Molecular Mechanisms Associated with Protecting IEC-6 Cells from Acrylamide-Induced Tight Junction Damage by Ganoderma atrum Polysaccharide
Abstract
Scope
The previous in vivo studies show Ganoderma atrum polysaccharide (PSG-F2) has a protective effect against the acrylamide (AA)-induced intestinal oxidative damage in rats. Now, this study aims to explore the protective mechanism with IEC-6 cell model.
Methods and results
Based on RNA Sequencing (RNA-Seq), the study screens MAPK signaling pathway as one of the most crucial pathways for pretreatment with PSG-F2 against AA-induced damage in IEC-6 cells. In total, six key MAPK signaling pathway-related proteins (p-P38/P38, p-ERK/ERK, and p-JNK/JNK), and three tight junction key proteins (Zonula Occludens protein-1, Claudin-1, and Occludin) are detected by Western blot and immunofluorescence, which verify the RNA-Seq data. Moreover, PD98059 interference inhibits critical proteins in the MAPK signaling pathway, thus uncovering the precise molecular mechanisms of MAPK/ERK signaling pathway involve in the protective effects of PSG-F2 against AA-induced intestinal barrier damage.
Conclusion
These findings confirm that PSG-F2 can be used as a daily dietary supplement to protect the intestinal cells from damage caused by thermal processing hazards AA.
1 Introduction
Acrylamide (AA) can be formed by Maillard reaction between the amino acid asparagine, and reducing sugars in food during food thermal processing at temperatures higher than 120 °C and low moisture conditions.[1, 2] Many studies demonstrated that AA has genotoxic and carcinogenic properties in different cell lines and animal models.[3, 4] Recently, many scientists focused on the toxicity of AA to intestine epithelial cells. Chen et al. reported that AA could raise the level of reactive oxygen species (ROS) and decrease the concentration of glutathione in the human colon cancer cell line Caco-2, which will cause DNA damage and cell death.[5-7] Our previous in vivo model also showed that AA could break small intestinal barrier via increasing the levels of nitric oxide, endothelin-1, and D-lactate, the anti-inflammatory cytokines like interleukin 10 in serum, DNA damage markers like 8-OHdG, and the pro-inflammatory cytokines like tumor necrosis factor-α, which prove an evident inflammatory intestinal injury in rats.[8, 9] However, the exact mechanism of toxicity is still not fully understood yet.
Moreover, in recent years, natural substances have been studied as protective agents to reduce the toxicity of AA.[10-12] According to our group studies, we isolated Ganoderma atrum polysaccharide (PSG-F2) with a purity of over 99.8%, as previously described.[13, 14] PSG-F2, with an average molecular weight of approximately 20.9 kDa, is composed of glucose, mannose, and galactose (the ratio is 70.7:16.2:13.1). It is a branched, and acidic heteropolysaccharide-protein complex which is linked by -(1→3), -(1→6), and -(1→4). And PSG-F2 has the potential capacity for antioxidant and immunomodulating both in vitro and in vivo.[15, 16] Our previous research has shown that the main acidic fraction PSG-F2 has a protective capacity on AA-induced intestinal epithelial oxidative damage in vitro.[17] However, up till now, the underlying mechanism still needs to be further investigated.
MAPK signaling pathway is a crucial signaling transduction pathway related to mitochondria-mediated apoptosis. Arthur et al. considered MAPK signaling pathway has potential for targeting treatment of autoimmune and inflammatory diseases.[18] The main subfamily of MAPK includes P38, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK).[19, 20] Numerous studies demonstrated that JNK and P38 are involved in stress-induced cell signal transduction, inflammation, and apoptosis.[21-23] ERK, involving in the regulation of cell proliferation and differentiation, is a key for the control of stress response or oxidative stress.[24-26] Moreover, several studies have shown that MAPK signaling pathway is of particular importance in regulating barrier function and transport across the intestinal barrier, by up- or down-regulating the expression of tight junction (TJ) proteins.[27-30] However, it is not clear whether the MAPK/ERK signaling pathway involves in the protective mechanism of PSG-F2 against AA-induced intestinal oxidative damage via improving the barrier function.
Therefore, in the present studies, we built an in vitro experimental model of IEC-6 cells to explore the mechanism of PSG-F2 alleviating the intestinal epithelial barrier dysfunction caused by AA. As a useful technique to explore the mechanisms and feasible regulators of bioactive compounds, RNA sequencing (RNA-Seq) was used to thoroughly discern the different expression of genes between the PSG-F2 pretreatment and the AA control of IEC-6 cells and highlight several signaling pathways. It is worth mentioning that the expression of numerous genes involved in MAPK/ERK signaling pathway is significantly changed in Group AA in IEC-6 cells. Thus, the expression of the related proteins was further checked to verify the involvement of MAPK/ERK signaling pathway.
