Effect of the Emulsifier Used in Dunaliella salina-Based Nanoemulsions Formulation on the β-Carotene Absorption and Metabolism in Rats
Abstract
Scope
Microalgae such as Dunaliella salina are a potential sustainable source of natural β-carotene due to their fast growth and high adaptability to environmental conditions. This work aims to evaluate the effect of the incorporation of β-carotene from this alga into different emulsifier-type nanoemulsions (soybean lecithin [SBL], whey protein isolate [WPI], sodium caseinate [SDC]) on its absorption, metabolization, and biodistribution in rats.
Methods and results
Nanoemulsions formulated with different emulsifiers at 8% concentration are obtained by five cycles of microfluidization at 130 mPa, then expose to an in vitro digestion or orally administer to rats. Feeding rats with nanoemulsions improves β-carotene uptake compared to control suspension, especially using SDC and WPI as emulsifiers. A greater presence of β-carotene and retinol in the intestine, plasma, and liver is observed, being the liver the tissue that shows the highest accumulation. This fact can be a consequence of the smaller droplets that protein-nanoemulsions present compared to that with SBL in the intestine of rats, which promote faster digestibility and higher β-carotene bioaccessibility (35%–50% more) according to the in vitro observations.
Conclusions
Nanoemulsions, especially those formulated with protein emulsifiers, are effective systems for increasing β-carotene absorption, as well as retinol concentration in different rat tissues.
1 Introduction
Microalgae are photosynthetic microorganisms with enormous potential to be used as a source of bioactive compounds in functional food production due to their rapid growth under a wide range of environmental conditions.[1] Moreover, these organisms present some advantages compared with higher plants, such as faster growth, higher yield, and shorter cultivation time, which make them an interesting source of bioactive compounds such as astaxanthin, fucoxanthin, β-carotene, omega-3 fatty acids, and polyphenols.[2-6] Among them, Dunaliella salina is one of the best sources of natural β-carotene, up to 14% of its total dry weight,[7] presenting the 50% of total β-carotene as a 9-cis isomer. Previous studies have reported that the administration of this β-carotene isomer presented a higher tissue accumulation, antioxidant, and biological effects than (all-E)-β-carotene.[8, 9] However, despite the higher biological value that β-carotene presents, it is easily degraded due to its highly unsaturated structure.[10, 11] The incorporation of lipophilic bioactive compounds into oil-in-water nanoemulsions has been shown to be effective to protect them from degradation, facilitate their incorporation in aqueous matrices, and increase their absorption.[12-15] These systems that consist of a lipid phase dispersed into an aqueous phase usually need an emulsifier for their stabilization, which can be classified according to their nature into proteins, phospholipids, polysaccharides, or others.
The election of emulsifier has been shown to highly affect the physicochemical properties of nanoemulsions; moreover in vitro studies revealed that it also affects the digestibility and the encapsulated compound bioaccessibility.[15-17] However, in vivo studies are required to elucidate the impact of emulsifiers on the oral bioavailability of β-carotene. Since the current consumption trend is towards natural products, the emulsifiers used in this study were different natural ingredients: soybean lecithin (SBL), whey protein (WPI), and sodium caseinate (SDC).[18-20] SBL has been chosen as a phospholipid-based emulsifier, while WPI and SDC have been chosen as protein-based emulsifiers.
Previous authors have studied the in vivo bioavailability of synthetic β-carotene enclosed in emulsions or nanoemulsions. These studies have concluded that emulsion-based delivery systems can enhance the bioavailability of the compound, and that by reducing the particle size, the absorption and metabolism of β-carotene can be improved.[21, 22] Nevertheless, as far as we know, there are no studies reporting the bioavailability of β-carotene from a raw material such as alga D. Salina enclosed in nanoemulsions.
Administration of Dunaliella algae species to mice has been shown to be effective in inhibiting atherogenesis and fatty liver formation, showing that the activity was 9-cis dependent.[23] Other authors reported that carotenoids obtained from an algal source have a higher antihepatotoxic effect in rats compared with synthetic β-carotene and with β-carotene alone extracted from a natural source.[24] Moreover, D. salina revealed a significant antifibrotic effect in induced-fibrosis rats via ameliorating the elevation of liver enzymes, inflammatory mediators, and fibrotic markers.[25] In addition, β-carotene is transformed into retinol (vitamin A) in the intestine and other organs, which is an essential vitamin with numerous biological functions[26]
The study of the biodistribution and accumulation of bioactive compounds in the body is essential, since the biological effectiveness of bioactive compounds is determined by their storage sites. Moreover, the microstructural changes that nanoemulsions undergo in the different parts of the in vivo gastrointestinal tract (GIT) can be of high interest to better understand how nanoemulsion stability is related to the bioavailability of the compound. Therefore, the aim of this work was to study the impact of the emulsifier (SBL, WPI, and SDC) on the in vitro and in vivo gastrointestinal stability and digestibility of nanoemulsions. In addition, the oral bioavailability and biodistribution of β-carotene extracted from microalgae D. salina and its metabolite (retinol) in rats was evaluated.
