and chloroquine led to improved survival for breast cancer patients with refractory metastases.393 In addition, some short-chain fatty acid HDACis, including sodium butyrate and valproic acid, have also yielded effective therapeutic effects against metastatic cancers in combination with other treatments.394-397 It can be seen that HDACis have shown great potential in clinical therapy against metastasis. However, several clinical trials based on romidepsin failed to achieve effective therapy in metastatic tumors, namely, metastatic head and neck cancer, metastatic prostate cancer, and colorectal cancer.398, 399
Mechanistically, monotherapy with HDACis might deprive tumor cells of their tolerance to the harsh microenvironment to some extent,400, 401 but this may not be enough to completely prevent malignant metastasis. Like DNMTis, some HDACis have shown potential for immunotherapy.402 It has been reported that HDACis and DNMTis can not only alter the gene expression patterns of tumor cells but also reshape the killing ability of T cells, transforming the tumor microenvironment into a hot tumor type that is more responsive to immunotherapy.403, 404 Based on this, a series of epigenetic therapies combined with immune checkpoint therapy combinations have been approved for clinical trials (NCT01928576, NCT03220477, NCT01928576, NCT03233724, NCT03220477, NCT03576963, NCT02901899, NCT02397720, and NCT04296942), reflecting the great potential of this combination therapy.
Strategies simultaneously targeting HDACs and oxidative stress can also produce synergistic antitumor effect.405, 406 For instance, valproic acid-modulated HDAC inhibition coupled with excessive intracellular ROS induced by doxorubicin effectively inhibited the progression of hepatocellular carcinoma.407 Codelivery of lactonic sophorolipids (HDAC inhibition) and ganetespib (Hsp90 inhibition) enhanced ROS accumulation and then effectively limited the metastasis of NSCLC.408 Besides, the combination of tubastatin A (HDAC6-specific inhibitor) and palladium nanoparticles (inducing oxidative stress) synergistically induced the apoptosis of breast cancer cells.409 Recently, several natural products with the ability to modulate oxidative stress were found to inhibit metastatic tumors by targeting HDAC. Curcumin, a natural HDACi, effectively retarded the metastatic process of various solid tumors. For example, curcumin markedly downregulated the expression of tumor markers, thus sensitizing breast cancer cells to chemotherapy.410 Additionally, resveratrol acetylated and reactivated PTEN to limit tumor metastasis by targeting and suppressing MTA1/HDAC.411 Quercetin treatment also partially potentiated cell death in leukemia by suppressing HDAC.412 Consistently, curcumin, in combination with other HDACis, including vorinostat and panobinostat, greatly enhanced the therapeutic effect compared with HDAC inhibitor monotherapy.413
4.3 Therapeutic strategies based on ncRNAs
NcRNA-based therapies have been extensively explored in recent years.414 In general, there are two main methods for targeting ncRNA against tumor metastasis: targeting ncRNAs that function as facilitators in tumor metastasis with ncRNA inhibitors, such as antagomirs (targeting mRNA)415 and miRNA sponges,416 and restoring the normal function of ncRNAs that are downregulated in metastatic tumors via synthetic ncRNA-like molecules,417 such as miRNA mimic agents. Growing numbers of antagomirs have been investigated for the treatment of tumor metastasis. For example, systemic treatment with a miR-10b antagomir effectively inhibited lung metastasis but did not reduce primary mammary tumor growth.418 Similarly, antagomirs targeting miR-1246 and miR-20a also limited the progression of metastatic tumors.419, 420 Although artificial miRNA or circRNA sponges are capable of silencing miRNA, their clinical utility is still lacking. It is worth mentioning that some natural products can also inhibit metastasis by regulating ncRNAs. An early study indicated that curcumin significantly changed the expression profiles of miRNAs under oxidative stress.421 Curcumin effectively