Cancer stem cells: Signaling pathways and therapeutic targeting
Abstract
Cancer stem cells (CSCs) constitute a minority cell population characterized by unbounded proliferative potential in both solid and hematological cancers. Despite sharing key stem cell attributes, CSCs possess unique traits, including the initiation and propagation of tumors and resistance to conventional therapies. The purpose of this review is to delve into the origins and fundamental characteristics of CSCs, emphasizing their role in tumor growth and metastasis. The focus extends to unraveling cellular signaling pathways driving oncogenic processes and understanding aberrant cellular crosstalk crucial for targeted cancer therapies. Beginning with an exploration of CSC properties and behavior, we progress to dissecting the cellular signaling network that fuels oncogenic pathways. The discussion spans the inception of CSCs, their survival strategies, and adaptation to new environments. We then transit to recent therapeutic advancements targeting CSCs, culminating in an exploration for precise therapeutic targeting. This review henceforth, underscores the vital significance of comprehending CSCs in cancer progression and treatment resistance. By unraveling the complex signaling pathways and survival mechanisms unique to CSCs, it paves the way for targeted therapeutic strategies that hold immense promise in enhancing cancer treatment efficacy while minimizing collateral damage.
Graphical Abstract
The activation of several critical signalling pathways cooperatively stimulates the cellular and molecular characteristics of cancer stem cells (CSCs). This is characterized by features like stemness, self-renewal, recurrence, and therapy resistance. Treatments are usually based on either blocking or dysregulating the signalling pathways, thereby inducing CSC death in several cases.
1 INTRODUCTION
Identified by John Dick in acute myeloid leukemia (AML), cancer stem cells (CSCs) are defined as cancer-origin cells possessing similar characteristics to the normal stem cells (NSCs).1 Later, isolated from the adult colon, breast, brain, skin, lung, pediatric cancer, oral squamous carcinoma, and neuroblastoma,2 these CSCs share the common self-renewal property as the NSCs. But, unlike NSCs, the CSCs progeny rarely undergo terminal differentiation and lack canonical mechanisms for cell maturation 2 due to deregulated epigenetic state.3 They seems to escape senescence, owing to the ability to extend their telomeres through aberrant telomerase activation.4
CSCs can be found in structural niches that provide regulation and maintenance cues (both intrinsic and extrinsic) to the “cellular hierarchy.”5 Stem cells occupy the top niche, terminally differentiated cells occupy the bottom, and transit-amplifying cells are positioned between the extreme niches.5 Several theories argue that CSCs are not exclusive to the top of the niche due to their differentiation and trans-differentiation abilities. Instead, their place in the niche is dynamic, implying CSCs can become specialized cancer cells and regain their stemness.6 This dramatically decreases their susceptibility to chemotherapy.7 CSCs are thus defined as unspecialized eukaryotic cells with high resistance to apoptosis and propagate hierarchically for rapid tumor formation8, 9 (Figure 1). These issues of CSCs origin are still debatable.
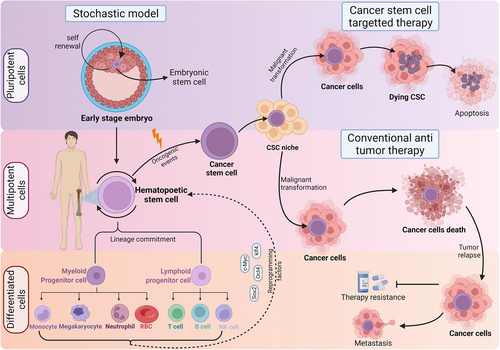
Since the origin of CSCs still remains debatable this review highlights the importance of understanding CSCs' role in cancer development and resistance to treatment. Deciphering their signaling pathways and survival mechanisms enables focused therapies, potentially improving treatment effectiveness with minimal damage to healthy cells.
2 IDENTIFICATION AND CHARACTERIZATION OF CSCS
-
Within the tumor, only a small fraction of cancer cells can exhibit tumorigenic potential when xeno-grafted in immune-deficient mice.
-
Specific distinctive surface markers are present for separating CSCs from the rest of the non-CSC population.
-
The tumors resulting from the CSCs contain both tumorigenic and nontumorigenic cells.
-
With self-renewal property, the CSCs subpopulation form tumors when serially transplanted through multiple generations.
The CSCs are hence proposed to persist in tumors as a distinct population, causing relapse and metastasis. Therefore, identifying these CSCs using specific surface markers followed by developments of targeted therapeutics is required to identify early prognosis and improve the quality of cancer patients lives.13
2.1 Cancer markers and isolation methods
2.1.1 Surface markers
-
Cluster of Differentiation 44 (CD44)—the large cell surface glycoprotein involved in cell adhesion and migration, which has been recognized as one of the CSC markers.14
-
CD133, another transmembrane glycoprotein, specifically localizes to cellular protrusions and is involved in cell growth and development.15
-
CD271, a marker for mesenchymal stem cells (MSCs)16 has also been highly expressed in xenograft squamous cell carcinoma of the head and neck (SCCHN)17 and human gastrointestinal tract cancer progression.18
-
T-cell receptor alpha locus-1-60 (TRA-1-60) is associated with prostate cancer,19 and stage-specific embryonic antigen-1 (SSEA-1) or CD15 is expressed in human glioblastoma (GBM) and human colonic adenocarcinoma.20, 21
-
CD24 has been studied as an independent prognostic marker for ovarian cancer and non-small lung cancer.22, 23
-
CD117 has been specified as a leukemia CSC marker, oral squamous cell carcinoma ovarian tumors, and gastrointestinal stromal tumor.24-26
-
Various CSC markers have been proposed with slight alterations in protein composition and structure in breast, prostate, brain, lung, esophageal, pancreatic, colon, head, and neck cancers27, 28 (Details in Table 1).
| CSC surface marker | Expression of CSCs in specific cancers | References |
|---|---|---|
| CD44 | Breast, colon, glioma, osteosarcoma, head, and neck, bladder, pancreatic, and prostate cancer, including leukemia | [29] |
| CD133 | Colorectal carcinoma | [18-21] |
| CD271 | Head and neck carcinoma, gastrointestinal tract cancer | [23, 30] |
| TRA-1-60 | Prostate cancer | [24] |
| SSEA-1 | Human glioblastoma and human colonic adenocarcinoma | [25, 26] |
| CD24 | Ovarian cancer and nonsmall lung cancer | [22, 31, 32] |
| CD117 | Leukemia, oral squamous cell carcinoma ovarian tumors, and gastrointestinal stromal tumor | [27, 33-37] |
| ALDH-1 | Breast cancer, colorectal cancer, esophageal squamous cell carcinomas, head and neck squamous cell carcinoma | [38-42] |
| ABCG-2 | Lung carcinoma, breast cancer, pancreatic carcinoma | [43-47] |
| Oct-4 | Prognostic brain, lung, bladder, prostate, ovarian, testicular, renal tumors, esophageal squamous cell carcinoma (ESCC) and Leukemia | [48] |
| Sox-2 | Brain, breast, lung, liver, testicular, prostate tumors, squamous cell carcinoma. gastric carcinoma, stage I lung adenocarcinoma, ovarian cancer, and small cell lung carcinoma | [49] |
| Nanog | Human germ-line cancers, colorectal gastric, lung, ovarian, and liver cancer | [50] |
| KLF4 | Colon, head, and neck squamous cell carcinoma, breast cancer, nasopharyngeal, and oral cancers | [51] |
| c-Myc | Breast, brain, colon, pancreas, salivary glands, head, and neck, ovarian, testis cancers, lymphoma, Leukemia, Hepatocellular carcinoma and uterine cervix early carcinoma | [41, 42, 52-55] |
| SALL4 | Acute and chronic myeloid leukemia, hepatocarcinoma, gastrointestinal, colorectal, breast, precursor B-cell lymphoblastic lymphoma glioma and lung cancers | [50] |
| BMI-1 | Breast cancer and oligodendroglial tumors | [56-58] |
| Nestin | Pancreatic, glioma and nonsmall cell lung carcinoma | [59-61] |
| CXCR12/CXCR4 | Gastrointestinal carcinoma | [62-65] |
2.1.2 Functional assays
The golden standard for CSCs identification is the Xeno-transplantation assay, which is used to assess the self-renewal and tumorigenic potential of the CSC population. CD34+ and CD38− were the first population of cell surface markers associated with leukemic stem cells isolated from human Leukemia, which were capable of regenerating the disease when induced by nonobese diabetic mice with severe combined immunodeficiency disease (NOD/SCID mice).66 Elevated aldehyde dehydrogenase (ALDH) activities in cells isolated from solid malignancies have also been found to have increased stem-like properties.