2 Experimental Section
2.1 Materials and Reagents
PSG-F2 was isolated from the Ganoderma atrum with a purity of over 99.8%, as previously described.[13, 14] Fetal bovine serum (FBS) was from Biological Industries (Israel). DMEM was bought from Solarbio Science & Technology Co., Ltd. (Beijing, China). AA and N-acetyl-L-cysteine (NAC) were from Aladdin Chemical Co. (Shanghai, China). The Cell Counting Kit-8 (CCK-8) was from Dojindo Molecular Technologies, Inc. (Shanghai, China). PD98059 (2’-amino-3’-methoxy flavone) was from MedChemExpress (New Jersey, USA). DMSO was purchased and from Sigma Co. (Saint Louis, USA). The ultra-pure water and distilled water were prepared by the Millipore Milli-Q Plus system ( New York, USA). All solutions are filtered by using a 0.20 µm filter membrane (Millipore, USA) to remove any impurities before being used in cell culture.
2.2 Cell Culture
Intestinal epitheloid cell lines from rats, IEC-6 cells, were from the American Type Culture Collection (CRL-1592, USA), and cultured in DMEM (high glucose), adding 10% v/v FBS, and 1% double antibiotics (Penicillin and Streptomycin) at 37 °C with 5% v/v CO2 in a constant humidity incubator (Thermo Fisher Scientific, USA).
Referring to the experimental protocol of the previously published research,[17] the study chose 5 mmol L−1 AA, 80–320 µg mL−1 PSG-F2, and 10 mmol L−1 NAC to further detect the protective effect of PSG-F2 on IEC-6 cells . In brief, the medium containing 10% FBS was replaced by the corresponding drug-containing culture medium after cells attachment (24 h). All the cells were divided into six groups randomly: Group PSG-F2 (treated with 80–320 µg mL−1 PSG-F2 for 24 h), Group PD98059 (treated with 1–30 µmol L−1 PD98059 for an hour), and Group PD98059 with PSG-F2 (after 24 h treated with 320 µg mL−1 PSG-F2 and then 10 µmol L−1 PD98059 for an hour), the following groups after the above treatment, add 5 mmol L−1 AA to culture 8 h. At the same time, the study set up Group control (treated with DMEM), Group AA (treated with 5 mmol L−1 AA), and Group PD98059 single (only pretreated with 10 µmol L−1 PD98059 for an hour).
2.3 Cell Viability Assay
The CCK-8 assay was usually used to analyze the influence of different drugs on cell viability, mainly referred to Qi et al.[31] The IEC-6 cells were inoculated in a 96-well plate (1.5 × 104 cells each well), processed as described in Section 2.2, and performed three biological repeats each group. Afterward, add 100 µL of CCK-8 working solution (stock solution:DMEM = 1:100) per well, then incubated in the constant humidity incubator for an hour. Finally, the microplate reader (Thermo Fisher Scientific, USA) was used to measure absorbance at 450 nm.
2.4 RNA-Seq Analysis and Genes Enrichment
The number of cells in Group control, Group AA (5 mmol L−1), and Group PSG-F2 (320 µg mL−1) should be over 107. The following steps referred to Ding et al.,[32] in short, the Trizol method was used to extract total RNA. The purified RNA was tested for the integrity and the situation of genetic contamination by gel electrophoresis using a Qubit 2.0 fluorometer. The library size was detected by Agilent 2100, and sequence cDNA was used by Illumina MiSeq System (n = 3). Based on the data from RNA-Seq, HTSeq was used to analyze the gene expression.[33] And the transcripts were identified as the differentially expressed genes (DEGs) with Fold Change (FC)> 2 and p-value <0.05. The Volcano map was implemented by R language (version 4.0.5). DEGs in Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was analyzed by cluster profiler. The heatmap and Venn plot were plotted in the genescloud platform (https://www.genescloud.cn).
2.5 Validation of Key Protein Expression by Western Blot
Cell samples were treated with 1% PMSF lysis buffer (v/v, the ratio of PMSF and Western & IP lysis buffer was 1:99) (Beyotime Biotechnology, China), centrifuging (10 000 × g) to take the supernatant, which was the whole-cell protein samples by freezing centrifuge (Sigma, Germany). Before electrophoresis, all the samples had measured the concentration by BCA Protein Assay Kit (Beyotime Biotechnology, China).