suppressed the lung metastasis of colorectal cancer by activating a ROS/KEAP1/NRF2/miR-34a/b/c cascade.422 Besides, curcumin administration greatly limited lncRNA H19-induced EMT in tamoxifen‑resistant breast cancer cells.423 Similarly, another natural antioxidant, resveratrol, showed great potential application in cancer management by targeting miRNAs or lncRNAs.424 On the other hand, the dysfunction of ncRNAs serving as tumor suppressors can be restored by synthetic ncRNA-like molecules such as miRNA mimic agents.425-427 For instance, miR34, serving as a suppressor in the metastasis of various solid tumors, has been taken to clinical trials. The results showed that the liposomal miR34 mimic has great potential to be applied in anticancer therapy.428 Moreover, the administration of miR-708-5p to mice with lung cancer resulted in efficient antitumor activity. miR-708-5p suppressed lung cancer invasion and metastasis by inhibiting the cytoplasmic localization of p21.429 Given this, artificial analogs of other miRNAs such as miR-503, miR-152, miR-145, miR-133a, miR-193b, miR-148b, miR-433, miR-127, and miR-200 still need to be explored and applied in the treatment of metastatic tumors.430-434
Importantly, with the development of nanodelivery technology, some ncRNAs with antitumor activity have been encapsulated into nanoparticles to achieve accurate delivery which is promising for use in overcoming tumor metastasis.435-437 A hydrogel-embedded, gold nanoparticle-based delivery vehicle successfully carried miR-96/miR-182 and achieved efficient local, selective, and sustained release, amplifying the antimetastatic ability of exogenous miR-96/miR-182.438 In addition to miRNAs, siRNA, and shRNA have also carried by delivery vehicles to realize antitumor therapy. For example, lipid nanoparticles carrying β3 integrin siRNA alleviated primary tumor growth and significantly inhibited metastasis in triple negative breasr cancer.439 Codelivery of siRNA and miRNA by liposome-polycation-DNA nanoparticles also significantly limited the lung metastasis of melanoma.440 In another study focusing on delivering lncRNA, plasmids encoding tumor suppressor lncRNAs were encapsulated into liposomes to prepare nanoparticles targeting renal cell carcinoma, which markedly inhibited tumor angiogenesis and metastasis.441 To further improve antitumor efficacy, many nano-drugs can combine ncRNA functional regulation with redox homeostasis regulation, such as the simultaneous delivery of miRNA and antioxidants (Table 1, Figure 6).
| Cancer type | ncRNA | ROS regulator | Mechanism | Refs. |
|---|---|---|---|---|
| Breast cancer | anti-miR-155 | Antioxidants (TEMPOL) | Scavenging ROS and inhibiting MMP-2 | [442] |
| Breast cancer | anti-miR-21; anti-miR-155 | Photosensitizer (Ce6) | Downregulating miR-21 and miR-155 and upregulating ROS levels | [443] |
| Breast cancer | miR-34a-m | Zn2+ | Enhancing apoptosis by ROS accumulation and Bcl-2 mRNA degradation | [444] |
| Melanoma | miR-30a-5p | Fe3O4 | Increasing ROS levels by Fenton reaction and targeting transcription factor E2F7 | [445] |
| Lung cancer | miR-3529-3p | MnO2 | Improving hypoxia environment and destroying mitochondrion function | [446] |
| Liver cancer | miR-195 | Ce6 | Trigger immunogenic cell death by ROS accumulation and miR-195 upregulation | [447] |
| Lung cancer | miRNA mimic let-7b | Triphenylphosphonium cation (TPP) | Targeting mitochondria and inhibiting mitochondrial respiratory chain | [448] |
| Breast cancer | Antisense-miR-10b | miR-34a-mimic | Inducing apoptosis and downregulating RhoC to suppress tumor metastasis | [449] |
| Ovarian cancer | miR-34a | Alexa Fluor 546C5 maleimide | Inducing apoptosis or cell cycle arrest | [450] |
| Hepatocellular carcinoma | miR-183 inhibitor | Au | Inducing immunogenic cell death by ROS accumulation | [451] |
| Esophageal cancer | miR181a | CeO2 | Improving hypoxia, inducing ROS accumulation, and causing DNA damage | [452] |
| Breast cancer | miR-34a | Ce6 | Synergistically promoting ROS accumulation and downregulating invasion proteins | [453] |