2.2 Characterization of CSCs
2.2.1 Self-renewal and differentiation protocol
CSCs exhibit self-renewal and tumorigenic potential along with Yamanaka Factors- Octamer-Binding Transcription Factor 4 (Oct-4), Sex-Determining Region Y-Box 2 (Sox-2), Krüppel-like factor 4 (Klf-4), Nanog Homeobox (Nanog), and Spalt-Like Transcription Factor 4 (SALL-4), associated with the core embryonic stem cells (ESCs) regulatory network self-renewal and maintenance.67 c-Myc is another important transcriptional factor (TF) that has been expressed in stem cells and overexpressed in several cancers.67 High expression of Oct-4 has been reported in poor prognostic brain, lung, bladder, prostate, ovarian, testicular, renal tumors, esophageal squamous cell carcinoma (ESCC), and leukemia as well.68 Sox-2 has been found in the brain, breast, lung, liver, testicular, and prostate tumors.68 Poor prognostic squamous cell carcinoma. gastric carcinoma, stage I lung adenocarcinoma, ovarian cancer, and small cell lung carcinoma further showed elevated levels of Sox-2.49 The Nanog promotes the epithelial–mesenchymal transition (EMT), an important feature of cancer cells' development to stem-like feature69 that has been reported to be elevated in human germ-line cancers.70 Further, overexpression of Nanog has been predicted in tumor progression and poor prognoses in colorectal gastric, lung, ovarian, and liver cancer.71 Klf-4, with diverse physiological functions including proliferation, differentiation, and development, has been reported to be a prognostic predictor of colon and head and neck squamous cell carcinoma.72, 73 SALL4 plays a crucial role in ESCs pluripotency and early embryonic development, serving as an integral component in the ‘stemness’ regulatory circuit alongside Oct-4, Sox-2, Nanog, and other factors. Its essential function lies in maintaining the self-renewal and pluripotency of embryonic stem cells. Conversely, in hematopoietic malignancies, abnormal reactivation of SALL4 expression is observed, correlating with worsened disease status in patients. Notably, SALL4 activation is implicated in the pathogenesis of tumor initiation and disease progression in these malignancies.74
2.2.2 Tumorigenicity and resistance to therapy
In the realm of tumorigenicity and resistance to therapy, various molecular targets have emerged. CD133, for instance, presents itself as a promising therapeutic target in metastatic melanoma, ovarian cancer, and colon cancer.62, 63, 75 It has also demonstrated utility as a target for antibody-drug delivery in gastric and hepatocellular carcinomas.64 ALDH1, on the other hand, has been actively pursued as a therapeutic target in non-small cell lung cancer and ovarian cancer.65, 76, 77 Similarly, Nestin emerges as a potential target for tumor angiogenesis,78, 79 and Musashi-1, a diagnostic lung cancer marker.79 The disruption of stroma-tumor cell interaction and the reduction of tumor growth and metastasis have been demonstrated using CXCR4 antagonists. CXCR4 is considered a therapeutic target for lung,80, 81 and breast cancer,82, 83 offering potential applications in noninvasive monitoring of disease progression and therapeutic guidance.84 Various CSC markers are associated with different cancers and their progression. c-Myc the oncogene has been reported to be overexpressed in 70% of human cancers, including breast, brain, colon, pancreas, salivary glands, head, and neck, ovarian, testis cancers, lymphoma, and leukemia.68, 85, 86 Poor prognosis in Hepatocellular carcinoma and uterine cervix early carcinoma has also been corelated with c-Myc.87-89 In conclusion, the detection of expression levels of these factors using various methodologies holds promise for early diagnosis, classification, and the development of targeted therapeutic strategies.67
2.2.3 Molecular and genetic profiles
SALL-4 can be one of the few genes that might establish this link (reviewed in Tatetsu et al.52). Several epigenetic studies and cellular model studies and cellular model studies have shown the oncogenic role of SALL-4 in several tumors like acute and chronic myeloid leukemia, hepatocarcinoma, gastrointestinal, colorectal, breast, precursor B-cell lymphoblastic lymphoma glioma, and lung cancers50 (Table 1). Elevated ALDH1 expression has been reported in prognostic significance of oesophageal squamous cell carcinomas (OSCCs) and head and neck squamous cell carcinoma (HNSCCs).90, 91 BMI1 is a protein required for hematopoietic stem cell (HSC) self-renewal and NSCs.92 Nestin and Musashi-1 both play a significant role in NSC self-renewal, and maintenance has been reported to be elevated in various malignancies.93 Chemokine CXCL12 (SDF1-alpha) and its receptor CXCR487 interaction play an important role in HSC migration. Overexpression of CXCR4 has been reported in several tumors and is associated with angiogenesis, recurrence, and therapeutic resistance.59, 94
These surface markers, stemness-related gene expression, and tissue factor expression levels may be useful for accessing patient prognosis.68 Detection of these factors expression levels by various methodologies might help in early diagnosis, classification, and targeted therapeutic strategies. These CSCs are maintained by several signaling pathways. Many of these pathways are nonlinear and interwoven networks of signaling mediators that feed on one another through crosstalk.82 De-annihilating this crosstalk is essential for carrying forward successfully targeted therapeutics.
3 SIGNALING PATHWAYS ASSOCIATED WITH CSCS
Signaling pathways that contribute to self-renewal, proliferation, survival, and differentiation in NSCs are abnormally either repressed or activated in the CSCs. These signaling pathways are not a single regulator but are intricate, with many intrinsic and extrinsic molecular signals and regulatory elements that regulate the growth and survival of the CSCs. The list of dysfunctional signaling pathways mainly includes—the Janus-activated kinase/signal transducer and activator of transcription (JAK/STAT), Hedgehog, Wnt, Notch, phosphatidylinositol 3-kinase/phosphatase, and tensin homolog phosphatidylinositol-3-kinase/protein kinase B/the mammalian target of rapamycin (PI3K/Akt/mTOR), nuclear factor-κB (NF-κB), transforming growth factor (TGF)/Smad signaling pathway in CSCs, signaling pathway in CSCs and peroxisome proliferator-activated receptors (PPAR) signaling pathways in CSCs.83, 95, 96
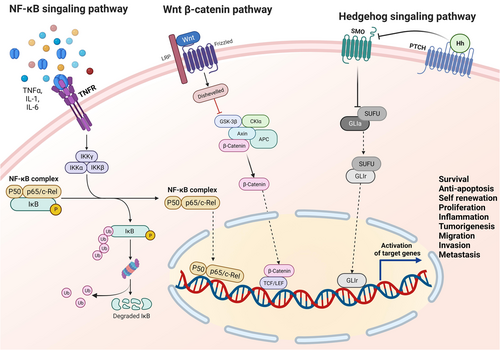
3.1 Wnt pathway
The Wnt/β-catenin pathway is highly evolutionary conserved, comprising of 19 Wnt ligands and more than 15 receptors.97 The Wnt ligands signal through β-catenin for their biological functions during embryonic development and tissue homeostasis98 and are known to regulate the CSC pluripotency (reviewed well in Bao and colleagues96, 99). This pathway, critical for self-renewal, dedifferentiation, apoptosis inhibition, and metastasis of CSCs,100 presents an attractive target for therapeutic interventions aiming to disrupt CSC maintenance and progression.
3.1.1 Implications for CSC maintenance
In the tumor microenvironment (TME), Wnt proteins and inhibitors like bone morphogenetic protein (BMP), and Delta are produced, activating CSC self-renewal capabilities.101 Proto-oncogenes, such as c-Myc, along with downstream upregulation of Cyclin D1, have been reported to induce the transformation of dormant CSCs into active CSCs through Wnt pathway activation in colorectal cancer.99, 102, 103 The Wnt pathway has been shown to be highly associated with the CD44+/CD133+ isolated colorectal CSCs as well.104 In addition to colorectal cancer, canonical Wnt signaling is also involved in maintaining other CSCs types.
3.1.2 Interplay of Wnt signaling and cellular communication network factor 5 (CCN5) (WISP-1) in the regulation of cancer stemness
CCN5, also known as Wnt-1 inducible signaling pathway protein 2 (WISP-2), is a member of the CCN family of proteins.105, 106 CCN proteins are involved in various cellular processes, including cell proliferation, differentiation, adhesion, and angiogenesis.107, 108 The CCN5 family of proteins, including CCN1 (Cyr61) and CCN5 (WISP-2), have been identified as modulators of stemness in breast cancer.109, 110 In breast cancer, CCN5 has been found to suppress the self-renewal and stem-like properties of CSCs, though some studies suggest that CCN5 may act as a tumor suppressor in breast cancer.10, 111 CCN5 seems to achieve this by downregulating key signaling pathways associated with cancer stemness, such as the Wnt/β-catenin pathway, which is known to play a role in maintaining CSCs.11, 112 CCN5 may also influence EMT, a process that is linked to cancer stemness and metastasis.113, 114 CCN5 has been reported to inhibit EMT in certain cancer cell lines, potentially reducing their stem-like properties.12 Understanding the role of CCN5 in regulating cancer stemness has clinical implications. It may lead to the development of targeted therapies aimed at enhancing CCN5 expression or activity to inhibit CSCs and reduce tumor aggressiveness. However, it is essential to note that the role of CCN5 in cancer stemness may be context-dependent and could vary among different cancer types and subtypes. Research on CCN5's specific role in regulating CSCs is still underway, and its mechanisms in different cancer contexts are still being elucidated. In summary, further research is needed to fully understand the molecular mechanisms underlying CCN5's impact on cancer stemness and its potential as a therapeutic target in cancer treatment in general and breast cancer in particular.
3.2 Notch pathway
This pathway is essential for angiogenesis, stem-cell fate determination, and cell–cell communication between adjacent cells through the transmembrane ligands and receptors. Discovered first in Drosophila back in 1917, mammals have four Notch receptors (Notch 1-4) and five Notch ligands (Delta-like 1, 3, and 4, Jagged 1, and Jagged 2).115 Dysregulation in this pathway is associated with chemoresistance, EMT induction, and the acquisition of a stem cell-like phenotype.67, 116 In pancreatic cancer and lung carcinoma, these Notch pathway components have been found to be upregulated.117, 118
Notch 1 and Notch 4 have been reported to promote self-renewal and metastasis in CD44+/CD24low isolated breast cancer cells in vitro and in vivo.116 The actin-related protein 2/3 complex has been found to maintain the stem-cell phenotype of glioma-initiating cells by activating the Notch 1 pathway.117 The Cargo Protein MAP17 (DD96, PDZKIP1), a nonglycosylated membrane-associated protein located in the Golgi apparatus and plasma membrane, has also been found to activate the Notch Signaling Pathway in cervical CSCs.118 Inducible nitric oxide (iNOS) has been associated with aggressive hepatocellular carcinoma. A study showed that this iNOS/NO promoted self-renewal capability through activation of Notch 1 signaling pathway in CD133+/CD24− liver CSCs in vitro by activating the Notch 1 signaling pathway in CD133+/CD24− liver CSCs in vitro as well as mouse xenograft tumor model.119 Even tumor necrosis factor-α (TNF-α) enhances the CSC-like phenotype in oral squamous cell carcinoma (OSCC) by Notch 1 signaling pathway activation. BRCA 1, the key regulator in breast cancer, has been seen to upregulate Notch ligands and receptors and maintain the stemness of breast CSCs.120 These findings indicate that Notch is important for regulating CSC self-renewal, growth, and metastasis.