2 Experimental Section
2.1 Materials
Freeze-dried alga D. salina was kindly provided by Monzón Biotech (Huesca, Spain). Pepsin (porcine), pancreatin (porcine), bile extract (bovine), lipase (porcine), Nile red, and SDC were obtained from Sigma–Aldrich (St. Louis, MO). Corn oil (Koipesol Asua, Deoleo, Spain) was purchased from a local supermarket. SBL was acquired from Alfa Aesar (Thermo Fisher Scientific, Massachusetts, USA). WPI was kindly provided by El Pastoret de la Segarra, S.L. (Spain).
2.2 Methods
2.2.1 Preparation of β-Carotene Enriched oil and β-Carotene Suspension
To obtain the β-carotene enriched oil, alga D. salina was mixed with corn oil at 65 °C (0.4 g alga mL−1 oil) and vortexed at 3000 rpm for 1 min. The mixture was treated with an Ultra-Turrax at 17 500 rpm for 2 min (IKA, Staufen, Germany) and submerged in a sonication bath for 5 min. Finally, the mixture was centrifuged at 9000 rpm for 15 min and the upper part was collected as the oil phase. The final concentration was 20 mg β-carotene g−1 oil, quantified by HPLC using the methodology described in Subsection 2.2.6.
To obtain the β-carotene suspension, the alga D. salina was mixed with water at 65 °C (0.4 g alga mL−1 water) and vortexed at 3000 rpm for 1 min. The mixture was treated with an Ultra-Turrax at 17 500 rpm for 2 min (IKA, Staufen, Germany) and submerged in a sonication bath for 5 min.
2.2.2 Nanoemulsion Preparation
To obtain the aqueous phase, 8% w/w of SBL, WPI, or SDC were added into ultrapure water and stirred for 4 h at room temperature. Then, 20% w/w of enriched β-carotene oil and 80% w/w of aqueous phase were mixed and homogenized using an Ultra-Turrax (IKA, Staufen, Germany) at 11 000 rpm for 2 min. Finally, nanoemulsions were obtained by passing coarse emulsions through a microfluidizer (LM10, Microfluidics, USA) at 130 MPa for five cycles. All formulated nanoemulsions presented a pH ≈6.
2.2.3 Nanoemulsions Characterization
Particle size. The particle size of nanoemulsions was measured using a Mastersizer 3000 (Malvern Instruments Ltd., Worcestershire, UK). Samples were diluted in ultrapure water and stirred in the dispersion unit with a constant speed of 1800 rpm. The mean particle size was expressed as surface area mean diameter (d32) in micrometers (µm), fixing a refractive index of the corn oil of 1.473 and 1.333 for water.
ζ-Potential. The ζ-potential was measured by phase-analysis light scattering (PALS) using Zetasizer laser diffractometer (Malvern Instruments Ltd Worcestershire, UK). Prior to the analysis, nanoemulsions were diluted (1:100) in ultrapure water. Gastric and intestinal samples were diluted maintaining its pH at 3 and 7, respectively. The results were reported in millivolts (mV).
Viscosity. Apparent viscosity of nanoemulsions was determined using an SV-10 vibro-viscometer (A&D Company, Tokyo, Japan), which produces a vibration of 30 Hz and a constant amplitude of 0.4 mm at controlled room temperature. The results were expressed in mPa s.
2.2.4 In Vitro Study
Gastrointestinal Digestion
To simulate the human digestion process, an in vitro GIT digestion based on an international consensus method[27, 28] with some modifications was used.