The protocol of Western blot was from the previous research.[17, 32] Based on the results of β-actin, the same amount of protein sample was prepared and subjected to 10% separating gel and 5% concentrated gel (SDS-PAGE) by Bio-Rad Mini-PROTEAN Tetra System (Bio-Rad, USA), then transferred the SDS-PAGE to the poly(vinylidene fluoride) (PVDF) membrane (Millipore, USA) via Bio-Rad Trans-Blot Turbo System (Bio-Rad, USA). And block the membranes with 5% w/v bovine albumin (Solarbio, China) dissolved by TBST (Tris-buffered saline adding 0.1% v/v Tween 20 [Solarbio, China]) for an hour at room temperature to prevent non-specific binding. Then, incubate the membranes with the corresponding anti-antibodies in TBST (1:1000) overnight at 4 °C: anti-p-P38, anti-P38, anti-p-JNK, anti-JNK, anti-p-ERK (Cell Signaling Technology, USA), anti-MAPK1 (ERK2, Boster Biological Technology Co. Ltd., China), anti-Claudin-1, anti-Occludin (Proteintech Group, Inc., USA), anti-Zonula Occludens protein-1 (ZO-1) (Abcam, Cambridge, UK), and anti-β-actin (ZSGB-Biotechnology Co. Ltd, Beijing, China). This was followed by washing three times in TBST, 10 min each, and incubating the membranes with corresponding species-specific secondary antibodies (ZSGB-Biotechnology Co. Ltd., Beijing, China) in TBST (1:10 000) for an hour. Then wash the membranes for 10 min three times. The protein signals were checked and pictured by Bio-Rad Chemi XRS+ (Bio-Rad, USA) with BeyoECL Plus (Beyotime Biotechnology, China). Image J software was used to measure protein quantification in optical density units and normalized to the corresponding actin sample expression.
2.6 Immunofluorescence
IEC-6 cells were seeded onto 24-wells plates (1.2 × 105 cells each well) for 24 h, and the next steps were as in Section 2.2,[28] referring to the methodology of the previous studies in the laboratory.[34] The cells were stained overnight at 4 °C with different primary antibodies (ZO-1, Occludin, and Claudin-1) after washing with PBS three times, treated with 4% paraformaldehyde fix solution for 10 min, immunostaining permeabilization buffer with saponin for 10 min, and blocking buffer for an hour, all the above reagents are from Beyotime Biotechnology (Shanghai, China). On the second day, incubated with goat anti-rabbit IgG H&L (Alexa Fluor 488) (1:200, ZSGB-Biotechnology Co. Ltd., Beijing, China) for an hour, and DAPI (Solarbio, China) for 10 min in the dark successively, and finally observed in a fluorescence microscope (Leica, Germany).
2.7 Statistical Analysis
The data were all represented as the means ± standard deviation (SD) for three biological independent parallel experiments. And use one-way analysis of variance (ANOVA) to analyze the data, and then carried out the least significant difference (LSD) test for multiple comparisons by the Social Science Statistical Program 25 software package. In all cases, *p < 0.05, **p < 0.01 versus solvent control group (Group control); #p < 0.05, ##p < 0.01 versus only treated with AA group (Group AA). The figures were completed by using GraphPad Prism 8.0.2. Pathway diagrams were realized by PowerPoint 2016, and PathwayBuilder Tool 2.0.
3 Results
3.1 PSG-F2 Ameliorated AA-Induced TJ Damage
PSG-F2, a naturally occurring polysaccharide obtained from Ganoderma atrum, has been considered a potent antioxidant and anti-inflammatory diet supplementation.[35, 36] In addition, our recent in vivo studies have shown that pretreatment with PSG-1 can protect the intestine from AA in rats.[8, 9] To investigate whether PSG-F2 has similar effects on protective effect on AA-induced intestinal barrier damage, we pretreated IEC-6 cells with PSG-F2 for 24 h, then AA damage for 8 h (Figure 1A).