3.3 Hedgehog (Hh) pathway
This pathway is considered a major regulator in vertebrate embryonic development, playing crucial roles in EMT, stem cell maintenance, tissue polarity, cell proliferation, and differentiation.121 This pathway's emerging role maintains self-renewal capabilities in both solid and liquid malignancies, with higher component levels in CSCs.96, 122-124 This network is relatively complex, comprising of the extracellular ligands (Desert Hh, Sonic Hh, and Indian Hh), transmembrane protein receptor PTCH (PTCH1, PTCH2), the transmembrane protein SMO, intermediate transduction molecules, and the downstream molecule Gli (Gli 1-3).122-124
In multiple myeloma CSCs, there is a higher expression of Gli1 and SMO in comparison to the non-CSCs depicting the activation of Hh signaling by the Hh ligands.125 Moreover, the higher activation of Hh signaling components has been observed in the human lung squamous cell carcinoma CSCs and glioma CSCs.126, 127 The Hh pathway activation has been observed to be involved with maintaining CML-CSCs in a murine CML model. In contrast, overexpression of SMO in an SMO-deficient mice CML model enhanced CML CSC expression to fourfold resulting in SMO deletion significantly reducing the CML CSCs.128 This signaling pathway is believed to drive the CSC phenotype in cancer by regulating the stemness-determining genes Nanog, Oct-4, Sox-2, Bmi-1, ALDH, and c-Myc.129-133 This pathway is henceforth associated with self-renewal, invasion, chemoresistance, and tumorigenesis of CSCs.129, 134, 135
3.4 JAK/STAT pathway
Studied first in Drosophila model, this signaling controls stem cell maintenance in the male germline stem cell microenvironment.136-138 This pathway is evolutionarily conserved. This pathway is also activated in CSCs.83, 139
Triggered by both cytokines and interferons, this pathway uses a novel mechanism where the cytosolic TFs termed as signal transducers, and activators of transcription (STATs) are tyrosine phosphorylated by the JAKs, allowing STAT protein dimerization and nuclear translocation. This rapidly allows the transduction of an extracellular signal into the nucleus that can modulate the downregulation or upregulation of the targeted gene.140 Seven STAT family members have been identified: STAT1-4, STAT5a, STAT5b, and STAT6. The JAK protein comprises four members: JAK1-3 and Tyk2.83
IL-10 has been reported to induce self-renewal, migration, and invasion in non-cell lung carcinoma.141 In endometrial carcinoma, IL-6 has been reported to activate the JAK1/STAT3 pathway in ALDHhigh/CD126+ CSCs.142 IL-6 and erythropoietin have been reported to activate the CD44+/CD24− CSC population in colorectal and breast cancer through JAK2/STAT3 pathway.143-146 In High-grade gliomas, a hypoxia-induced pathway that utilizes the hypoxia-inducible factor 1α (HIF-1α) TF and the JAK1/2-STAT3 axis has been reported to enhance the self-renewal capability of glioma stem-like cells.147 Thus, the role of JAK/STAT pathway signaling is linked with the survival, self-renewal, and metastasis of CSCs (Figure 2).
3.5 PI3K/Akt/mTOR pathway
The PI3K/Akt is an intracellular phosphatidylinositol kinase, and mTOR signaling pathways are crucial to stem cell proliferation, metabolism, and differentiation.67, 148 They consist of the regulatory subunit p85 and catalytic subunit p110, which has serine/threonine (Ser/Thr) kinase and phosphatidylinositol kinase activities.134 There are three isoforms of Akt (Akt1-3), which are crucial effectors of PI3K and directly activated in response to PI3K.135 mTOR complex is directly downstream target genes with two multiprotein complexes (mTORC1 and mTORC2).148 mTORC2 phosphorylates Akt at serine residue 473, which leads to a full Akt activation.149 Although abnormal activation of PI3K/Akt/mTOR pathway has been studied in several cancer forms, like breast cancer, prostate cancer, esophageal adenocarcinoma, colorectal cancer, and several other cancer forms136-138 yet few studies have been done on and it's linked with CSCs.149 To state a few examples, this pathway has been found to be involved in the EMT in chemoresistant epithelial ovarian cancer cells.137 In pancreatic and prostate cancer, migration and invasion of CSCs resulted from the activation of this signaling pathway.150, 151 CD133+/CD44+ prostate CSCs showed maintenance of stemness and tumorigenicity on activation of PI3K pathway activation.152 This signaling pathway has also demonstrated activation of ALDH+/CD44+ head and neck CSCs with proliferative and migration capability.150 Activation of mTOR promoted the survival and proliferation of breast CSCs and nasopharyngeal CSCs.146, 151 In colorectal carcinoma, mTORC1 activation promoted ALDH1 activity, activating colorectal CSCs.153 EpCAM (epithelial cellular adhesion molecule) and tumorigenicity in hepatocellular CSCs is increased on activation of mTORC2.154 Hence a link between PI3K/Akt/mTOR pathway and CSCs is clearly evident.
3.6 NF-κB signaling pathway
The NF-κB is a rapidly inducible TF with five different proteins (p65, RelB, c-Rel, NF-κB1, and NF-κB2). The main physiological function of NF-κB is the p50-p65 dimer.155, 156 The complexes’ activity is regulated by two major pathways (canonical NF-κB signaling and noncanonical NF-κB signaling) (Discussed in detail in Yang et al.83). Several cytokines, angiogenic and growth factors, and proteases are responsible for NF-κB signaling during tumor development and progression.157 Cytokines contributing to tumor-promoting inflammation, such as—IL-1, IL-6, TNF-α, MCP1, iNOS, and COX2 and other factors like Cyclin D1, Cyclin E, proto-oncogene c-Myc are responsible for NF-κB pathway activation leading to the proliferation of the cancer cells.158 NF-κB promotes apoptosis inhibition by transcriptional regulation of the cellular inhibitor of apoptosis (CIAPs) 1,2 and XIAP, as well as Bcl-2 and Bcl-xL.159, 160 Over-activation of this pathway has been reported in several cancer forms, like pancreatic cancer, melanoma, and ovarian cancer.161-163 Once activated, this NF-κB regulates a variety of genes that significantly overlap with the hallmarks of cancer.164 Further, NF-κB pathway activation further contributes to EMT (Vimentin, Twist) remodeling of the extracellular matrix through angiogenesis induction (IL-8, VEGF), resulting in the promotion of invasion and metastasis (MMP2, MMP9, uPA).165 One of the earliest examples of NF-κB involvement in CSC was found in primary AML samples where the CD34+ AML CSCs exhibited enhanced NF-κB DNA binding activity.166 Later, studies done in the prostate and GBM showed that the CSCs expressed higher levels of acetylated and total p65 marked the elevated NF-κB activation in CSC subsets of tumors.167, 168,p.3 A panel of selected representative proinflammatory signatures had been significantly associated with NF-κB regulation of CSCs in a variety of tumors such as breast, ovarian, GBM, and prostate cancers.169-173 Henceforth, this pathway is essential for regulating inflammation (an important hallmark of cancer progression), self-renewal and maintenance, and metastasis of CSCs.174
3.7 TGF/Smad signaling pathway
This pathway is involved in several cellular processes associated with embryonic development, including cell differentiation, proliferation, apoptosis, and homeostasis.175 TGF-β family is encoded by 33 genes encoding structurally related polypeptides corresponding to ligand precursors.175 One of the key characteristics of both normal and cancerous cells in acquiring stem cell properties is EMT induced by the TGF-β.176, 177 Several studies have revealed the critical role of TGF-β family signaling in CSC maintenance and differentiation in various cancer forms, including breast, squamous cell carcinoma, ovarian cancer, glioma, and leukemia.178-182 These TGF-β superfamily ligands bind to a type II receptor, which recruits and phosphorylates a type I receptor. This type I receptor phosphorylates receptor-regulated Smads (R-Smad), binding to the common pathway Smad (co-Smad). This complex of R-Smad/Co-Smad acts as a TF and accumulates in the nucleus to regulate the target gene expression.175 There are eight Smads (Smad1-8) in vertebrates.183 There are two types of Smad Complexes: AR-Smads activated by TGF-β (Smad2 and 3) and BR-Smad (1, 5, 8, and 9). Co-Smad-4 is a shared medium in various TGF-β signal transduction processes. I-Smads (Smad6 and 7) bind to the activated type I receptors and inhibit or regulate signal transduction of the TGF-β family.183 TGF-β/Smad plays a significant role in the cell proliferation of CSCs. Cyclin D1 activates Smad2/3 and Smad 4, promoting the Cyclin D1-Smad 2/3-Smad 4 signaling regulating the self-renewal capability of liver CSCs.184 TGF-β/Smad signaling induces quiescence mediating chemo-resistivity in squamous cell carcinoma CSCs.185 Upregulation of TGF-β1 induced expression of Smad4, p-Smad2/3, and CD133 in liver CSCs.186 Although there are few studies on the TGF-β/Smad signaling pathway in CSCs, this pathway does play a significant role.
3.8 PPAR signaling pathway
The PPARs are the ligand-activated nuclear TFs, cloned first from mouse liver.187 There are three subtypes of PPARs—PPARα, β, and γ (encoded by the PPARA, PPARD, and PPARG genes, respectively). They are reported to regulate the expression of target genes involved in many cellular functions, including cell proliferation, differentiation, and inflammation.188 PPAR signaling has also been reported to play a pivotal role in tumorigenesis and cancer development. They are reported to be involved in the modulation of the EMT process in CSC initiation and regulation of CSC functions (reviewed well in Kuenzli and Saurat188). PPARs have been closely related to CSC metabolism. In GBM, lung carcinoma, and mouse mammary gland carcinoma, the CSCs are reported to be regulated for metabolic reprogramming by PPARα and PPARβ/δ.189 The transcription coactivator (PPAR gamma-coactivator 1α), also known as PGC-1α, has been found to promote breast CSC proliferation and invasion by enhancing oxidative phosphorylation and mitochondrial biogenesis.190 However, the exact mechanisms still remain unknown.191 These studies so far do suggest that PPARs play an essential role in the growth of CSCs.