| Ovarian cancer; breast cancer | miRNA-139-5p | Ce6 | Synergistically inducing tumor apoptosis by ROS accumulation | [454] |
| Breast cancer | miR-21and miR-155 detection | Photosensitive metal-organic frameworks | Accurately targeting and killing tumors by ROS accumulation | [455] |
| Breast cancer | Antago3 (long noncoding RNA ASBEL) | Curcumin | Inhibiting cell proliferation and metastasis by regulating Wnt/β-catenin signaling and inducing apoptosis | [456] |
| Non-small cell lung cancer | lncRNA (MT1DP) | lncRNA (MT1DP) and erastin | Modulating NRF2 by stablizing miR-365a-3p and inducing ferroptosis | [457] |
| Oral cancer | MTHFD1L shRNA | 5-aminolevulinic acid | Inducing mitichondrion dysfunction and apoptosis | [458] |
| Hepatocellular carcinoma | USP22 shRNA | Sorafenib | Promoting ROS generation and suppressing multidrug resistance-associated protein 1 (MRP1) | [459] |
| Cervical cancer | Bcl-2 siRNA | Curcumin | Synergistically inducing apoptosis | [456] |
| Skin cancer (melanoma) | STAT3 siRNA | Curcumin | Synergisticcally suppressing tumor progression | [460, 461] |
| Leukemia | BCR-ABL siRNA | Resveratrol | Synergistically inducing apoptosis | [462, 463] |
| Bladder cancer | elF5A2 | Curcumin | Silencing the expression of FEIF5A2 to inhibit tumor metastasis | [464] |
| Colorectal cancer | PD-L1 siRNA | Resveratrol | Inhibiting glycolysis and upregulating mitochondrial oxidative phosphorylation | [465] |
| Lung cancer | MRP1 siRNA | Doxorubicin | Downregulating multidrug-resistant protein 1 and inducing the depletion of GSH | [466] |
| Hepatocellular carcinoma | NRF2 siRNA | NRF2 siRNA and Sorafenib | Inhibiting antioxidant factor NRF2 and suppressing GSH synthesis | [467] |
| Intestinal cancer | SNEDDS-siRNA-APIs | Rhodamine b and protoporphyrin IX | Downregulating SNEDDS-siRNA-APIs and inducing ROS accumulation | [468] |
| Breast cancer | PDL1 siRNA | Photosensitizer | Inducing “self-synergistic” immunogenic cell death by ROS accumulation | [469] |
| Ovarian cancer | Plk1 siRNA | Platinum(IV) (Pt(IV))-backbone polymers | Inducing cell apoptosis | [470] |
| Ovarian cancer | VEGF siRNA | Alternating irradiation | Improving hypoxic tumor microenvironment and inhibiting angiogenesis to limit liver and lung metastasis | [471] |
| Breast cancer | PDL1 siRNA | Indocyanine green | Inducing immunogenic cell death and downregulating PDL1 to improve immunotherapy | [472] |
| Prostatic cancer | FAM-siRNA | Porphyrin-lipid | Remarkedly improving transfection efficacy of siRNA and inducing ROS accumulation | [473] |
| Breast cancer | GPX4-siRNA | Ce6 | Promoting lipid ROS accumulation to induce ferroptosis | [474] |
| Melanoma | PDL1 siRNA | Photosensitizer PPA | Facilitating ROS generation to induce immunogenic cell death and downregulating PDL1 to avoid tumor immune evasion | [475] |
| Head and neck cancer | EGFR siRNA | Pyropheophorbide phosphatidic acids | Downregulating EGFR and inducing ROS accumulation | [476] |
| Liver cancer | HIF-1α siRNA | Linear poly porphyrin | Downregulating HIF-1α to sensitize tumor to photodynamic therapy | [477] |
| Breast cancer | siRNA inhibitor apoptotic protein | Porphyrin | Promoting tumor apoptosis | [478] |
| Endometrial cancer | Ferrochelatase-siRNA | 5-aminolevulinic acid | Accelerating PpIX accumulation to generate ROS | [479] |
| Breast cancer | HIF 1α siRNA | Porphyrin | Enhancing the photodynamic therapy efficacy | [480] |
| Breast cancer | FOXA1 siRNA | Porphyrin | Inducing tumor apoptosis by ROS accumulation | [481] |
| Fibrosarcoma | c-myc siRNA | Liposome | Augmenting c-myc silencing and delaying tumor progression by ROS accumulation | [482] |
| Lung cancer | Ad5/F35-APE1 siRNA | Hematoporphrphyrin derivative | Inhibit tumor proliferation and apoptosis | [483] |
| Pancreatic cancer | ABCG2 siRNA | 5-aminolevulinic acid | Accelerating PpIX accumulation to generate ROS | [484] |
| Hepatocellular carcinoma | RRM2 siRNA | Ce6 | Inhibiting tumor growth and enhancing cell apoptosis | [485] |
| Ovarian cancer | Plk1 siRNA | Hypocrellin A | Boosting the gene therapy efficiency and facilitating apoptosis by ROS accumulation | [486] |
| Breast cancer | Bcl-2 siRNA | Curcumin | Inducing tumor apoptosis | [487] |