In Vitro Bioaccessibility
2.2.5 In Vivo Study
Animals and Study Design
Female Sprague Dawley rats weighing 200–250 g were used for the in vivo study. The animal procedures were conducted in accordance with EU Directive 2010/63/EU for animal experiments and approved by the Animal Ethics Committee of Universitat de Lleida (CEEA 01-04/18). After an acclimatization period, animals were randomly divided into four groups (n = 5) (suspension, SBL nanoemulsion, WPI nanoemulsion, and SDC nanoemulsion). Rats were fasted for 12 h before the experiment with free access to water. Rats were anesthetized with isoflurane and blood samples were taken by cardiac puncture. Plasma was immediately separated by centrifuging the blood samples at 4000 rpm for 10 min at 4 °C and stored at −80 °C. Rats were sacrificed by exsanguination and the stomach, duodenum, jejunum, ileum, and colon were removed and their content was collected and immediately characterized by microscopy. Then, the mentioned tissues, liver, kidney, white adipose tissue (mesenteric), and brown adipose tissue (cervical) were collected, rinsed with phosphate buffered saline, weighted, and stored at −80 °C.
Dosage Information
Rats were orally administered at a volume of 20 mL kg−1 with a feeding needle. All animals were fed with a dose of 60 mg of β-carotene per kilogram body weight, irrespective of the vehicle used, which is only achievable through supplements in humans. Control suspension consisted on the β-carotene from alga diluted in water. Sample administration was conducted in five times with 30 min intervals. Rats were sacrificed 30 min after the last administration.
2.2.6 Carotenoid Extraction and Quantification
Nanoemulsions and micellar samples. β-Carotene extraction was performed according to a previously reported method with some modifications.[29] Aliquots of 1 mL of sample were mixed with 2 mL of ethanol/hexane (2:3 v/v), vortexed at 1500 rpm for 1 min, and centrifuged at 4000 rpm for 5 min at 4 °C. Afterwards, the collected upper organic layers were collected and evaporated under N2 and stored at −40 °C. The quantification of β-carotene was carried out using an HPLC system equipped with a 600 Controller and a diode array detector (Waters, Milford, MA) following a previouslyreported method.[30] β-Carotene was identified using C30 column 250 × 4.6 mm i.d., 5 µm (Bischoff Chromatography, Leonberg, Germany) at room temperature. The injection volume was 20 µL and the flow rate was 1 mL min−1. Separation was carried out in 23 min under the following conditions: 0 min, 70% B; 10 min, 20% B; 20 min, 6% B; 21 min, 6% B; 23 min, 70% B. Water was kept constant at 4%. A β-carotene standard was used to identify analytes by retention times and ultraviolet–visible spectra. The HPLC–UV chromatograms were acquired, selecting the 450 nm wavelength.
Plasma and tissue samples. Retinol and β-carotene extraction was performed in a dark room to avoid compound degradation, following a previously reported method with some modifications.[31] For plasma samples, 150 µL were mixed with the internal standard trans-β-apo-8′-carotenal and 600 µL of ethanol/hexane (1:2 v/v) containing 0.1% BHT. Then, the mixture was vortexed at 1000 rpm for 1 min, centrifuged at 9000 rpm for 5 min at 4 °C, and the organic fraction was collected. Afterwards, the collected upper organic layers were collected and evaporated under N2 and stored at −80 °C. For tissue samples, 250 mg of tissue was mixed with 600 µL of milli-Q water and homogenized using an Ultra-Turrax at 9000 rpm for 1 min. After that, tissue homogenates were mixed with the internal standard trans-β-apo-8′-carotenal and 1000 µL of ethanol/hexane (1:2 v/v) containing 0.1% BHT, and the same procedure as for plasma samples was followed. The quantification of β-carotene and retinol from plasma and tissue samples was performed using a Acquity UPLC with a photodiode array detector from Waters (Milford, MA, USA) equipped with a binary solvent delivery system. The analysis was performed using a reverse-phase C18 column (ACQUITY UPLC® BEH 1.7 µm 150 × 2.1 mm) kept at 32 °C. The volume injected was 7.5 µL and the mobile phase consisted of a gradient of (A) acetonitrile/methanol (70:30 v/v) and (B) water/acetonitrile (95:5 v/v). The flow rate was 1 mL min−1. The gradient profile of the mobile phase was set at 90% A and increased linearly to 100% A over 5.5 min with a 6.5-min hold, after which the mobile phase was changed back to 90% A over 2 min and the held for 2 min. β-Carotene was detected at 450 nm and retinol at 325 nm.