3.9 CROSSTALK BETWEEN THE SIGNALING PATHWAYS
As previously mentioned, these complex signal transduction pathways have crosstalk in between them to regulate the CSCs.192 The CSC cell proliferation and survival are maintained when the NF-κB and Wnt/β-catenin signaling work together. β-catenin has been found to regulate proton-oncogene c-Myc, TNFRSF-19 (a member of the TNF receptor superfamily), and its receptor molecules further activate the NF-κB signaling, studied in colorectal cancer.193 NF-κB activation leads to upstream regulation of inflammatory genes, which initiates increased stemness, a key characteristic of CSCs.169, 194 CD146 (biomarker and therapeutic target of clinical significance)195 silencing inhibits NF-κB/p65-initiated GSK-3β expression. This subsequently promotes nuclear translocation, and activation of β-catenin which restores stem cell phenotypes in differentiated colorectal carcinoma cells.76 Wnt/β-catenin and Hedgehog signaling pathways play crucial roles in embryogenesis, stem cell maintenance, and tumorigenesis. A novel mechanism through which the Wnt/β-catenin signaling pathway stimulates the Hedgehog signaling was studied in colorectal carcinoma by Noubissi et al. In this study, the Wnt/β-catenin signaling induced expression of an RNA-binding protein, CRD-BP, that in turn bonded and stabilized Gli1 messenger RNA (mRNA), which caused the elevation of Gli1 expression and transcriptional activity. In turn, this phenomenon promoted the survival and proliferation of colorectal CSCs.196 Even the TGF-β signaling pathway is no exception. One mechanism by which the EMT initiation is initiated in cancerous cells is by removal of the β-catenin by TGF-β from adhering junctions. This procedure involves the TGF-β-dependent PTEN dissociation resulting in β-catenin and Akt activation giving rise to CSC-like phenotypes as studied in colon carcinoma in vivo models.197 A study on CD133+ cells isolated from primary human cutaneous squamous cell carcinoma specimens expressed a repertoire of stemness-related genes, including NOTCH and NOTCH1 mediated NF-κB pathway signaling along with Wnt pathway-related genes, growth receptors, PI3K/mTOR, STAT pathways, and chromatin modifiers as well.198 The JAK/STAT3 pathway is required for TGF-β-induced EMT, cancer cell migration, and invasion via upregulation of p-Smad-3, SNAIL expression. Further, the TGF-β/Smad and IL-6/JAK/STAT3 signal synergistically to enhance EMT in lung carcinomas.199 IL-17E binding to IL-17RB activates NF-κB and JAK/STAT3 pathways that promote proliferation and sustain the self-renewal capability of CSCs in hepatocellular carcinoma (HCC).200 Supporting this importance of crosstalk during EMT, the TGF-β signaling is indirectly regulated by Erk, JAK/STAT, and p38.201 TGF-β plays a central role in mediating EMT by activating the Smad pathway.202, 203 In addition, various accumulating evidence suggests that TGF-β-induced EMT is NF-κB-dependent in various cancer types.204-206 TGF-β/BMP and the signaling pathways of MAPK/Akt, Wnt, Hedgehog, Notch, and the interleukin/interferon-gamma/TNF-α cytokines are also interlinked that play crucial roles in embryonic development and CSC maintenance as well.207, 208 This increasing evidence shows that the crosstalk regulates the self-renewal and metastasis of CSCs (Figure 3). Further, with a growing list of new regulatory factors and targets being identified, it's evident that the pathways are interwoven into a vast signaling network. This crosstalk between pathways can be direct, indirect, unidirectional, or bidirectional and often occurs as a part of a feedback loop. Hence, in-depth mechanistic studies are necessary to identify the specific convergence point of the pathways for the targeted therapeutic approach to the CSCs.
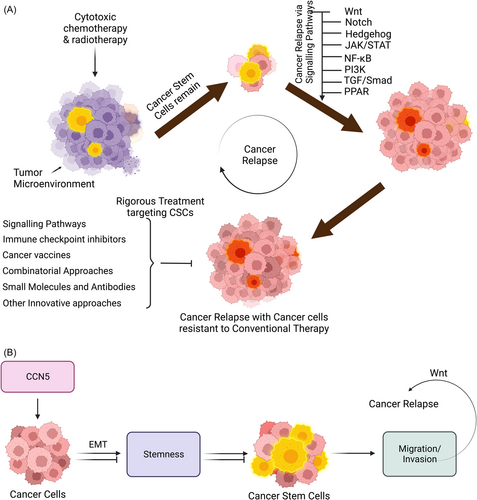
4 TARGETING CSC SIGNALING PATHWAYS
Conventional therapies focusing on cancer as elaborated in several review papers, center on the intricate interplay within the tumor ecosystem and microenvironment. As discussed earlier, the tumor ecosystem and microenvironment regulated CSC proliferation and survival through essential direct cell-to-cell contacts, in which various paracrine factors are secreted. Microenvironmental factors maintain the stemness of CSCs through pathways that promote self-renewal, such as the Wnt/β-catenin, Notch, and Hh pathways. Consequently, disrupting these support systems emerges as a potential strategy to eliminate CSCs210, 211 (refer to Tables 2 and 3 for details).
| Compound | Tumor type | Trial phase and number | References |
|---|---|---|---|
| Agents targeting the Wnt/β-catenin signaling pathway in clinical trials | |||
| LGK974 | Solid malignancies | Phase 1 (Completed): NCT02278133 | [212] |
| ETC-159 | Advanced solid tumors | Phase 1 (Recruiting, Ongoing): NCT02521844 | [213] |
| PRI-724 | Advanced solid tumors | Phase 1b (closed due to low recruitments): NCT01302405 | [214] |
| SM08502 | Advanced solid tumors | Phase 1 (Recruiting, Ongoing): NCT05084859 | [215] |
| CGX1321 | Advanced gastrointestinal tumors | Phase 1 (Recruiting, Ongoing): NCT03507998 | [216] |
| GNF6231 | Breast carcinoma | Preclinical | [217] |
| OMP-54F28 in Combination with Paclitaxel and Carboplatin | Recurrent platinum-sensitive ovarian cancer | Phase 1 (Completed): NCT02092363 | [218] |
| Niclosamide | Prostate cancer, colorectal cancer | Phase 2 & Phase 1 (Recruiting, Ongoing): NCT03123978, NCT03521232 | [219-221] |
| ONC201 | Glioblastoma | Phase 2 (Recruiting, Ongoing): NCT02525692 | [222] |
| CWP232291 | Myeloma | Phase 1b (Completed): NCT02426723 | [223] |
| DKN-01 | Multiple myeloma | Phase 1/2 (Completed): NCT01457417 | [224] |
| Agents targeting the Notch signaling pathway in clinical trials | |||
| MK-0752 | Breast cancer | Phase 1/2 (Completed): NCT00645333 | [225] |
| LY-900009 | Advanced cancer | Phase 1 (Completed): NCT01158404 | [226] |
| RO4929097 | Advanced nonsmall cell lung cancer | Phase 2 (Terminated, because of in-appropiate number of subjects): NCT01193868 | [227] |
| PF-03084014 | Desmoid tumors/aggressive fibromatosis | Phase 1 (active): NCT01981551 | [228] |
| Agents targeting the Hedgehog signaling pathway in clinical trials | |||
| Glasdegib | Newly diagnosed acute myeloid leukemia or high-grade myelodysplastic syndrome | Approved | [229] |
| LDE225 (Sonidegib) | Triple-negative (TN) advanced breast cancer (ABC) Patients | Phase 1 (Completed): NCT02027376 | [230] |
| Vismodegib | Triple-negative breast, pancreatic, and colorectal carcinoma | Phase 2: NCT02694224, NCT01064622, NCT00636610 | [231, 232] |
| Agents targeting the JAK/STAT signalling pathway in clinical trials | |||
| Ruxolitinib | Metastatic pancreatic ductal adenocarcinoma, breast cancer, colorectal cancer, nonsmall cell like lung cancer, gliomas | Completed: NCT01423604, NCT01594216, NCT02145637, NCT03514069 |
[233] |
| WP1066 | Recurrent malignant glioma | Phase 1: NCT01904123 | [233] |
| AZD4205 | Nonsmall cell like lung cancer | Phase 2: NCT03450330 | [233] |
| Agents targeting the PI3K/Akt/mTOR pathway in clinical trials | |||
| BEZ235 | Refractory acute leukemia | Phase 1 completed: NCT01756118 | [234] |
| OSI-027 | Advanced solid tumors | Phase 1 completed: NCT00698243 | [235] |
| Everolimus | Refractory malignant brain tumors | Phase 1 completed: NCT03387020 | [236] |
| Agents targeting the NF-κB pathway in clinical trials | |||
| Bortezomib | Metastatic neuroendocrine tumors | Phase 2: NCT00017199 | [237] |
| Curcumin | Invasive breast cancer | Phase 1: NCT03980509 | [238] |
| Agents targeting the TGF-Smad pathway in clinical trials | |||
| R1-Ki | Mammary tumor | Preclinical | [28] |
| SB431542 | Melanoma | Preclinical | [28] |
| Vactosertib | Solid tumors, colorectal cancer, nonsmall cell like lung cancer, urothelial cancer | Phase 1/2: NCT02160106, NCT03724851, NCT03732274, NCT04064190 | [28] |
| Agents targeting the PPAR pathway in clinical trials | |||
| Pioglitazone | Head and neck cancer | Phase 2a: NCT00099021 | [239] |
| Troglitazone | Liposarcoma | Phase 2: NCT00003058 | [240] |
| Attempts to target some CSCs | ||||
|---|---|---|---|---|
| Target | Compounds and drugs | Cancer type | Trial phase and number | References |
| ALDH1 | Nifurtimox Cyclophosphamide Topotecan |
Relapsed neuroblastoma or medulloblastoma | Phase II (not-recruiting): NCT00601003 | [241] |
| Chemotherapy +/− disulfiram | Nonsmall cell lung carcinoma | Phase II/Phase III: NCT00312819 | [242] | |
| CD47 | AO-176 AO-176 + Paclitaxel AO-176 + Pembrolizumab |
Multiple solid tumor malignancies | PhaseI/Phase II (recruiting): NCT03834948 | [243] |
| CC-90002 | AML & myelodysplastic syndromes | Phase I (Terminated): NCT02641002 | [244] | |
| Hu5F9-G4 | Solid tumor | Phase I (completed): NCT02216409 | [245] | |
IBI188 Azacitidine Decitabine |
AML | Phase I/Phase II (recruiting): NCT04485052 | [246] | |
| CD44 | RO5429083 | Neoplasms | Phase 1: NCT01358903 | [247] |
| RG7356 | AML | Phase I: NCT01641250 | [247] | |
| Hyaluronic acid-conjugated Chemotherapeutics | Ovarian, prostate, breast cancer | Preclinical | [248-250] | |
| IL-3Rα | TAGraxofusp | Blastic plasmacytoid dendritic neoplasm | Completed: NCT04317781 | [251] |
| EGFR and c-Met | Amivantamab Lazertinib Carboplatin Pemetrexed Direct oral anticoagulant (DOAC) Low molecular weight heparin (LMWH) |
NSCLC | Phase II: NCT05498428 | [252] |
| CD19 | Tisagenlecleucel Lymphodepleting Chemotherapy Bridging Therapy |
Pediatric non-Hodgkin lymphoma | Completed: NCT03610724 | [253] |
4.1 Limitations of conventional treatments on CSCs
