Autophagy Activation by Resveratrol Reduces Severity of Experimental Rheumatoid Arthritis
Abstract
Scope
Previous work reported that dietary supplementation with resveratrol lowers synovial hyperplasia, inflammatory and oxidative damage in an antigen-induced arthritis (AIA) model. Here, it is investigated whether resveratrol can regulate the abnormal synovial proliferation by inducing autophagy and controlling the associated inflammatory response.
Methods and results
Animals treated with resveratrol 8 weeks before AIA induction show the highest significant signal for microtubule-associated protein 1 light chain 3 by confocal microscopy. Besides, resveratrol significantly reduces p62 expression, but it does not increase the signal of beclin-1. Also, active caspase-3 expression, as well as poly(ADP-ribose) polymerase, is upregulated in the AIA group, and is significantly reduced in resveratrol-treated AIA group. Resveratrol also mitigates angiopoietin-1 and vascular endothelial growth factor signals. Finally, resveratrol significantly reduces the serum levels of IL-1β, C reactive protein, and prostaglandin E2, as well as nuclear factor κB synovial tissue expression, which shows a significant correlation with p62 expression.
Conclusion
Dietary supplementation with resveratrol induces the noncanonical autophagy pathway and limits the cross-talk with inflammation, which in consequence modulates the synovial hyperplasia. Preventive strategies that incorporate dietary intervention with resveratrol may offer a potential therapeutic alternative to drugs to influence the risk of rheumatoid arthritis and influence its course.
1 Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disorder of the synovial joints. It is characterized by the abnormal synovial hyperplasia with local infiltration of various types of immune and inflammatory cells, all in conjunct leads to the formation of an abnormal tissue called rheumatoid pannus, which finally destroys the adjacent cartilage and bone.[1] Interestingly, it has been suggested that a reduced rate of programmed cell death could provide one explanation for the pannus formation.[2]
Initially, autophagy was identified as a cellular housekeeping pathway for cell survival strategy during nutrient starvation or other stresses. However, autophagy not only induces cell survival, but can also lead to a form of cell death that is characterized by the accumulation of autophagic vacuoles and termed type II programmed cell death or autophagy cell death (vs type I programmed cell death or apoptosis).[3, 4] Autophagy plays a delicate role in the regulation of cell survival and death. During the autophagy process, a high number of autophagosomes (a double-membrane cytoplasmic vacuoles) are created, which fuse with lysosomes to form autolysosomes. Microtubule-associated protein 1 light chain 3 (LC3), beclin-1, and ubiquitin-binding-protein p62 (p62), are three major regulators of the autophagy pathway. The LC3, an autophagy executor, presents two forms: the inactive LC3-I (located in cytoplasm), which is converted to the lipidated form LC3-II (localized in autophagosomal membranes). Then, the amount of LC3-II correlates with the extent of autophagosome formation.[5] Beclin-1 participates in the early stage of autophagosome formation, and p62 is a substrate of autophagy whose degradation may reflect upregulated autophagic clearance.[6]
Similar to other autoimmune diseases, autophagy plays a dual role in RA,[7, 8] showing a therapeutic and pathogenic role.[9, 10] Thus, increased levels of autophagy have been described in the synovium of patients with active RA and are correlated with disease severity.[11] Moreover, increased autophagy in RA CD4+ T cells results in T-cell hyperactivation and apoptosis resistance.[12] However, other studies have suggested that severe endoplasmic reticulum stress in RA synovial fibroblasts leads to cell death through the formation of autophagic vacuoles.[7] Also, it has been demonstrated that a combination of the mammalian target of rapamycin (mTOR) (suppressor of autophagy) inhibitor everolimus and methotrexate (MTX) may be useful for the treatment of RA in patients who have an inadequate response to MTX monotherapy.[13] Notably, autophagy seems to be related to RA; however, this relationship is still controversial.
Although the past decade has seen remarkable advances in treatment strategies for RA, some patients do not reach low disease activity or become non-responders, and most of the treatments induce multiple side effects. With this scenario, natural compounds that exhibit anti-oxidant and anti-inflammatory properties have gained medicinal potential for the development of new drugs. Resveratrol is a polyphenol present in our diet, which has been widely recognized for its anti-inflammatory, anti-oxidant, anti-cancer, and anti-ageing properties.[14-17] Previously, others and we described that resveratrol exerts potent anti-proliferative, immunomodulatory, anti-inflammatory, and anti-oxidant effects in an in vivo acute arthritic model.[16, 18-20] However, conflicting results were reported about the role of resveratrol on cell death induction.[21-23]
In this way, the aim of our study was to increase the understanding of the anti-proliferative and anti-inflammatory capacity of dietary supplementation with resveratrol in the acute phase of an antigen-induced arthritis (AIA) model that shares many histopathological similarities with human RA arthritis. We hypothesized that resveratrol might also have a protective effect by enhancing autophagic flux and modulating the cross-talk existence with inflammation.[24] The results of this study are of great interest, because prevention is one of the challenges in RA, and diet could potentially be modifiable.