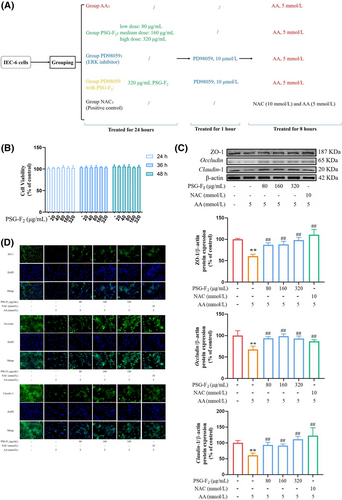
Firstly, PSG-F2 exhibited nearly no toxic effects at 20–320 µg mL−1 on viability in IEC-6 cells (Figure 1B), which is in agreement with our previous study.[17] Therefore, we subsequently focused on exploring the protective role of PSG-F2 on the expression of three key TJ proteins (ZO-1, Occludin, and Claudin-1) in the IEC-6 cells by using Western blot (Figure 1C), and immunofluorescence (Figure 1D). Notably, TJ proteins was mainly reduced by using 5 mmol L−1 AA. As a toxic compound, AA caused cell nucleus crumpling, division, and even disappeared, which is consistent with the results of RNA-Seq based on the DAPI staining. In addition, AA-induced a down-regulation of TJ protein compared with Group control by 37 ± 6%, 38 ± 8%, and 41 ± 7%. In our previous studies, pretreatment with PSG-F2 at 20–160 µg mL−1 plays a significant protective role against AA-induced cytotoxicity via a mitochondria-mediated intrinsic apoptosis. But this time, only pretreatment with 80, 160, or 320 µg mL−1 of PSG-F2 could efficiently block AA-induced intestinal barrier dysfunction with effects of positive control, NAC (p < 0.05, p < 0.01). Especially the pretreatment with PSG-F2 at a high dose of 320 µg mL−1 showed significant effects on the release of Occludin, Claudin-1 compared with that at low or middle doses. The expression of three key TJ proteins in IEC-6 cells treated with PSG-F2 was improved in a dose-dependent manner compared with Group AA. The protein levels of TJ were increased by: ZO-1 (17 ± 2%, 23 ± 1%, and 30 ± 5%), Occludin (18 ± 9%, 21 ± 8%, and 27 ± 9%), and Claudin-1 (18 ± 6%, 24 ± 5%, and 32 ± 5%) in cells treated with 80, 160, and 320 µg mL−1 of PSG-F2, respectively, compared with Group AA. The immunofluorescence analysis of TJ proteins demonstrated that AA could induce most of the TJ loss, cell deformation, and even cell death. While pretreatment of PSG-F2 could prevent this damage, the effect was similar to that of the positive control NAC. In total, these findings suggest that PSG-F2 could protect the intestinal barrier from AA-induced damage by up-regulating the expression of TJ.
3.2 Differentially Expressed mRNAs by RNA-Seq Data Analysis
To ensure that RNA-Seq could accurately determine the protective mechanism of PSG-F2, we chose the highest concentration of PSG-F2 (320 µg mL−1). As we know, RNA-Seq has become an efficient and widely-used method in molecular biology, with details about transcriptome profiling like gene expression profiles to explore the expression at the gene level.[33] The results showed obvious alterations in the gene expression profile of IEC-6 cells after 320 µg mL−1 PSG-F2 protecting from AA. There were 1559 genes differentially expressed in a significant manner between Group control and Group AA (FC > 2, p < 0.05). As can be seen from the Volcano plot of DEGs (Figure 2A), the up and down-regulated genes were 621 and 938, respectively. The Volcano plot of Group AA and Group PSG-F2 is shown in Figure 2B, and the up and down-regulated genes were 133 and 305, respectively. In total, 438 differential genes were expressed (FC > 2, p < 0.05). After comparison, there were 235 DEGs consistent between the two comparison pairs. The heatmap representation of DEGs changed mRNA transcripts in IEC-6 cells (Figure 2C), the number of DEGs in Group control and Group PSG-F2 compared with that of Group AA at different fold-change cutoffs are presented in Table 1. It can be observed that PSG-F2 can change the number of DEGs in IEC-6 cells compared with AA-treated.