Conventional treatments for malignant diseases, including surgery, chemotherapy, and radiotherapy, constitute the traditional “three pillars” of cancer therapy. Integrating immunotherapies with these conventional treatments has emerged as a crucial strategy in cancer therapy. Conventional treatments like radiotherapy and chemotherapy have been found to possess additional immune activation mechanisms, such as depleting immunosuppressive Tregs and MDSCs and releasing tumor antigens, which are recognized and presented to T lymphocytes by antigen-presenting cells.254, 255
CSCs, recognized as tumor-initiating cells within a tumor, possess unique properties, including self-renewal and the ability to differentiate into various cell types, and are believed to play a significant role in cancer initiation, progression, recurrence, and resistance to traditional cancer treatments such as chemotherapy and radiation therapy.256, 257 They create an immunosuppressive TME through interactions with various cells, including regulatory T cells (Tregs), tumor-associated neutrophils (TANs), and myeloid-derived suppressor cells (MD-CSs).258 The TME, characterized by factors like pH and hypoxia, also contributes to immune evasion.259, 260 Preclinical studies have emphasized the significance of combination therapies for treating CSCs. For example, combining a DNA methylation inhibitor (AZA) with CD47 blockade via 5F9 mAb increased macrophage-mediated phagocytosis, inhibited AML growth, and improved survival in xenograft mice models.254 Clinical studies have validated this approach, demonstrating higher complete response rates and reduced leukemic stem cell levels in AML patients receiving the combination therapy.261 Similarly, combining cirmtuzumab and ibrutinib, targeting receptor tyrosine kinase-like orphan receptor 1 (ROR1) and B-cell receptor signaling, respectively, was more effective in reducing CLL cells in preclinical models and showed promising results in clinical trials.262 Furthermore, small-molecule inhibitors targeting fat mass and obesity-associated protein not only suppress AML stem cell self-renewal but also inhibit immune checkpoint expression and immune evasion, highlighting the broad potential of anti-CSC therapy.255 The transient nature of stemness and the potential for non-CSC populations to dedifferentiate and replenish CSCs support the rationale for combining conventional therapies with CSC-targeting treatments to enhance therapeutic efficacy. Although the potential of immunotherapy in targeting CSCs is promising, challenges remain, including identifying CSC-specific markers and overcoming the immunosuppressive microenvironment often associated with CSCs.263 Further research is needed to refine and optimize immunotherapeutic approaches for effectively eradicating CSCs and improving overall cancer treatment outcomes.
4.2 Targeting CSCs
4.2.1 Immune checkpoint inhibitors
CSCs employ strategies like downregulating tumor-associated antigens, upregulating immune checkpoint proteins like PD-L1, and reducing major histocompatibility complex class I (MHC-I) expression to escape immune surveillance.264-276
Significant examples are: AML stem cells suppress T-cell function through CD200 receptor overexpression.277 In head and neck squamous cell carcinoma (HNSCC), PD-L1 is significantly overexpressed in CD44+ CSCs.267 PD-L1 also regulates CSC stemness in breast cancer.271 CSCs transition to a dormant state, reduce immunogenicity, and avoid immune surveillance. Circulating tumor cells (CTCs), similar to CSCs, evade natural killer (NK) cell cytotoxicity through interactions with tumor-associated neutrophils (TANs).278, 279 MHC-I expression on normal cells inhibits NK cell activation, but some CSCs upregulate MHC-I, potentially influencing NK-cell regulation.280, 281 Immunotherapies, including cancer vaccines, adoptive T- and NK-cell therapies, monoclonal antibodies, bispecific antibodies (bsABs), and immune checkpoint inhibitors (ICIs), hold promise in targeting CSCs.282 However, targeting CSCs is challenging due to shared signaling pathways with NSCs.283-285 Cell-surface targets for CSCs have gained attention, with markers like CD44, CD133, CD117, CD123, CD47, CD98hc, and others identified.286 CD44v6, a CD44 isoform, has been targeted with anti-CD44v antibodies but faced skin toxicity issues.287 CD47 is overexpressed on cancer cells, including CSCs, promoting immune evasion.288, 289 CD47-targeted therapies, including IBI188 and HX009, are in clinical trials.290 CD123, highly expressed on cancer cells, particularly in hematological malignancies, is targeted by Talacotuzumab (JNJ-56022473) and IMGN632.291 TAGraxofusp, an IL-3Rα-targeted therapy, is approved for blastic plasmacytoid dendritic neoplasms (BPDCN).292 Combining CD123 and CD3 in bsABs, like Flotetuzumab, shows promise in AML.293 As such the bsABs are engineered to have dual specificity for different epitopes. CD3/T cell receptor (TCR)-engaging bsABs redirect T cells to tumor cells. BsABs targeting CD47 and PD-1/PD-L1 are in trials.294 Amivantamab, targeting EGFR and c-Met, is approved for NSCLC with EGFR ex20-ins mutations.295
4.2.2 Cancer vaccines
These encompass dendritic cell vaccines and peptide vaccines, that are designed to train the immune system to recognize and attack CSCs.296-298
Adoptive cell therapy (ACT)
In contrast to readily available antibody-based immunotherapy, ACT stands as a promising strategy to enhance immune responses against CSCs. It involves using CSCs as a source of antigens to stimulate antigen-presenting dendritic cells (DCs) and develop DC-based vaccines targeting CSCs. DCs play a pivotal role in bridging innate and adaptive immune responses by presenting processed epitopes to CD4+ and CD8+ T cells via MHC II and MHC I, respectively, while also secreting cytokines crucial for T-cell survival, proliferation, and tumor infiltration.299 DC vaccination has shown promise in various cancer types. It involves the ex vivo differentiation of autologous precursor cells into immature DCs, maturation with cytokines, and pulsing with cancer antigens, such as antigen peptides, tumor cell lysates, exosomes, or mRNAs, followed by administration to patients, resulting in antigen-specific T-cell activation.299 Numerous clinical studies have affirmed the safety and efficacy of DC vaccinations in cancer therapy, inducing immune responses, increasing tumor-infiltrating lymphocytes, and improving overall survival.300, 301 Additionally, a specific approach targets ALDH peptides in DC-based vaccines, as ALDH enzymes, particularly ALDH1A1 and ALDH1A3, are overexpressed in CSCs and play crucial roles in tumor development and therapy resistance.302-304 DCs loaded with ALDH epitope peptides have demonstrated the ability to inhibit tumor growth and metastasis, especially when combined with immune checkpoint inhibitors (ICIs) like anti-PD-L1 therapy.304 Utilizing the entire tumor cell lysate, which includes a wide range of tumor antigens, for pulsing DCs is another promising approach for CSC-specific DC vaccines. High ALDH activity in CSCs can be used to identify and isolate these cells, providing a source of antigens for developing CSC-targeted therapies.305 Furthermore, mRNA derived from CSCs have shown efficacy in DC vaccines, inducing a potent cytotoxic T-cell response compared to mRNA from the entire tumor cell population.265 Clinical trials have demonstrated the potential of CSC-targeted DC vaccines in treating GBM and various other cancers, with some studies indicating an enhanced therapeutic effect when combined with therapies that stimulate immune responses and inhibit immune suppression, such as ICIs.306 Combining CSC-targeted DC vaccines with radiation therapy or conventional therapies like surgery and chemotherapy has also shown promise in preventing local recurrence, reducing metastasis, and diminishing CSC populations in preclinical models.307, 308
Chimeric antigen receptor (CAR)-T cell therapy
(CAR)-T therapy involves engineering patients' T cells to express receptors specific to CSC markers. (CAR)-T cells can directly target and destroy CSCs expressing the targeted markers, potentially reducing the CSC population and inhibiting tumor growth.210, 309, 310 (CAR)-T cell therapy targets cancer cells with engineered T cells. Kymriah (Tisagenlecleucel), approved by the FDA in 2017, was the first anti-CD19 (CAR)-T therapy. It was initially authorized for treating relapsed or refractory pediatric and young-adult B-cell acute lymphoblastic leukemia (ALL) and later for adult relapsed or refractory diffuse large B-cell lymphoma.311 Since then, four additional anti-CD19 (CAR)-T-based therapies and one anti-B-cell maturation antigen (BCMA) (CAR)-T product have received approval for treating B-cell lymphomas/leukemias and multiple myeloma.312, 313 (CAR)-T cells targeting solid tumor antigens, like MUC1 and EpCAM, are under investigation.314 While successful in hematological malignancies targeting CD19 and BCMA,315 (CAR)-T therapy faces challenges in solid tumors, including antigen loss, on-target off-tumor toxicity, exhaustion, and TME barriers.316 Further, side effects like cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are observed.317, 318 However, manufacturing (CAR)-T cells is costly and variable.319
4.2.3 Combinatorial approaches