2.2.7 Fluorescence Optical Microscopy
Initial nanoemulsions, nanoemulsions at the different phases of in vitro digestion, and nanoemulsions from the different GIT of rats were collected and dyed with Nile Red, previously dissolved at 0.1% w/v in ethanol. Then, micrographs were obtained using fluorescence with an optical microscope (Olympus BX41, Olympus, Göttingen, Germany) with a 100× objective lens. The images were obtained using a digital camera (Olympus DP74) and processed with the software CellSens (Olympus Göttingen, Germany)
2.2.8 Statistical Analysis
All experiments were assayed in duplicate and three repetitions of each analysis were carried out on each parameter in order to obtain mean values. Analysis of the variance (ANOVA) was performed to compare treatments. Least significant difference (LSD) test was employed to determine differences between means. The confidence interval was set at 0.95 and all results were analyzed using the Statgraphics Plus v.5.1 Windows package (Statistical Graphics Co., Rockville, Md).
3 Results and Discussion
3.1 Characteristics of Nanoemulsions
The electrical charge of all nanoemulsions was negative being about −48 mV in both SBL and SDC nanoemulsions and about −35 mV in that with WPI (Table 1). The negative electrical charge of the phospholipid (SBL) nanoemulsion was due to the negatively charged phosphate groups at the pH of the nanoemulsions (≈6)[32]. In protein-based nanoemulsions (those containing WPI or SDC), the negative ζ-potential was because the pH was above the pI of the proteins (≈5), so the ionizable groups were deprotonated and the net charge was negative.[33, 34] The smaller mean particle size values of protein-based nanoemulsions (≈0.250 µm) in comparison to that with the phospholipid (≈0.380 µm) may be attributed to the higher capacity of high weight emulsifiers (such as WPI or SDC) to form a viscoelastic film surrounding the droplets than low weight emulsifiers like SBL.[35] As can be observed in the microscope images (Figure 1), protein-based nanoemulsions presented aggregated droplets and a polydisperse distribution (Figure 2). In contrast, nanoemulsion formulated using the phospholipid showed bigger but well dispersed droplets than protein-based nanoemulsions, and therefore, a less polydisperse distribution (Figure 2). Flocculation observed in protein-based nanoemulsions may be a consequence of the strong hydrophobic attraction generated between protein-coated droplets promoted by the increased surface hydrophobicity caused by the conformational changes that proteins suffer when they are adsorbed at the interface.[19] Phospholipid-based nanoemulsion showed the lowest viscosity, followed by that formulated using WPI, being about 4 and 7 mPa s, respectively. SDC nanoemulsion showed a significantly higher viscosity than the other nanoemulsions, which was about 50 mPa s. Previous authors have also reported a relatively higher viscosity using SDC rather than other emulsifiers like SBL.[15] Such a high viscosity obtained using SDC may be a consequence of the formation of star-like aggregates that jam in dense aqueous suspensions and lead to a sharp increase of the viscosity.[36]
| Emulsifier | Particle size [µm] | ζ-potential [mV] | Viscosity [mPa s] | |
|---|---|---|---|---|
| Initial | SBL | 0.377 ± 0.015Ac | −49.90 ± 2.71Aa | 3.96 ± 0.35Ba |
| WPI | 0.262 ± 0.003Ab | −35.07 ± 0.90Ac | 7.28 ± 1.97Ca | |
| SDC | 0.232 ± 0.009Aa | −47.21 ± 1.39Ab | 50.0 ± 6.9Bb | |
| Gastric | SBL | 11.62 ± 0.64Cb | −21.07 ± 1.48Ca | 2.88 ± 0.67Aa |
| WPI | 1.70 ± 0.31Ba | 13.43 ± 0.77Cb | 4.55 ± 1.20Bb | |
| SDC | 12.48 ± 0.53Cc | 14.90 ± 1.30Cc | 5.62 ± 0.20Ac | |
| Intestinal | SBL | 3.10 ± 0.15Bc | −33.25 ± 1.97Ba | 2.56 ± 0.32Ab |
| WPI | 0.39 ± 0.02Aa | −33.41 ± 1.79Ba | 1.75 ± 0.04Aa | |
| SDC | 0.66 ± 0.06Bb | −27.73 ± 1.11Bb | 1.54 ± 0.02Aa |
- Values are expressed as mean ± standard deviation. Different capital letters indicate significant differences (p < 0.05) between different digestion phases of the same nanoemulsion. Different lowercase letters indicate significant differences (p < 0.05) between nanoemulsions with different emulsifier.