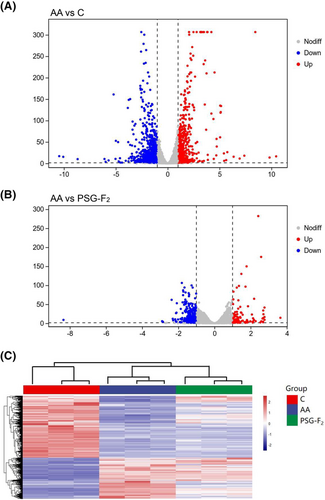
| Number of differentially expressed genes | FC>2 | FC>3 | FC>4 | FC>5 | FC>10 | FC>20 | FC>50 | |
|---|---|---|---|---|---|---|---|---|
| AA vs C | Upregulated | 621 | 217 | 123 | 75 | 36 | 21 | 7 |
| Downregulated | 938 | 377 | 201 | 117 | 30 | 14 | 6 | |
| AA vs PSG-F2 | Upregulated | 133 | 39 | 23 | 14 | 1 | 0 | 0 |
| Downregulated | 305 | 58 | 14 | 4 | 1 | 1 | 1 | |
3.3 Analysis of DEGs with GO and KEGG Enrichment
Based on RNA-Seq, KEGG enrichment was identified for DEGs altered by PSG-F2 protecting IEC-6 cells from AA. A total of 7089 DEGs were annotated with Gene Ontology (GO). GO analysis of DEGs was divided into three parts: biological process (BP), cell components (CC), and molecular function (MF), respectively. The DEGs of Group Control compared to Group AA are mainly concentrated in the BP of regulation of nucleus division, the cellular component of autolysosome, and the molecular function of transcription activator activity, which revealed that AA can destroy the intestinal permeability by affecting nucleus division and transcription resulting in autophagy (Figure 3A). According to GO enrichment analysis of Group PSG-F2 and Group AA (Figure 3B), the DEGs are mainly concentrated in the BP of chromosome segregation, CC of chromosome and centromeric region, and MF of ATP-dependent microtubule motor activity, which showed that PSG-F2 may protect intestinal barrier in AA-induced IEC-6 cells by changing chromosome separation and microtubule activity. According to the result of KEGG pathway enrichment in AA-induced damaged IEC-6 cells, compared with Group control, 330 pathways were involved in DEGs (Figure 3C), and the primary gene expression clustering for DEGs from the 67 significant signal pathways (p < 0.05) are as shown in Table S1, Supporting Information which mainly participated in signaling pathways linked to the cell life activities (such as proliferation, metabolism, and necroptosis). MAPK signaling pathway was one of the most significantly enriched pathways linked to AA-induced damage. As shown in Figure 3D, between Group PSG-F2 and Group AA, the KEGG enrichment results showed that 212 signaling pathways were significantly enriched, and there were 21 differential pathways. The most crucial pathways are presented in Table S2, Supporting Informationwhich indicated that PSG-F2 could play a preventive role in AA-induced damage.
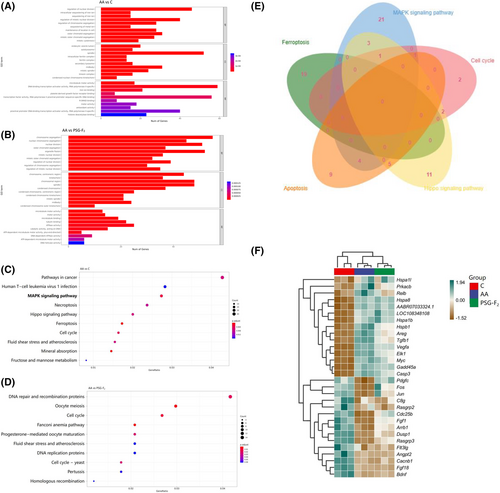
Based on the Venn plot of the primary signaling pathway (Figure 3E), we found that the number of DEGs in MAPK signaling pathway involved in cell cycle, apoptosis, and hippo signaling pathway were 2, 5, and 4, respectively. MAPK signaling pathway was significantly affected in IEC-6 cells by AA, with changes in expression observed for 35 DEGs, among which increasing and decreasing genes were 20 and 15, respectively (Figure 3F). In addition, MAPK signaling pathway is the most classical intracellular signal transduction pathway.[37] Therefore, this study focused on the protective mechanism of MAPK signaling pathway. Further analyses are necessary to fully investigate the involvement of MAPK signaling pathway at the protein level.
3.4 Regulated the Protein Expression of MAPK Signaling Pathway in IEC-6 Cells
To identify the role of MAPK signaling pathway, six representative protein members of the MAPK families (p-P38/P38, p-ERK/ERK, p-JNK/JNK) were analyzed by Western blot. Obviously, the phosphorylation level of MAPK protein was remarkably down-regulated in Group PSG-F2 (80, 160, and 320 µg mL−1) by as follows: p-ERK/ERK (62 ± 9%, 62 ± 5%, and 68 ± 6%), p-P38/P38 (36 ± 4%, 42 ± 6%, and 49 ± 5%), and p-JNK/JNK (45 ± 8%, 34 ± 6%, and 30 ± 4%), respectively, compared with those treated with AA (Figure 4, p < 0.05, p < 0.01). Taken together, pretreatment of PSG-F2 significantly reduced the ratio of p-ERK/ERK, so we used an ERK inhibitor to validate the mechanism of PSG-F2 at a later step.