| Cervical cancer | Bcl-2 siRNA | Curcumin | Targeting multiple signaling pathways, including cell cycle, apoptotic, and autophagic pathways | [488] |
| Breast cancer | VEGF siRNA | Lactate oxidase/Catalase (LOx/CAT) | Promoting lactate consumption and suppressing tumor proliferation and angiogenesis | [489] |
| Colorectal cancer | PD-L1 siRNA | FdUMP | Inducing large amounts of ROS to enhance immunogenic cell death | [490] |
| Breast cancer | HIF 1α siRNA | Doxorubicin | Downregulating HIF-1α and inducing apoptosis by ROS accumulation | [491] |
| Breast cancer | HIF 1α shRNA | Gold nanoparticles | Triggering immunogenic cell death and enhancing the therapeutic anticancer efficacy | [492] |
| Leukemia | Mcl-1 siRNA | Hydrophobic PCPDTBT | Inducing ROS accumulation and downregulating Mcl-1 to damage activated macrophages | [493] |
| Breast cancer | P-gp siRNA | Doxorubicin and photosensitizer | Downregulating P-gp to overcome drug resistance and inducing ROS accumulation to enhance chemotherapy | [494] |
| Breast cancer | P-gp siRNA | Selenium | Downregulating P-gp to overcome drug resistance and inducing tumor apoptosis | [495] |
| Breast cancer | P-gp siRNA, Bcl-2 siRNA | Doxorubicin and DNA nanostructure | Overcoming drug resistance and accelerating tumor cell death | [496] |
| Non-small-cell lung cancer | YAP-siRNA | Photosensitizer (Ppa) | Inducing tumor apoptosis by ROS accumulation and modulating and suppressing Bcl-2 EGFR bypass signaling pathway by inhibiting YAP | [497] |
| Ovarian cancer | Sod2 siRNA | Tetraphenyl porphine sulfonate | Synergistically promoting ROS accumulation to induce apoptosis | [498] |
| Oral cancer | Wnt-1 siRNA | Ce6 | Inhibiting the Wnt/β-catenin signaling pathway to enhance phototherapy | [499] |
| Oral squamous cell carcinoma | HIF 1α siRNA | Titanium dioxide | Causes lysosomal damage, HIF-1α gene silencing, and OSCC cell elimination efficiently | [500] |
| Head-and-neck cancer | HIF 1α siRNA | Photosan | Downregulating HIF 1α to improve photodymic therapy | [501] |
| Breast cancer | VEGF siRNA | AIE photosensitizer | Synergistically inducing tumor cell death by ROS accumulation and VEGF silence | [502] |
4.4 Therapeutic strategy targeting m6A methylation modulators
Under oxidative stress, dysfunction of certain m6A methylation regulators frequently results in abnormal reduction or increase of overall levels of m6A methylation, thus affecting tumor metastasis. Therefore, targeting these m6A modulators has great potential in therapeutic applications against tumor metastasis.503-505 Recently, the potential of targeting m6A regulators has been explored in many studies, most of which have shown satisfactory effects against tumor metastasis. For instance, the administration of small-molecule inhibitors targeting m6A regulators can slow down or even reverse metastasis-associated biological events. The inhibitor of FTO, Dac51, can effectively block tumor immune evasion by activating CD8+ cells.506 Similarly, Alk-04, a selective inhibitor of ALKBH5, significantly enhanced melanoma therapy by decreasing suppressive immune cell accumulation.507 A new sodium channel blocker, imidazobenzoxazin-5-thione MV1035, has been found to attenuate the migration and invasive ability of glioblastoma cells by inhibiting ALKBH5.508 MiRNA mimics can also be applied as potential inhibitors targeting m6A modulators. It has been reported that miR-186 inhibited hepatoblastoma metastasis by inhibiting the METTL3-modulated Wnt/β-catenin pathway.509 Moreover, miR-135 was also reportedly to suppress the EMT of breast cancer cells by silencing ZNF217 and inactivating METTL3.510