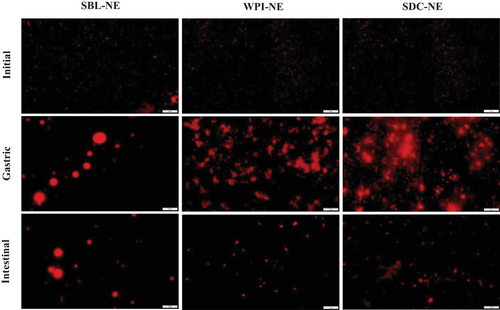
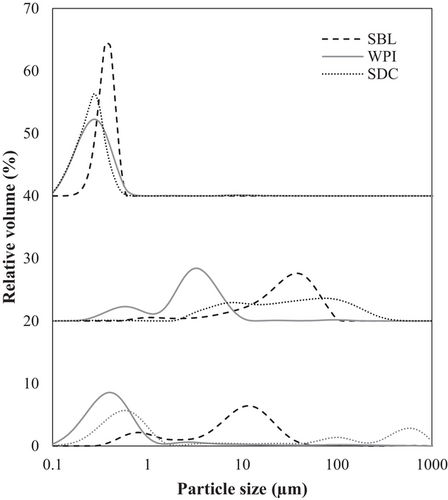
3.2 In Vitro Gastrointestinal Digestion of β-Carotene Nanoemulsions
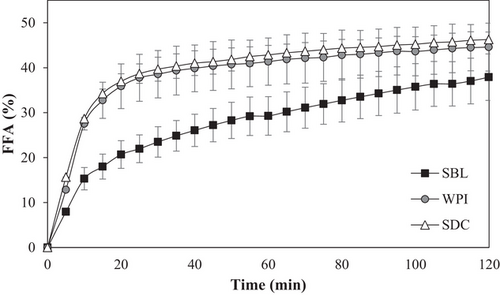
Nanoemulsions formulated with a protein emulsifier (WPI or SDC) presented a higher initial rate of FFA release than that formulated with a phospholipid (SBL), although no differences were observed in the final extent (≈43%) (Figure 3). These results evidenced that the election of emulsifier has an impact on the digestibility of nanoemulsions and is in agreement with previous works.[37] The differences in the initial rate of FFA release observed between protein- and phospholipid-based nanoemulsions may be attributed to the particle size presented during gastrointestinal digestion. When entering the intestine, protein-based nanoemulsions presented small particle sizes due to the redispersion of the gastric aggregates as a consequence of the electrostatic stabilization[38] (Table 1 and Figure 1). Such small particle sizes increased the surface area of lipid exposed to lipase and facilitate lipid digestion.[39] Conversely, the phospholipid-based nanoemulsion presented a higher particle size due to the coalescence phenomenon (Figure 1), and therefore, a lower surface area and reduced access of lipase to the lipid substrate. Moreover, protein-based emulsifiers presented a lower surface activity than the phospholipid, so they were more likely to be partially digested by pepsin. Thus, protein nanoemulsions may present a relatively weak interfacial layer and facilitate absorption of bile salts and or/lipase.[16]
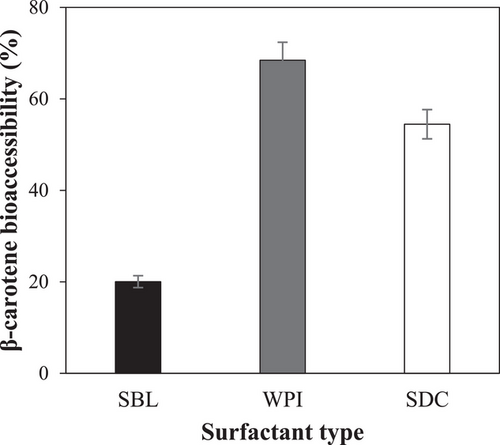
The highest β-carotene bioaccessibility was found in WPI nanoemulsion (≈68.5%), followed by SDC nanoemulsion (≈55.5%), and SBL nanoemulsion (≈20%) (Figure 4). These results highlight that the emulsifier nature significantly impacted the bioaccessibility of β-carotene, although all nanoemulsions presented the same final lipid digestibility (Figure 3), as observed previously by other authors.[15] Moreover, the results obtained in this work evidenced that the incorporation of β-carotene into nanoemulsions can greatly enhance its bioaccessibility. Nonencapsulated β-carotene has been reported to present a very low stability under gastrointestinal conditions, being mostly degraded during its pass through the GIT.[40]
The higher β-carotene bioaccessibility achieved using WPI or SDC compared to SBL could be a consequence of the antioxidant activity provided by the peptides resulting from the protein digestion.[41] In fact, previous studies have also reported a high β-carotene bioaccessibility using WPI as emulsifier of nanoemulsions.[42] Moreover, the higher β-carotene bioaccessibility of WPI compared to SDC nanoemulsions despite the same FFA release profile (Figures 3 and 4) could be due that WPI can better inhibit lipid oxidation than SDC.[43] The affinity for iron of phosphate groups of casein molecules is greater and stronger than that of carboxylate groups present in other proteins.[44] Thus, SDC can promote lipid oxidation due to the chelating properties and electrostatic interactions of adsorbed casein molecules that favor the positioning of transition metal ions on the surfaces.