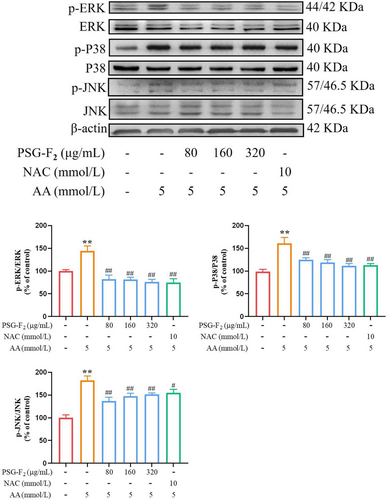
3.5 ERK Signaling Pathway Inhibitor PD98059 Suppressed the TJ Damage via Down-Regulating Expressions of MAPK/ERK Signaling Pathway
In addition, combined with our previous studies,[17] AA exposure increased the production of ROS and activated MAPK signaling pathway, but pretreatment with PSG-F2 could effectively reduce the expression of ERK. To further verify the significant role of MAPK/ERK signaling pathway in AA-induced intestinal barrier damage, ERK signaling pathway-specific inhibitor PD98059 was utilized. PD98059 was pretreated with different concentrations (1, 5, 10, 20, and 30 µmol L−1) for an hour before AA exposure for 8 h. As shown in Figure 5A, PD98059 (10–30 µmol L−1) could significantly relieve cytotoxicity of AA, and rebound cell viability by 9% to 16% (p < 0.01), which showed that the inhibition of ERK effectively protected AA-induced cell viability. Pretreatment with 10 µmol L−1 PD98059, the toxicity of AA can be alleviated, so 10 µmol L−1 of PD98059 was chosen to perform the following experiments. Also, the cell death of pretreatment with PSG-F2 and PD98059 was decreased compared to Group AA (Figure 5B, p < 0.01).
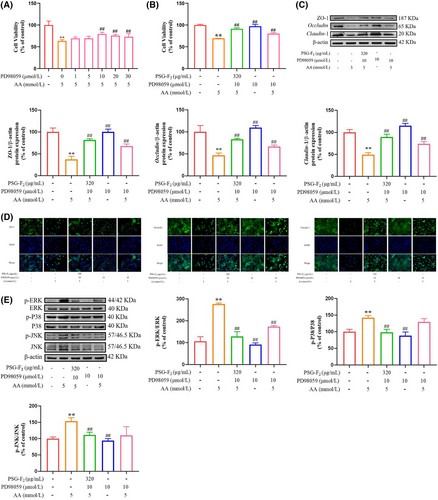
As shown in Figure 5C,D, TJ proteins expression levels were increased in the IEC-6 cells treated with PD98059compared with Group AA. The protein level of ZO-1, Occludin, and Claudin-1 were decreasing by 63 ± 7%, 53 ± 6%, and 50 ± 5%, respectively, compared with Group control (100%). The cells treated with PD98059 and PSG-F2 contributes to improvement in expression of TJ-related protein compared with Group AA (p < 0.05, p < 0.01). The immunofluorescence results also showed that the fluorescence intensity was almost absent in the Group AA, while the TJ between cells was more obvious with PD98059 pretreatment, and the fluorescence intensity of the cells treated with PSG-F2 and PD98059 approached that of the Group control. The results suggested that PSG-F2 improved TJ protein expression levels via MAPK/ERK signaling pathway.
The protein levels of MAPK signaling pathway were down-regulated in IEC-6 cells treated with PD98059 compared with Group AA (Figure 5E). The ratio of phosphorylation protein (p-ERK, p-P38, and p-JNK) in IEC-6 cells pretreated with PD98059 and AA decreased by 104 ± 5%, 36 ± 9%, and 33 ± 2%, respectively, compared with Group AA. Meanwhile, compared with the Group PD98059 (AA-induced damage cells treated with PD98059 alone), the levels of p-ERK decreased by 44 ± 5% after treatment of cells with PD98059, PSG-F2, and AA (Group PD98059 with PSG-F2), but the levels of P38 and JNK were not significant. The results suggested that MAPK/ERK signaling pathway was one of the main pathways for AA-induced damage and PSG-F2 protection in IEC-6 cells.