Some drugs with certain antioxidant abilities specifically target m6A modulators, thus limiting tumor metastasis. For example, maclofenamic acid remarkedly increased m6A methylation levels by suppressing FTO activity, subsequently inhibiting the survival of cancer stem cells.511, 512 Another drug with antioxidant capacity, simvastatin, downregulates m6A methylation of EZH mRNA by targeting METTL3, limiting EMT in lung cancer.513 It was also reported that resveratrol can attenuate Aflatoxin B1-induced ROS accumulation, thus leading to the reprogramming of m6A and eventually impacting hepatic function.514 Therefore, resveratrol might serve as a kind of potential antitumor drug, or at least as a drug to prevent tumorigenesis. Given that oxidative stress participates in the modulation of the m6A regulator during tumor metastasis, the combined intervention of these regulators and oxidative stress-associated signals might contribute to improving the antitumor therapy effect. For example, compared to targeting IGF2BP3 alone, combined suppression of IGF2BP3 and HIF-1α further prevented stomach cancer angiogenesis and metastasis.515 Codelivery of YTHDF1 siRNA with photosensitizer remarkedly enhanced photodynamic therapy efficiency by modulating the m6A modification level of HSP70 and lysosomal proteases.516
4.5 The challenges and limitations of epigenetic therapy
Currently, a major challenge for epigenetic therapies lies in achieving therapeutic specificity, extending beyond mere target selectivity. This lack of specificity can lead to the overactivation of oncogenes while simultaneously inhibiting tumor suppressor genes, resulting in genomic instability.517 Moreover, poor efficacy is notably evident in solid tumors, as DNMT inhibitors rely on DNA incorporation, which is primarily effective in actively dividing cells, rather than in solid tumors with relatively slow division rates. Additionally, HDACis display tissue-dependent effects: while HDAC1 overexpression correlates with poor prognosis in lung and pancreatic cancer, it may paradoxically improve survival outcomes in breast cancer.518 Such observations underscore the importance of tissue-specific target validation for achieving successful outcomes. Additionally, cellular mechanisms affecting drug uptake and metabolism may lead to resistance, posing another significant limitation.519
NcRNA therapies have shown promise both in vitro and in vivo, yet their clinical application remains hindered by challenges related to immunogenicity, specificity, and delivery.520 Ongoing clinical trials for miRNA therapies highlight immune-related adverse effects as a major concern. For instance, a Phase I trial of MRX34, a liposomal miR-34a mimic, was halted after severe immune-related events led to the deaths of four patients.428 Serious adverse events, including sepsis, hypoxia, cytokine release syndrome, and liver failure, predominantly occurred in the later treatment stages, indicating immune-mediated toxicity. Off-target effects are another pressing issue, as nontarget cell uptake or excessive dosing can lead to aberrant expression beyond physiological protein levels.521 The issue of dose of RNA therapy is also an important consideration in the off-target problem, where overdose can saturate the system and inhibit the function of endogenous miRNAs.522 Ensuring efficient delivery of ncRNA to target organs and cells while ensuring intracellular RNAi activity remains a significant challenge. A study by Sugo et al. successfully delivered siRNA targeting myostatin to mouse skeletal muscle using anti-CD71 antibody conjugation, yet the siRNA was trapped in endosomes, limiting its efficacy.523
Targeting dysregulated m6A regulators with small molecule agonists or inhibitors holds potential as a therapeutic strategy in cancer treatment. However, research on the underlying mechanisms by which m6A regulators influence tumorigenesis remains limited.524 The functions and in-depth mechanisms of m6A regulators in cancer remain largely unestablished and need future investigations. Moreover, findings regarding the roles of m6A regulators (such as METTL3, METTL14, FTO, and ALKBH5) in tumors are contradictory; some factors may act as both tumor suppressors and oncogenes. This complexity reduces the clarity of m6A as a singular therapeutic target, delaying the development of related small-molecule drugs. Notably, combining m6A modification inhibitors with chemotherapy, radiotherapy, and immunotherapy is expected to overcome the problem of tumor chemotherapy resistance,505 but further basic research and clinical cohort studies are still needed.