[45] In contrast, WPI has a more compact structure and a lack of phosphate groups, which could limit the ability to bind iron, avoid oxidation,[46] and prevent lipid oxidation by inactivating peroxyl radicals.[47, 48] Finally, the markedly reduced bioaccessibility observed using the phospholipid-based nanoemulsion may be attributed to the low capacity of the phospholipid emulsifier to inhibit oxidation. In addition, previous authors have observed reduced β-carotene bioaccessibility by using phospholipid-based emulsifiers such as soy lysolecithin due to the sedimentation of the compound during the centrifugation.[49]
3.3 In Vivo Digestion of β-Carotene Nanoemulsions in Rats
3.3.1 Microstructural Changes in Rat Digesta
The microstructure of the SBL, SDC, and WPI nanoemulsions in the digesta of the different regions of the GITs (stomach, duodenum, jejunum, ileum, and colon) of the rats were studied by analyzing the digesta of the different regions post-mortem, using an optical microscopy (Figure 5). Microscope images of digesta from rats fed with the suspension were also taken as a control.
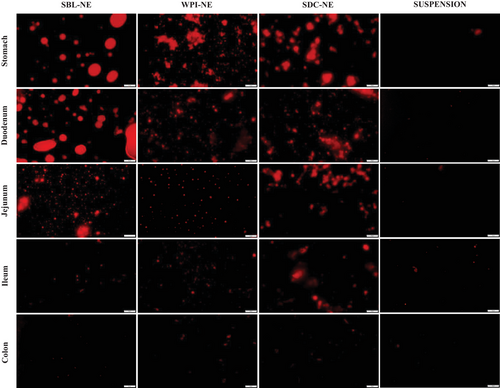
In the gastric digesta, protein-based nanoemulsions presented aggregation of droplets, while phospholipid-based nanoemulsion showed droplet coalescence (Figure 5). Other in vivo studies also showed flocculation in the stomach of rats using nanoemulsions containing SDC as emulsifier.[50] In addition, these observations are consistent with the in vitro results from the present study (Figure 1). These instability phenomena may be promoted by the reduction in the ζ-potential of nanoemulsions (Table 1).
Duodenum digesta microstructure of rats was also different depending on the emulsifier used. Microscope images showed that nanoemulsion containing the phospholipid presented higher particle sizes than those formulated using proteins. Similarly, to in vitro observations, aggregated droplets of protein-based nanoemulsions were dispersed in the duodenum due to the electrostatic stabilization promoted by an increase of the ζ-potential (Table 1 and Figure 1). In fact, previous authors[50] observed the same effect in rats fed with nanoemulsions containing SDC as emulsifier at different oil concentrations. In contrast, the particle size of phospholipid-based nanoemulsion increased as a result of droplet coalescence.
As the nanoemulsions progressed throughout the distal parts of the small intestine, fewer and smaller oil droplets were observed (Figure 5). This indicates that oil droplets were successfully digested and absorbed through the enterocytes of the intestinal mucosa by being incorporated into the mixed micelles.[51] Even so, in the jejunum digesta of rats fed with phospholipid-based nanoemulsion were still larger droplets than in those of rats fed with protein-based nanoemulsions (Figure 5), which may be attributed to the presence of lipid droplets, micelles, and vesicles.[50] It seems that nanoemulsion containing the phospholipid was digested more slowly than protein nanoemulsions, as can be seen in Figure 3. At the end of the gastrointestinal digestion, very few lipid droplets were observed in the colon of rats, irrespective of the emulsifier used. This indicates that all nanoemulsions were successfully digested and lipids were absorbed.