4 Discussion
Nowadays, natural plant extracts are considered a vital source of diet daily supplements due to their diversity of chemical structures, biological activities, and their overall reduced toxicity and side effects compared to chemically synthesized drugs.[23, 38, 39] Reveal functional mechanisms of natural plant extracts have become the focus of scientific research. Here, we report that PSG-F2, a natural polysaccharide isolated from Ganoderma atrum, exhibited a protective ability in intestinal epithelial IEC-6 cells. Recently, our in vitro study has shown that PSG-F2 could reduce cell apoptosis and oxidative stress in AA-induced damage of IEC-6 cells.[17] Mechanistically, we provided the evidence that PSG-F2 could protect IEC-6 cells via MAPK/ERK signaling pathway in vitro based on RNA-Seq, verifying through Western blot and immunofluorescence now. These findings not only reveal a potential for the protection of PSG-F2 in intestinal epithelial cells but also shine a light on the relationship between MAPK/ERK and TJ.
The intestinal barrier, which can effectively block the harmful substances such as bacteria and toxins passing through the intestinal mucosa, is mainly composed of the intestinal epithelial cells and the TJ between the IECs.[40] TJ functions to regulate epithelial permeability and integrity.[41] It is composed of a variety of transmembrane proteins (such as Occludin, Claudins) and cytoplasmic protein proteins (like ZO-1). The cooperation between Occludin and ZO-1 plays a key role in maintaining normal TJ structure and intestinal epithelial function.[42] Numerous studies have demonstrated that antioxidants such as procyanidin B2, myricetin, rhein, and quercetin can promote the intestinal barrier function,[23, 38, 39, 43-46] but their precise mechanisms are different, interestingly. Pterostilbene improves DSS-induced intestinal epithelial barrier destruction by inhibiting the NF-κB-mediated MLCK-MLC signaling pathway in mice.[47] Rhein regulates Nrf2 and MAPKs to ameliorate the intestinal barrier damage induced by lipopolysaccharide.[39] Quercetin inhibits the activation of PKC-δ and protects the intestinal barrier by improving the expression of TJ-related proteins.[48] In this study, pretreatment with PSG-F2 ameliorated AA-induced down-regulation of TJ protein. AA could reduce expression of ZO-1, Occludin and Claudin-1, indicating that AA impaired TJ and thus reduced cell viability. In contrast, pretreatment with PSG-F2 inhibited the AA-induced damage, thereby protecting IEC-6 cells. Therefore, it is reasonable that PSG-F2 has a preventive effect on AA-induced intestinal barrier damage by enhancing the expression of three important TJ proteins.
By RNA-Seq analysis, we identified MAPK signaling pathway as a possible target of PSG-F2. Recent evidences indicate that the permeability of the intestinal barrier and TJ-related proteins are regulated by a variety of cellular signaling pathways, such as MAPK cascades reactions.[49] Our result was in agreement with previous researches that showed that AA up-regulated the phosphorylation proteins level of P38, JNK, and ERK induced oxidative stress in the Caco-2 cells at the same concentration (5 mmol L−1) or PC12 cells at 10 mmol L−1, and the natural products (Olive oil hydroxytyrosol, Procyanidin B2, a cocoa polyphenolic extract, and Canolol) have ameliorative effects of AA-induced oxidative stress via MAPK signaling pathway.[4, 20, 23] Kinugasa et al. found that activation of ERK1/2 could improve trans epithelial electrical resistance and the mRNA expressions of Claudin-1 and -2 in the human colon cancer-derived cell line T84[50] and renal epithelial LLC-PK1 cells.[51] And the latest studies revealed that MAPK signaling pathway is one of the widest-regulated cellular pathways that regulate a variety of life activities of different kinds of cells, including proliferation, survival, apoptosis, inflammation, immunity, and differentiation.[52, 53] ERK, JNK, and the p38 subfamilies are the most extensively characterized MAPK members.[54, 55] The MAPK subfamily is involved in different signaling pathways. For example, ERK regulates the growth and differentiation of cells, and JNK and P38 play an important role in stress responses like inflammation and apoptosis. In particular, targeting ERK kinase may present promising prospects for repairing apoptosis-related cellular functions,[56] for example GINS2 could induced cell cycle arrest and apoptosis in PANC-1 cells via the MAPK/ERK signaling pathway.[57] And TNF-α promoted apoptosis by increasing mitochondrial fission through MAPK–ERK–YAP signaling pathway in A172 cells.[58] Butyrolactone-I could significantly alleviate LPS-induced TJ loss and inflammatory respond via MAPK signaling pathway in vivo and in vitro. Therefore, MAPK signaling pathway is closely related to apoptosis and intestinal barrier injury.[27, 28, 30, 59] Similar to natural plant extras mentioned above, PSG-F2 also induced down-regulated expression of MAPK signaling pathway key protein within 24 h of pretreatment, which led to balance of redox and even prevented oxidative stress in IEC-6 cells. Thus, PSG-F2 also functions as a protective diet daily supplements mainly through suppression of MAPK signaling pathway.