5 CONCLUSIONS AND PROSPECTS
Continuous oxidative stress is a hallmark of tumor biology, playing a pivotal role in all stages of metastasis, from EMT to the colonization of distant organs. Although the high-level oxidative stress microenvironment during the metastasis process leads to the death of most detached tumor cells, some surviving tumor cells adapt to the oxidative stress state through mechanisms such as genetic mutations, metabolic remodeling, and epigenetic remodeling, thereby enhancing their metastatic potential. The ROS levels fluctuate dynamically across different phases of metastasis, making oxidative stress regulation complex and highly context-dependent. Epigenetic remodeling, as a reversible gene regulation strategy, is well-suited to respond to the shifting oxidative stress landscape, providing tumor cells with enhanced adaptability. Unlike gene mutations, epigenetic modifications are more dynamic and responsive to environmental cues, such as those experienced during metastatic progression. For instance, during the invasion of surrounding tissues, an EMT program occurs in tumor cells, enhancing their motility and invasiveness. However, upon colonization in distant organs, these cells can revert from a mesenchymal state back to an epithelial state. Existing studies have demonstrated that the DNA methylation level of the E-cadherin promoter plays a crucial role in regulating this EMT. There is no doubt that epigenetic changes are involved in the modulation of tumor metastasis influenced by oxidative stress.
This review focuses on how DNA methylation, histone modifications, ncRNAs, and m6A modifications influence tumor metastasis by altering the transcription and translation processes of oncogenes and tumor suppressor genes under oxidative stress. Numerous studies indicate that various forms of oxidative stress, such as hypoxia and viral infections, can finely tune the expression of critical genes by reshaping DNA methylation, histone acetylation, m6A levels, and ncRNAs, thereby facilitating tumor metastasis. However, the roles of emerging modifications like histone sulfation, lactylation, and ADP-ribosylation, as well as ncRNAs like piRNA and circRNA, in tumor metastasis during oxidative stress remain to be fully explored. Unraveling these mechanisms across cancer types is essential for developing and enhancing cancer therapies.
While epigenetic therapy holds theoretical promise, its clinical implementation faces significant challenges, primarily concerning the specificity of target. Similar epigenetic changes can occur in both normal and cancerous cells, and certain cancers may depend on specific epigenetic alterations, making them sensitive to perturbation. Even minor disruptions in this regulatory balance can lead to severe cellular consequences, whereas normal cells may have compensatory mechanisms to offset these changes. Thus, pinpointing the most critical epigenetic modifications in various cancers is crucial. Additionally, issues of heterogeneity, immunogenicity, and delivery present further hurdles, particularly for ncRNA-based therapies. Therefore, personalized approaches combined with advanced nanotechnology for targeted delivery may improve the precision and efficacy of epigenetic therapies.
Moreover, given the significant role of ROS in altering the tumor epigenetic landscape, certain oxidants and antioxidants can epigenetically regulate tumors by modulating ROS levels, thereby inhibiting metastasis. The combination of ROS modulators and epigenetic drugs has also shown notable antitumor effects. However, most research has concentrated on nano-therapeutic strategies involving photosensitizers and miRNA mimics, with few approaches progressing to clinical trials. Further exploration of how ROS drives tumor progression via epigenetic modifications, and the crosstalk between these modifications, will be critical for optimizing existing treatments and developing new strategies to combat metastasis.
AUTHOR CONTRIBUTIONS
Peilan Peng: Conceptualization (equal); writing—original draft (lead); writing—review and editing (equal). Siyuan Qin: Conceptualization (equal); writing—review and editing (equal). Lei Li: Methodology (lead); writing—review and editing (equal). Zhijun He: Supervision (equal). Bowen Li: Supervision (equal); writing—review and editing (equal). Edouard C. Nice: Methodology (equal); writing—review and editing (equal). Li Zhou: Writing—review and editing (lead). Yunlong Lei: Writing—review and editing (lead). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
All images are created in whole or in part using BioRender (www.biorender.com), and they have all obtained licenses. This work was supported by the National Key Research and Development Project of China (2023YFC3402100), the Guangdong Basic and Applied Basic Research Foundation (2019B030302012), the National Natural Science Foundation of China (81821002, 82130082, 82341004, 82403105, 82303838), 1⋅3⋅5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD22007), the Natural Science Foundation of Chongqing (CSTB2024NSCQ-MSX0889), Science and Technology Research Project of Chongqing Education Commission (KJQN202400405), and Postdoctoral Research Project, West China Hospital, Sichuan University (2024HXBH110).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.