3.3.2 Retinol and β-Carotene Concentration in Rat Plasma and Tissues
β-Carotene is absorbed in the intestinal cells (enterocytes) mainly via passive diffusion or through facilitated transport via scavenger receptor class B member 1 (SR-BI) and possibly other lipid transporters, such as CD36.[52] As can be observed in Table 2, the β-carotene concentration in the duodenum was greatly increased when the bioactive compound was enclosed in nanoemulsion (≈1289–2000 ng g−1) rather than administered in suspension (≈125 ng g−1). The presence of oil in nanoemulsions could have enhanced the absorption of β-carotene, since the coingestion of carotenoids with lipids has been reported to enhance its absorption.[53, 54] Moreover, according to previous works, the presence of emulsifiers could have reduced the interfacial tension and increased the membrane fluidity enhancing the absorption of the bioactive compounds in the intestinal cells.[55] Higher absorption was detected in rats fed with protein-based nanoemulsions than those fed with phospholipid-based nanoemulsion. Indeed, these results are in accordance with our in vitro observations, in which the bioaccessibility was higher using WPI or SDC rather than SBL (Figure 4). As mentioned in the previous section, this fact can be attributed to the higher capacity of protein emulsifiers than phospholipids to prevent β-carotene degradation during the GIT and increase the bioaccessibility of the compound. In fact, some authors have reported that the presence of proteins such as WPI or SDC can increase the solubilization and transition of β-carotene into the mixed micelles.[56, 57]
| β-Carotene [ng g−1 tissue or mL plasma] | ||||
|---|---|---|---|---|
| SUSP | SBL-NE | SDC-NE | WPI-NE | |
| Duodenum | 125.2 ± 69.0a | 1289.0 ± 565.5b | 1903.9 ± 759.0bc | 2088.6 ± 621.9c |
| Jejunum | 1724.5 ± 1599.6a | 6742.6 ± 1924.6ab | 7175.9 ± 5061.8b | 14 200.4 ± 4453.0c |
| Ileum | 1599.6 ± 1829.1a | 1737.0 ± 784.2a | 3404.9 ± 293.6b | 8381.5 ± 1390.3c |
| Colon | 105.7 ± 80.8ab | 148.5 ± 92.9ab | 182.2 ± 36.9ab | 96.7 ± 15.6a |
| Liver | 64.1 ± 43.8a | 1853.2 ± 292.1b | 1684.7 ± 636.4b | 1760.7 ± 408.4b |
| Kidney | ND | ND | ND | ND |
| White adipose | ND | ND | ND | ND |
| Brown adipose | ND | ND | ND | ND |
| Plasma | 42.9 ± 29.9a | 1410.0 ± 344.9b | 1259.7 ± 224.4b | 1201.0 ± 108.4b |
- Values are expressed as mean ± standard deviation. Different letters indicate significant differences (p < 0.05) between vehicles.
Once absorbed in the intestine, β-carotene is mainly cleaved to retinol and other metabolites by the β-carotene-15,15′-oxygenase (BCO1) but also through β-carotene-9′,10′-oxygenase (BCO2) enzyme in the intestinal cells, where rats present the highest activity of both enzymes.[26] In fact, this could be the reason why β-carotene could have not been quantified in some tissues such as the kidney, white adipose, and brown adipose tissue of rats (Table 2). However, although rodents are very efficient in cleavage of β-carotene to retinol in the intestine, it is possible to detect circulating β-carotene when it is administered in supraphysiological amounts,[58] as is the case of the present study, where β-carotene was detected in plasma and liver tissues (Table 2), being the latter one of the most important sites of β-carotene accumulation.[59]
Table 3 shows that the orally administered β-carotene was conversed to retinol and was found mainly in the latter form in rat tissues. Higher retinol concentrations in rats administered with nanoemulsions than in those fed with the suspension were observed (Table 3), as a consequence of the greater β-carotene absorption using nanoemulsions rather than the suspension (Table 2). Again, differences were observed depending on the emulsifier used, being SDC and WPI nanoemulsions the systems that promoted the highest retinol concentrations, especially in the duodenum, liver, and kidney of rats (Table 3). This fact can be attributed to the presence of higher levels of β-carotene in the intestinal cells of rats fed with SDC or WPI nanoemulsions rather than with SBL nanoemulsion (Table 2). However, although adipose tissue is an important site of β-carotene accumulation in humans, it was not detected in rats in this study (Table 2). In fact, previous studies have also failed to detect this compound in rat adipose tissue after administration, even after chronic feeding.[60, 61] In contrast, retinol was quantified in the adipose tissue of rats, although no differences were detected among vehicles (Table 3). The presence of retinol in rat adipocytes could be related to the presence of the retinol-binding protein (RBP) in these cells.