Numerous clinical trials explore combination therapies that include immunotherapy alongside traditional or targeted therapies. These combinations aim to target CSCs and non-CSCs within the tumor, reducing the likelihood of tumor recurrence and resistance.320, 321 Combining immunotherapy with radiotherapy has also shown potential. A combination of fractionated photon irradiation and CD98hc-directed UniCAR treatment exhibited a synergistic cytotoxic effect on radioresistant HNSCC spheroids, suggesting improved antitumor efficacy.322 Clinical trials combining conventional treatments like FOLFOXIRI drug therapy and bevacizumab with chemoradiotherapy are ongoing in patients with advanced rectal adenocarcinoma [NCT03085992]. However, combined anticancer immunotherapy's success hinges on overcoming immune evasion mechanisms exhibited by CSCs since CSCs create an immunosuppressive TME and employ various strategies to evade immune surveillance. Combining CSC-targeted immunotherapies with immune checkpoint blockade holds promise for stimulating antitumor immune responses and represents a potentially more effective cancer therapy. Addressing tumor cell plasticity and heterogeneity, targeting multiple CSC antigens, and integrating conventional treatments can further optimize these approaches. Developing relevant preclinical models and robust CSC analysis in patient-derived specimens are essential for assessing clinical responses accurately. While several clinical trials on combined CSC-targeted therapies have shown promising results, the specific targeting of CSCs requires further exploration to become a clinical reality.323, 324
4.2.4 Small molecules and antibodies
In recent years, the growing prominence of nanotechnology has also spurred the advancement of nanodrug delivery systems (NDDS), which have found extensive applications in cancer treatment. Nanoparticles (NPs) are crucial components of NDDS due to their small size, biocompatibility, and biodegradability, serving various roles including drug delivery, imaging, photothermal therapy (PTT), recognition, and gene delivery.325 NDDSs precisely release therapeutic agents in the body, improving drug solubility, bioavailability, and efficacy.326 Nanocarriers enable precise targeted drug delivery through active and passive mechanisms.327, 328 Active targeting involves NP conjugation with antibodies, peptides, aptamers, or other molecules, reducing toxicity to healthy cells, preventing drug degradation, and offering advantages such as specificity, biocompatibility, reduced cytotoxicity, extended drug half-lives, controlled release, and high drug loading capacity compared to traditional chemotherapy.329 Passive targeting relies on the enhanced permeability and retention (EPR) effect, allowing NPs to accumulate slowly in tumor tissue while sparing healthy cells.330 Diverse nanocarriers include lipid-based NPs, polymer/nonpolymer NPs, carbon nanotubes (CNTs), graphene oxide, nanocapsules, dendritic macromolecules, polymer micelles, and quantum dots (QDs), enhancing therapeutic delivery with biocompatible payloads.331, 332 Ongoing developments in nanotherapeutics continue to explore various nanomaterial carriers, advancing efficient drug and therapeutic delivery.329, 330
As such various nanodrug delivery systems and strategies for targeting CSCs using nanoparticles which has been exclusively discussed in details in the review by Yue et al.333 Hyaluronic acid (HA) has been used in drug delivery systems to target CD44, a common receptor on CSCs. Various studies have utilized HA-based nanocarriers to enhance drug delivery to CSCs and inhibit their growth.333 CD44-targeted nanocarriers, such as HA copolymerized with styrene maleic acid and 3,4-difluoromethylcurcumin, showed improved uptake by triple-marker positive (CD44+/CD133+/EpCAM+) pancreatic CSLCs compared with triple-marker negative (CD44−/CD133−/EpCAM−) counterparts and inhibited their proliferation.334 HA conjugated with 6-mercaptopurine (MP) and doxorubicin (DOX) effectively targeted colon cancer cells and CSCs, leading to significant tumor growth inhibition.335 Mineralized HA-SS-tetradecyl nano-carriers nanocarriers (M-HA-SS-TA) loaded with sulforaphane (SFN) exhibited strong inhibition of breast cancer cells bearing CD44+ CSC-like properties in response to tumor niches.336 Magnetic fluid hyperthermia (MFH) mediated by anti-CD44 antibody-modified superparamagnetic iron oxide NPs (SPIONPs) was effective in killing CSCs and inhibiting tumor growth in head and neck squamous cell carcinoma (HNSCC) Cal-27 cell lines 3D culture.337 For effective colorectal cancer therapy targeting cancer stem-like cells (CSLCs), PEG-PCL-based nanoparticles loaded with the topoisomerase inhibitor SN-38 were developed. CD133 (prominin-1), a potential marker for colorectal CSLCs, was targeted using anti-CD133 antibody-conjugated SN-38-loaded nanoparticles (CD133Ab-NPs-SN-38). These nanoparticles exhibited enhanced cytotoxicity against CD133+ cells, inhibited colony formation, suppressed tumor growth, and reduced CD133 expression, offering a promising approach for CSLC-targeted therapy in colorectal cancer.338 In the context of metastatic triple-negative breast cancer (TNBC), where CSCs are pivotal for cancer spread, broadly inhibiting the Wnt/β-catenin pathway can harm normal cells. To address this challenge, a targeted nanoparticle approach focused on integrin α5 (ITGA5) was developed. ITGA5 showed high expression in TNBC cells and their lung metastatic sites, justifying the use of ITGA5 ligands like RGD for precise drug delivery. These RGD-modified lipid-polymer hybrid nanoparticles, carrying diacidic norcantharidin, effectively curtailed TNBC tumor growth and metastasis, offering a promising strategy for treating metastatic TNBC by selectively attenuating β-catenin.339 Codelivery of DOX and salinoMycin (SAL) through a redox-triggered dual-targeted liposomes (CEP-LP@S/D) was used for synergistic liver cancer treatment, targeting CD133+-EpCAM+ CSC for both in vitro and in vivo models.340 Polymeric micelles that coloaded gold nanorods (GNRs) and AdriaMycin, creating an innovative therapeutic and diagnostic approach was created for targeting CSCs in hepatocellular carcinoma. Recognizing the significance of EpCAM as a surface marker for CSCs, GNRs were utilized for photoacoustic imaging to facilitate precise GNR identification and tissue mapping. This nanosystem effectively achieved targeted destruction of cancer cells, particularly when subjected to laser ablation. Additionally, ex vivo photoacoustic imaging exhibited the capability to distinctly delineate tumor regions through the use of GNR contrast.341 Utilizing biological nanoparticles like extracellular vesicles for hepatic delivery of RNA-based therapeutics in hepatocellular cancer, specifically targeting liver CSCs marked by EpCAM, holds potential for improving treatment outcomes. RNA nanotechnology paired milk source nanocapsules with synthetic oligonucleotide aptamers effectively reduced β-catenin expression and proliferation in vitro. In vivo studies using tumor xenograft models demonstrated the therapeutic efficacy of these RNA nanotechnology-based biological nanotherapeutics, offering a promising approach for controlling LCSC proliferation.341, 342 MSN drug loading enhanced the cytotoxicity of anticancer drugs with low potency by modifying the surface with positive polymer polyethylenimine for enhanced cellular uptake. PEI-modified MSNs (PMSNs) were used for the double delivery of cisplatin and hepatocyte nuclear factor 4 alpha (HNF4α)-encoding TF to inhibit CD133+ HUH7 cells and reduce the proportion of CSCs. These dual-loaded PMSNs, effectively arrested cells in the S-phase and induced apoptosis, displaying efficient suppression of tumor growth in vivo. This study highlights the potential of a nanoparticle-based delivery system for the dual role of chemotherapy and gene-based therapy in treating hepatocellular carcinoma.343 A versatile nanocarrier using DNA origami to combat drug resistance in cancer treatment was created. This carrier co-delivers doxorubicin (Dox) and two distinct antisense oligonucleotides (ASOs) targeting B-cell lymphoma 2 (Bcl2) and P-glycoprotein (P-gp) into drug-resistant cancer cells (Hela/ADR, MCF-7/ADR), enhancing therapeutic efficacy. The origami was aptamer-functionalized using staple strands with 5’-end extended MUC1 sequences to enhance targeting. The resulting Apt-Dox-origami-ASO demonstrated controlled release of Dox and ASOs in specific conditions. Additionally, it effectively protected ASOs from degradation and efficiently delivered Dox and ASOs into the cytoplasm of cancer cells, resulting in significant downregulation of target genes and enhanced cancer therapy. This innovative multifunctional origami-based codelivery nanocarrier offers a promising strategy for overcoming drug resistance in cancer therapy.344 A targeted theranostic nano vehicle was designed using manganese-doped MSNs with dual targeting via folate receptor and CD44, promoting cancer cell and CSC uptake.345 N-(2-hydroxypropyl) methylacrylamide (HPMA) cyclopamine delivery system improved drug solubility and reduced systemic toxicity, effectively eliminating CD133+ tumor stem cells in pancreatic and liver cancer.346, 347 Glioma stem cells (GSCs) drive chemotherapy resistance in GBM, often triggered by hypoxia. Disulfiram, an antialcoholism drug, exhibits potent NF-κB inhibition and anti-GSC effects. Poly lactic-co-glycolic acid nanoparticle-encapsulated disulfiram (DS-PLGA) were reported to effectively inhibited GCSs demonstrating efficient anti-GSC activity in vitro and anti-GBM efficacy in vivo, targeting the hypoxia (Hif-1α and Hif-2α)-induced GSC network and highlighting potential for GBM treatment.348 Despite the limited treatment options for GBM, advancements in cell fate regulation and nanomedicine offer promise. Bioreducible poly (β-amino ester) nanoparticles efficiently deliver microRNA (miRNA) to CSCs, inhibiting the stem cell phenotype of human GBM cells in vitro and promoting tumor growth inhibition in orthotopic human GBM xenografts when intratumorally infused. Co-delivery of specific miRNAs within these nanoparticles (nano-miRs) resulted in extended survival from GBM in mice, illustrating potential for targeted GBM treatment.349 Subsequently, various nanocarriers, such as composite magnetic nanocubes and polymeric nanoparticles, have been developed for targeted drug delivery to CSCs in HCC.350 Dual pH-sensitive polymeric drug-conjugated nanoparticles showed enhanced inhibition of drug-resistant CSCs.350 Additionally, gold nanoparticles conjugated to doxorubicin (AuNPs-Adr) effectively targeted and delivered doxorubicin to CSCs.351 Nanoparticles loaded with sorafenib and glucose oxidase, designated SG@GR-ZIF-8, exhibited significant antimetastatic HCC activity.352 The use of nanocarriers like mesoporous silica nanoparticles (MSNs) integrated with chemotherapy and immune checkpoint blocking therapy-termed as cocktail therapy demonstrated enhanced therapeutic efficacy against breast CSCs. It was reported to reduce the proportion of CSC and enhance the T cells infiltration in tumor tissues, and thus prolonged the survival of mice.353