2 Results
2.1 Resveratrol Suppresses Synovial Hyperplasia by Enhancing Autophagic-Flux in AIA
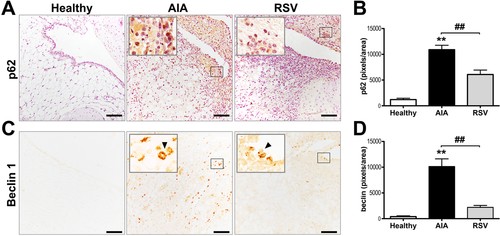
Given that our previous results showed that resveratrol modulates the abnormal proliferation of synovial tissue by decreasing synovial cell proliferation,[16] we now evaluated whether resveratrol could modulate cell death pathways. We first explored whether resveratrol can increase the autophagic flux. Results showed a higher expression of LC3 (p ≤ 0.001), mainly located in the cellular infiltrate from AIA synovium, compared with healthy synovial samples (Figure 1), in which just a low basal signal was observed dispersed in the cytoplasm from the cells of the lining zone. However, the highest expression of LC3 was reached in the resveratrol group, with a significant larger signal for LC3, compared with the AIA samples (p ≤ 0.05) in which its expression was observed in cells from all synovium and even the presence of autophagic vacuoles were easily observed in the tissue (Figure 1C). Additionally, to have a more accurate detail of autophagic activity, we also assessed p62 and beclin-1 autophagy markers. In agreement with the LC3 expression, in AIA group, both p62 and beclin-1 showed a greater expression (p ≤ 0.001) in the cellular infiltrate and in cells of the lining zone than in healthy controls (Figure 2). However, p62 and beclin-1 signals were markedly decreased by resveratrol treatment compared with AIA samples (p ≤ 0.005 and p ≤ 0.001, respectively). Thus, the results suggest that resveratrol could promote the non-canonical (beclin-1 independent) autophagy pathway in the synovial tissue of an AIA model.
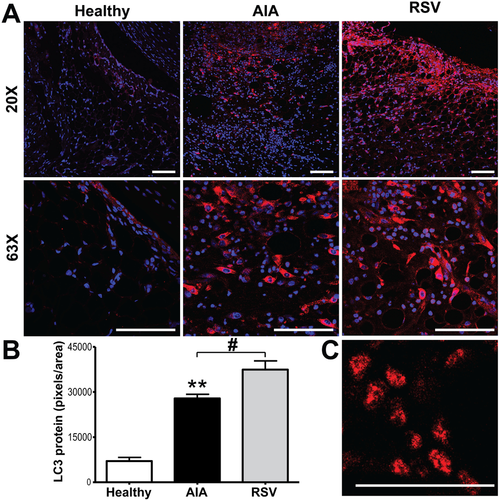
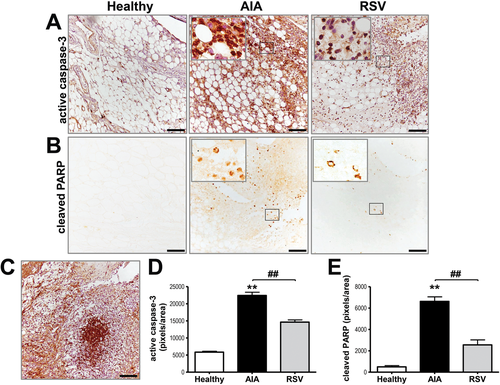
We next sought to determine the effect of resveratrol on synovial tissue apoptosis by measuring the expression of active caspase-3, as well as its substrate, the poly(ADP-ribose) polymerase (PARP). Figure 3 shows synovium from AIA samples had the highest level of both, compared with the healthy and resveratrol groups. These signals were mainly observed in cells from the infiltrate and the lining area. In the resveratrol-treated AIA group, the expression of active caspase-3 was significantly reduced (p ≤ 0.001) compared with AIA samples, but maintaining a higher level compared with healthy group. Consistent with active caspase-3 staining, resveratrol treatment also significantly (p ≤ 0.001) lowered PARP expression. Thus, oral administration of resveratrol down-regulates apoptosis but upregulates autophagy in the synovial tissue of an AIA model, suggesting that resveratrol can modulate synovial hyperplasia by enhancing autophagic-flux in AIA.