| Retinol [ng g−1 tissue or mL plasma] | ||||
|---|---|---|---|---|
| SUSP | SBL-NE | SDC-NE | WPI-NE | |
| Duodenum | 147.8 ± 43.4a | 123.8 ± 48.7a | 250.6 ± 70.8b | 238.6 ± 45.2b |
| Jejunum | 58.7 ± 54.7a | 204.0 ± 73.7b | 248.7 ± 65.3b | 312.5 ± 169.3b |
| Ileum | 217.5 ± 104.2a | 280.1 ± 91.9a | 262.8 ± 85.1a | 263.3 ± 77.5a |
| Colon | 56.3 ± 17.4a | 62.4 ± 27.6a | 87.2 ± 27.8a | 51.4 ± 42.4a |
| Liver | 745.8 ± 514.3a | 1825.0 ± 355.7b | 1962.3 ± 690.9b | 3253.5 ± 645.2c |
| Kidney | 634.3 ± 294.3a | 821.4 ± 324.7a | 1242.8 ± 305.8b | 2324.0 ± 507.2c |
| White adipose | 212.8 ± 22.9ab | 225.1 ± 48.8b | 123.8 ± 36.4a | 189.5 ± 82.7ab |
| Brown adipose | 214.1 ± 93.0a | 189.3 ± 51.7a | 234.6 ± 54.2a | 249.7 ± 78.9a |
| Plasma | 104.5 ± 49.9a | 232.7 ± 33.3b | 369.5 ± 62.0c | 473.2 ± 23.0d |
- Values are expressed as mean ± standard deviation. Different letters indicate significant differences (p < 0.05) between vehicles.
Interestingly, although retinol and β-carotene concentrations in the liver and plasma were higher using nanoemulsions than the suspension, they showed different trends depending on the vehicle used. Feeding the rats with the suspension, retinol concentrations were higher than those of β-carotene in these tissues. In contrast, using nanoemulsions, a higher concentration of β-carotene rather than retinol was observed in the plasma of rats and similar values were detected in the liver (Tables 2 and 3). This suggests that the metabolism of β-carotene could be different when enclosed in nanoemulsions than in suspension. Although β-carotene is usually cleaved in the intestine, it can also follow another pathway. Intact β-carotene can escape the intestinal cleavage, be incorporated into chylomicrons and released by exocytosis to the lymphatic system for delivery to the bloodstream and eventually directed to the liver.[52] Therefore, it seems that by using the suspension, the β-carotene is more likely to be cleaved into retinol in the intestinal cells, whereas the use of nanoemulsions appears to promote the lymphatic pathway, therefore, showing high levels of intact β-carotene in the plasma and liver (Table 2). Once β-carotene arrives in the liver, it can be cleaved into retinol since this organ also contains BCO1 and BCO2 enzymes, although the activity that they present in this tissue is reduced compared to that in the intestinal tissue.[62] In fact, the liver was the tissue that presented the highest retinol accumulation, followed by the kidney (Table 3).
4 Concluding Remarks
All studied emulsifier-type nanoemulsions showed to be effective to encapsulate and protect β-carotene extracted from alga D. salina. Feeding rats with nanoemulsions increased the intestinal absorption of β-carotene, increasing the concentration of the compound in the enterocytes compared to the suspension. Moreover, the retinol concentration was higher using nanoemulsions than the suspension, both in the intestine and in the liver, kidney, and plasma. Among the nanoemulsions studied, those formulated with protein emulsifiers (WPI or SDC), promoted a higher concentration of β-carotene in the intestine of rats rather than that with the phospholipid (SBL). Indeed, by administering nanoemulsions formulated with WPI as emulsifier, the highest concentration of retinol was detected in plasma, duodenum, liver, and kidney. The low efficiency of SBL to enhance β-carotene absorption could be related to the coalescence phenomena observed in the stomach of rats and the reduced β-carotene bioaccessibility that revealed in vitro experiments.
Therefore, protein-based nanoemulsions, especially those with WPI, appear to be promising encapsulation systems to increase the absorption of β-carotene from alga D. salina and enhance the bioavailability and biodistribution of β-carotene and retinol in rats. Nevertheless, more studies should be done to better understand the specific mechanisms involved in the β-carotene metabolism when ingested enclosed in nanoemulsions, as well as to do further steps to scale the results obtained up to humans.
Acknowledgements
This work was funded by the project AGL2015-65975-R (FEDER, MINECO, UE) and project RTI2018-094268-B-C21 (MCIU, AEI; FEDER, UE). Author Júlia Teixé Roig thanks the University of Lleida for the pre-doctoral grant. The authors would also thanks Monzón Biotech (Spain) for kindly providing alga Dunaliella salina.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
J.T.R. conducted the in vitro and in vivo experiments. All authors contributed to the design of the study, interpretation of the data and edition and revision of this manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