4.2.5 Innovative approaches
Liposomes: The nontoxic and biodegradable vesicles, have also been employed as effective drug delivery systems to improve therapeutic outcomes by stabilizing compounds and enhancing their distribution at target sites.354 Liposomes encoding TNF-associated apoptosis-inducing ligands (TRAILs) through plasmid DNA showed potential in eliminating CSCs and inhibiting tumor growth in in vitro colon cancer cell lines and tumorospheres.355 Liposomal nanocarriers loaded with paclitaxel and SAL were found to effectively induce apoptosis in breast CSCs in in vitro models, suggesting their potential for breast cancer treatment.356
CNTs: CNTs with high surface-to-volume ratio, enhanced electrical conductivity, strength, biocompatibility, and ease of functionalization have even been explored as promising platforms for drug and gene delivery. CNTs exist as cylindrical tubes composed of sp2 hybrid carbon atoms and can be categorized into single-walled CNTs (SWCNTs) and multiwalled CNTs (MWCNTs) based on the number of layers.357 CSCs, notably resistant to traditional hyperthermia, can be effectively targeted using PTT mediated by amino-modified multiwalled CNTs (MWCNTs). Additionally, specific CSC markers like CD133 in GBM can be targeted by functionalizing CNTs with CD133 monoclonal antibodies, effectively killing CSCs through PTT. CNT-based drug delivery systems functionalized for gastric CSCs show promise for targeted therapy.358 Utilizing CNTs for combined delivery of chemotherapeutic agents like paclitaxel (PTX) and SAL also have demonstrated enhanced therapeutic effects, particularly in breast cancer (BC) CSCs. pH-responsive release mechanisms in the acidic TME further optimize drug delivery for improved efficacy.359
GNRs and QDs: GNRs and QDs are emerging materials for targeted drug delivery due to their anisotropic shape and unique optical properties.360, 361 A study explored reduced graphene oxide-silver nanoparticle nanocomposites (rGO-Ag) synthesized using R-phycoerythrin (RPE) as an alternative therapy to conventional chemotherapy. rGO-Ag effectively targets and reduces viability of ovarian cancer cells and ovarian CSCs (OvCSCs) while triggering apoptosis and mitochondrial dysfunction. rGO-Ag exhibied notable toxicity towards OvCSCs, reducing the number of ALDH+CD133+ colonies and inducing apoptosis, possibly through reactive oxygen species generation, mitochondrial membrane potential reduction, and enhanced apoptotic gene expression. Combining rGO-Ag with SAL significantly enhances apoptosis levels, suggesting potential for selective OvCSC elimination. As such the study underscored rGO-Ag as a promising nano-therapeutic for specific targeting and elimination of highly tumorigenic ALDH+CD133+ cells, highlighting its potential for targeted therapy against tumor-initiating cells, presenting a new approach in cancer treatment.362
PTT: PTT utilizes metal NPs to eliminate CSCs by converting light into heat, inducing a hyperthermal physiological response. Studies highlight the potential of PTT in inhibiting tumor growth, with applications such as using amino-modified multiwalled CNTs for effective CSC eradication.363, 364 Researchers have successfully targeted CSCs by functionalizing nanoparticles (NPs). For instance, CD133 monoclonal antibody-grafted CNTs target CD133-positive GBM-CSCs, effectively eliminating them via PTT. Additionally, nanoparticles loaded with bimodal metal cages and photodynamic therapy (PDT) target CSCs by reducing cell mobility under laser irradiation.357, 365 Combinatorial approaches, such as using PTT properties of CNTs and metallic materials, along with chemotherapy, show promise in producing synergistic effects for CSC eradication. Other strategies involve utilizing manganese dioxide/carbon nanocomposites and PDT to modulate the TME and improve therapeutic resistance.366 Innovative approaches, including functionalized nanoprobe-facilitated surface-enhanced Raman scattering and MoS2 nanosheets combined with moderate PTT, demonstrate potential in predicting cancer dissemination by CSC-based surveillance and modulating CSC signaling pathways, respectively.367, 368 While PTT effectively hampers tumor growth by targeting CSCs, complete tumor eradication is often challenging due to the shallow penetration of near-infrared (NIR) light. Hence, integrating PTT with complementary therapies is anticipated to surmount these limitations.
MFH: MFH harnesses the efficient thermal conversion ability of magnetic NPs under an alternating magnetic field to induce localized hyperthermia within tumors. This technique creates a high-temperature zone, effectively killing tumor cells and triggering apoptosis.369 In various experiments, MFH has demonstrated significant potential in targeting CSCs. For instance, anti-CD44 antibody-modified superparamagnetic iron oxide nanoparticles (SPION) induced hyperthermia, leading to the demise of CSCs and notably inhibiting Cal-27 tumor growth in mice.348 Additionally, MFH, when integrated with nuclear targeting systems coated with MSNs of superparamagnetic iron oxide-based NPs, directly targets CSCs and facilitates a combination of thermotherapy and hypoxia-activated chemotherapy, resulting in complete apoptosis of CSCs.370 Biomimetic magnetic NPs have exhibited the ability to induce apoptosis in stress-escape CSCs and impede their proliferation and metastasis both in vitro and in vivo. This occurs through a combined therapeutic effect involving DOX chemotherapy and magnetic MSNs MFH under the influence of an alternating magnetic field.371 Moreover, antibody-modified NPs, particularly those targeting lung CSCs, have shown enhanced cellular uptake and prolonged tumor accumulation, effectively eliminating up to 98% of lung CSCs in in vivo models. The combination of hyperthermia and chemotherapy exhibited significant inhibition of tumor growth and metastasis in mice carrying lung CSC xenografts, with minimal side effects and adverse reactions.372 In summary, MFH exhibits promising potential in selectively targeting and eradicating tumor stem cells.
Gamma-secreatase inhibitors (GSIs): GSIs are a class of pharmaceutical compounds designed to inhibit the activity of the gamma-secretase enzyme.373, 374 Gamma-secretase is a multi-subunit protease complex found in cell membranes, particularly in the endoplasmic reticulum and Golgi apparatus.375 It plays a critical role in various cellular processes, including the cleavage of specific transmembrane proteins. GSIs were initially developed for their potential therapeutic applications in various diseases, particularly in Alzheimer's disease and cancer.376, 377 GSIs have also been investigated for their potential in cancer therapy.378 Notably, GSIs can inhibit the Notch signaling pathway, crucial in cell differentiation, proliferation, and survival.379 In certain cancers, abnormal Notch signaling is implicated in tumor growth and resistance to therapy.380 GSIs may disrupt Notch signaling in cancer cells, potentially reducing tumor growth and sensitizing cancer cells to other treatments. In addition to APP and Notch, gamma-secretase has other substrates, including E-cadherin, ErbB4, and CD44. Inhibition of these substrates can have various effects on cell adhesion, proliferation, and signaling, and GSIs have been explored for their potential in these areas.
These nanodrug delivery systems and strategies show great potential in targeting CSCs and improving cancer treatment efficacy. However, further research and clinical studies are needed to fully validate their effectiveness and safety in human patients. Isolation and enrichment of CSCs forms the fundamental basis for a rational approach to target these CSC-based-targeted therapy.381 Fortunately, this field has developed considerably due to the technological advancement of flow cytometric-based differential sorting and analysis of CSC-specific cell surface markers, Hoechst 33342 dye exclusion, ALDH enzymatic activities, as well as biochemical and functional assay such as colony-forming assay, formation of cancer spheres cancer induction in nude mice models.5, 28, 382 Once isolated and characterized, one of the potentially effective combined theraputical approach can/might be applied the to target the specific CSCs associated with particular cancer.