Combined pretreatment of PSG-F2 together with a classical ERK inhibitor, PD98059, showed synergistic positive effects on cell viability, and intestinal barrier. The effect caused by PSG-F2 in preventing AA-induced TJ damage could be further amplified by PD98059 since PD98059 could also protect IEC-6 cells from AA. Mechanistically, the protective efficacy was improved by the combined pretreatment of PSG-F2 and PD98059, which enhanced the inhibition of MAPK signaling pathway in AA-treated IEC-6 cells.
Notably, we found that AA-induced damage may involve the activation of ferroptosis, which was another remarkable pathway between Group AA and Group control in KEGG enrichment. As we know, ferroptosis is quite different from the normal cell death mechanism (such as apoptosis, necroptosis, etc.), mainly manifested by the imbalance of intracellular iron ion content and the high degree of lipid peroxidation.[60] From GO enrichment results, we found that ferroptosis-related GO terms made up a large percentage of the top 10 significantly enriched, such as “intracellular sequestering of iron ion,” “sequestering of iron ion,” and “iron ion binding,” which may indicate that AA could induce ferroptosis through changing the content of intracellular iron ion in IEC-6 cells. In connection with our previous study, AA can indeed cause an imbalance of the oxidative stress system in cells, and some crucial studies provided evidence that excessive iron can induce ferroptosis by the production of ROS via Fenton reaction.[61, 62] However, after pretreatment with PSG-F2, compared with the Group AA, the DNA repair-related pathway is more significant, which may be because that PSG-F2 can also affect the expression of some DNA repair proteins or genes, so as to achieve the purpose of protecting IEC-6 cells from AA. In addition, Wei et al. also showed that tagitinin C could induce ferroptosis via endoplasmic reticulum stress-mediated activation of PERK/Nrf2/HO-1 pathway.[61] Therefore, ERK may be directly linked to the protective mechanism of PSG-F2, and the next step is to investigate the relationship between PSG-F2 and ferroptosis.
Combined with the previous study of PSG-F2,[17] the protective mechanism of PSG-F2 was improved (this study is presented in the yellow box). We found that the pathway that is involved in the PSG-F2-protection of IEC-6 cells from apoptosis was not only the caspase cascade reaction but may also be mediated by the MAPK/ERK signaling pathway. PSG-F2 can alleviate the destruction of cell membrane integrity via MAPK/ERK signaling pathway, thereby relieving the cell apoptosis (Figure 6). Further research is needed to determine the PSG-F2 receptor and other proteins involved in the PSG-F2 mediated activation pathway to improve the integrity of the intestinal barrier via MAPK/ERK signaling pathway in IEC-6 cells.

In summary, combined with previous research, we reported that AA activated oxidative stress and apoptosis by MAPK/ERK signaling pathway, thereby destroying the intestinal epithelial barrier and permeability. Interestingly, PSG-F2 plays a protective role in AA-induced damage of IEC-6 cells by regulating MAPK/ERK signaling pathway. Besides, as PSG-F2 is involved in many signaling pathways, future work is needed to focus on the complex protective mechanism of PSG-F2, such as ferroptosis.
Acknowledgements
The financial support from the National Key Research and Development Program of China (2019YFE0106000), the National Natural Science Foundation of China (No: 21866021), the Natural Science Foundation Key Projects of Jiangxi Province (20212ACB205012), the Technology Innovation Leading Program of Jiangxi (20212BDH80001), and the Research Project of State Key Laboratory of Food Science and Technology (SKLF-ZZB-202115) are gratefully acknowledged. Furthermore, the Federal Ministry of Education, Science and Research (BMBWF) of the Republic of Austria as well as the Austria's Agency for Education and Internationalisation (OeAD) is acknowledged for the financial support via the Scientific and Technological Cooperation project “Study on simultaneous inhibition of typical thermal food processing contaminants in baked foods and their combinatory toxicity with mycotoxins” (CN 13/2020). The Data Availability Statement was added on March 20, 2023.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
J.L.: data curation, formal analysis, writing original draft; Y.Y.: investigation, review & editing; E.V.: methodology, review & editing; D.M.: investigation, review & editing; Q.Y.: visualization, software; J.X.: visualization, software; C.L.: methodology, software; Y.C.: review & editing, conceptualization, supervision.
Open Research
Data Availability Statement
Data available on request from the authors.