4.2.6 Targeting multiple pathways and overcoming therapeutic resistance of CSCs
Utilizing in silico mining and intelligent approaches for drug chemo-informatics or pharma-informatics presents a pertinent strategy to target various pathways linked to CSCs and address therapeutic resistance. However, the tracking of CSCs and targeted therapeutics associated with them is still under investigation. CSC is heavily influenced by growth and developmental signaling involving TFs like Oct-4, Sox-2, KLF-4, Nanog, and c-Myc. Signaling cues from extracellular matrix factors, integrins, ECM components, and local factors like hypoxia, local acting substances, tumor-infiltrating lymphocytes, tumor-associated macrophages, cancer-associated mesenchymal stem cells have been reported to influence the biology of the CSCs.83 Targeting CSCs is of paramount importance to cancer therapy. In this context, one needs to understand that the interaction between CSCs and TME is significant. Unless one considers them and their dynamic nature together, it is impossible to target CSCs.383 Through intrinsic and extrinsic mechanisms, TME influences the CSCs physiology. The extrinsic actions involve the growth factors and cytokines dependency of CSC, while the inherent mechanisms include DNA methylation or demethylation, and other epigenetic changes. While some tumors initially respond nicely, they eventually become resistant. This has been extensively researched and characterized in a variety of solid tumor, as well as in liquid tumors, including AML and CML. It is believed that therapy resistance is due to CSC. CSCs generally are stubborn to therapy, which allows them to escape from the treatment regime.384, 385 The mechanisms by which CSCs achieve therapeutic resistance involve their enhanced capacity to repair the DNA damage along with amplification of multiple drug resistance (MDR) transporters, antiapoptotic proteins, signaling pathways activating the CSCs self-renewal like NOTCH, Hh, and Wnt/β-catenin as well as through the expression of other efflux proteins on their membrane, like the ABC transporters.5, 386 The common ABC transporters responsible for drug resistance in CSCs are ABCG2, ABCB1, and ABCC1. Response of cancer to conventional chemotherapy and radiotherapy is quite variable and unpredictable. While some cancers initially show good responses, they eventually show resistance. These are called refractory cancers. This is well documented and reported in several solid tumor fields387, 388 as well as liquid cancers like AML and CML.
A prerequisite for targeting CSC is the identification of cell surface receptors that can serve as a specific target location. This strategy for drug delivery exhibit significant promise in targeting CSCs and enhancing the effectiveness of cancer treatment. Nevertheless, thorough research and clinical studies are required to comprehensively validate their safety and efficacy in human patients. The isolation and enrichment of CSCs form the fundamental basis for a rational approach to implementing targeted therapies focused on CSCs.
5 CONCLUSION AND FUTURE PERSPECTIVES
5.1 Recap of CSC signaling pathways and therapeutic targeting
Frequent recurrence of cancer, distant metastasis, and refractory to conventional chemo-radiation therapy seem to be the bottleneck in the process of eradicating cancer. Decades of fruitful research have identified the CSC as one of the main drivers. Without a mechanism to address these elusive cell populations, recurrence and resistance to conventional therapy will persist. A combination of cell surface markers and their biochemical and functional properties has been exploited to identify and isolate CSCs. Despite this progress, there is still a lack of reliable and accurate CSC markers for many cancers that exists in reality. Robust assays are needed to assess CSC functions in tumor samples, crucial for evaluating CSC-targeted immunotherapies due to their heterogeneity and adaptability.389, 390 Defining the entire CSC population within a tumor is complex as no single surface marker covers all types, and high phenotypic variability exists due to mutations, epigenetic changes, and microenvironment influences.28, 391 Moreover, the scarcity of tumor-specific antigens, with over 70% of CSC markers present on normal cells, poses challenges, potentially causing toxicities during immune therapy.392, 393 Researchers are exploring multispecific antibody and multispecific (CAR)-T cell immunotherapy strategies to enhance treatment specificity and overcome tumor antigen escape, offering promising options for malignancy treatment.382 Overcoming this hurdle is crucial for developing therapeutic strategies with higher sensitivity, specificity, and fewer off-target effects. Small interfering RNA, short hairpin RNA, or lentiviral-based vectors are used to knock down CSC-associated key oncogenes and signaling pathways, resulting in the selective killing of these cell types. Recently CRISPR-Cas9-based gene editing platforms have proved to be very helpful in selecting to achieve a conditional knockdown.394
5.2 The ongoing pursuit of effective CSC-targeted therapies
- (a)
Enhancing CCN5 expression396: One potential therapeutic approach is to enhance the expression of CCN5 in cancer cells. This could be achieved through genetic manipulation or drugs that promote CCN5 transcription or stability.
- (b)
Modulating signaling pathways397: CCN5 is known to interact with several signaling pathways, including the Wnt/β-catenin pathway. Therapies aimed at modulating these pathways could indirectly affect CCN5 activity. Small molecules or biologics that target specific components of these pathways could be explored.
- (c)
Stemness inhibition398: Given CCN5's potential role in inhibiting cancer stemness, therapies targeting CSCs may indirectly involve CCN5. These therapies could include CSC-targeted drugs, such as those that disrupt CSC-specific signaling pathways.
- (d)
Combinatorial approaches: Combinations of therapies that target multiple aspects of cancer, including CCN5-related pathways, may be more effective. Combining CCN5-targeted strategies with existing cancer therapies (e.g., chemotherapy, immunotherapy) could enhance treatment outcomes.
- (e)
Patient stratification: Identifying patients whose tumors express low levels of CCN5 and are more likely to benefit from CCN5-targeted therapies could be essential for personalized treatment strategies.
- (f)
Clinical trials: Clinical trials are necessary to evaluate the safety and efficacy of CCN5-targeted therapies in cancer patients. These trials can help determine the optimal dosage, treatment duration, and potential side effects of CCN5-related treatments.
- (g)
Biomarker development: Developing biomarkers that can reliably measure CCN5 levels or tumor activity could help in patient selection and monitoring of treatment responses.
5.3 The clinical impact and future prospects
It's important to note that therapeutic targeting of CCN5 is complex and may be context-dependent. The role of CCN5 can vary among different cancer types, and its functions in normal tissue biology also need to be considered to avoid potential side effects. Further research is needed to better understand the mechanisms of CCN5 in cancer and to develop safe and effective therapeutic strategies that harness its potential as a target for cancer treatment.
Even with the identification of novel anticancer targets, often one of the biggest challenge is targeted and precision delivery. Nanoparticle-mediated drug delivery is a promising approach that can be utilized to target CSCs, the subset of cancer cells that are often resistant to conventional cancer treatments and are believed to drive tumor growth and recurrence.399-401 Nanoparticles can be engineered to encapsulate and deliver therapeutic agents, such as chemotherapy drugs or targeted therapies.402, 403 Functionalizing nanoparticles with specific ligands, antibodies, or peptides can enable them to target surface markers or receptors overexpressed on CSCs, ensuring selective drug delivery to these cells.402 Nanoparticles protect the encapsulated drugs from degradation and premature release, improving drug stability during circulation in the bloodstream.403, 404 Some anticancer drugs have limited solubility, making their administration challenging. Nanoparticles can increase drug solubility, allowing for more efficient delivery.405 Nanoparticles are small enough to penetrate the tumor tissue, reaching CSCs within the tumor's core, enhancing their therapeutic efficacy (Figure 4).
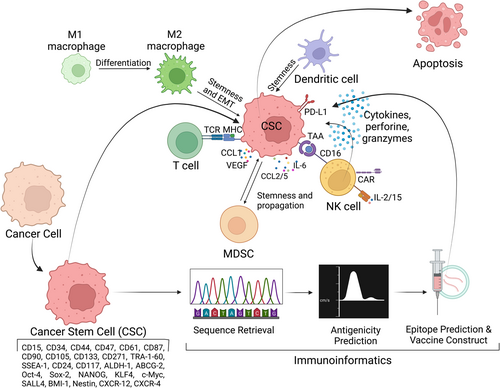
Immunotherapy and (CAR)-T-based therapy is the latest development in this area to target cancer cells using the body's own immune cells or to engineer them to produce cytotoxic cytokines. (CAR)-T cells are engineered and manipulated cells to identify and eradicate tumors by recognizing tumor-associated antigens on cancer cells. (CAR)-T cells have evolved over several years to become more sophisticated and specialized. The first-generation (CAR)-T had just the CD3ζ domain for cytoplasmic signaling, which was then modified with an additional costimulatory domain along with the CD3ζ domain, which commonly is 4-1BB (CD137) or CD28. The third generation of (CAR)-Ts has two costimulatory signaling domains, while the fourth generation of CARs (TRUCKs) is engineered to secrete inflammatory cytokines like IL-6, IL-12, and IL-15.406 In addition, by leveraging bioinformatics tools to immunotherapy, unique antigens expressed on the surface of certain CSCs can be identified, allowing for the design of precise immunotherapies. This approach would enable the development of personalized vaccines that stimulate the immune system to recognize and eliminate CSCs, addressing the root cause of cancer recurrence and resistance. Moreover, immunoinformatics facilitates the prediction of immunogenic epitopes and optimization of immunotherapeutic strategies, contributing to the advancement of tailored and effective cancer treatments.407 Therefore, through a careful selection of targeting both TME and the tumor, the desired effect may be achieved. Combination therapy with immunomodulatory agents, immune checkpoint inhibitors, and targeting cell surface molecules will be of future interest to enhance the clinical and therapeutic effects.
Targeted therapies that specifically inhibit signaling pathways associated with CSCs are being explored as a way to reduce treatment resistance and improve cancer outcomes. These therapies aim to disrupt CSC self-renewal and differentiation, ultimately reducing the ability of tumors to regenerate and spread. However, the complexity of CSC signaling networks and their context-dependent roles pose challenges in developing effective CSC-targeted treatments. Ongoing research in this field continues to uncover new insights into CSC signaling and its potential as a therapeutic target in the fight against cancer.
AUTHOR CONTRIBUTIONS
Joyeeta Talukdar: Methodology (equal); software (equal); writing—original draft (equal). Tryambak P. Srivastava: Methodology (equal); software (equal). Om S. Sahoo: Methodology (equal); software (equal). Abhibroto Karmakar: Methodology (equal); software (equal). Avdhesh K. Rai: Methodology (equal); software (equal); supervision (equal). Anupam Sarma: Methodology (equal); software (equal); supervision (equal). Gayatri Gogoi: Methodology (equal); software (equal); supervision (equal). Mohammed S. Alqahtani: Methodology (equal); software (equal). Mohamed Abbas: Methodology (equal); software (equal). Ruby Dhar: Conceptualization (equal); project administration (equal); supervision (equal); validation (equal). Subhradip Karmakar: Conceptualization (equal); project administration (equal); supervision (equal); validation (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
J.T. thank ICMR for providing fellowship (ICMR RA fellowship number-BMI/11(49)2022). The authors are grateful to DHR and ICMR for supporting our research vide grant no I-1291 (Reference Number: 382019000741) provided to S.K.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.