2.2 Preventive Effect of Resveratrol on Inflammation of AIA Samples and Its Correlation with p62 Synovial Expression
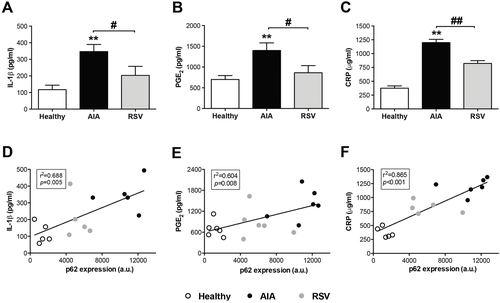
Given that autophagy could regulate the cytokine IL-1β via inflammasome, and IL-1β is considered one of the cytokines that orchestrate RA, we next assessed the effect of resveratrol on IL-1β levels in serum. As shown in Figure 4, the IL-1 β levels, which increased in the serum of AIA animals in relation to healthy animals (p ≤ 0.005), were significantly prevented by the oral administration of resveratrol (p ≤ 0.05). Similar results were obtained with prostaglandin E2 (PGE2 ) and C reactive protein (CRP), inflammatory key mediators, in different forms of inflammatory arthritis (Figure 4). Furthermore, we analyzed if the inflammatory serum markers evaluated were correlated with autophagy through p62 synovial expression (Figure 4). The mean p62 expression level was significantly and positively correlated with serum IL-1β (p ≤ 0.005 and r = 0.688), PGE2 (p ≤ 0.01 and r = 0.604), and PCR levels (p ≤ 0.0001 and r = 0.865).
Since nuclear factor κB (NF-κB) contributes not only to inflammation, but also to cell proliferation, angiogenesis, and subsequently pannus formation, we also evaluated the effect of resveratrol on synovial tissue NF-κB p65 protein expression (Figure 5). Results showed an increased expression of p65 in the cellular infiltrate and in cells from the lining zone from AIA samples, compared with healthy samples (p ≤ 0.001). In contrast, the treatment with resveratrol reduced significantly its expression (p ≤ 0.05), but maintains a higher signal in relation to the healthy group. Finally, when correlation between p65 and p62 expression was evaluated, a stronger positive correlation was observed (p ≤ 0.0001), which reached a correlation coefficient of 0.876 (Figure 5C). Thus, oral administration of resveratrol can help to control the systemic and synovial inflammation in AIA via enhancing autophagy flux and reducing the NF-κB pathway.
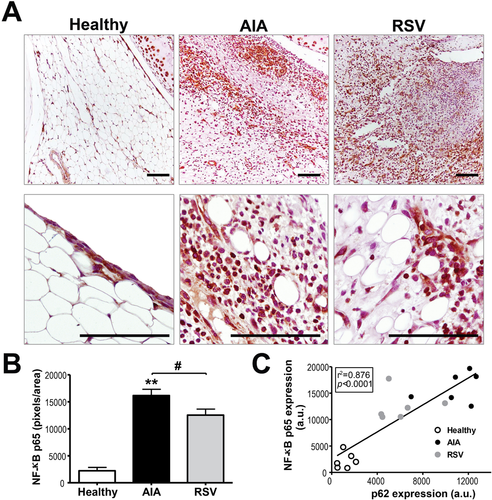
2.3 Anti-Angiogenic Effects of Resveratrol in AIA Synovial Tissue
Angiogenesis plays a pivotal role in inflammatory arthritis since it is required for the development of proliferative synovial tissue. Our recent study revealed that resveratrol reduces the serum concentration of cytokine-induced neutrophil chemoattractant (CINC-1), a murine homologue of human IL-8 chemokine that plays a central role in RA angiogenesis.[16] To help to unravel whether resveratrol could also modulate the development of the rheumatoid pannus through the expression of key pro-angiogenic factors, we evaluated the expression of vascular endothelial growth factor (VEGF) and angiopoietin 1 (Ang-1). Results showed that healthy samples presented an almost null expression of VEGF, which was mainly located in cells from the lining, while the AIA group showed a high expression of VEGF (p ≤ 0.001) that was homogenously distributed in all tissue (Figure 6). Although the resveratrol group showed a tendency to reduce the VEGF expression, non-significant expression changes were observed compared with the AIA samples. Therefore, we identified the blood vessel maturation present in synovial tissue through the expression of Ang-1 (Figure 6). Healthy samples showed a low basal expression of Ang-1 that was mainly located in the blood vessel cells. As expected, the expression of Ang-1 was highly increased in AIA samples (p ≤ 0.001), showed a homogeneous localization in all synovial membrane; while its expression was significantly reduced (p ≤ 0.05) after resveratrol treatment, showing a limited expression in the blood vessel cells. Resveratrol could also contribute to modulate synovial hyperplasia by decreasing the formation of new blood vessels in the synovial membrane.
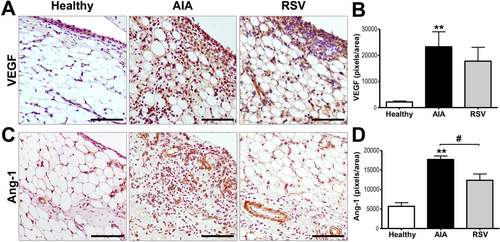
3 Discussion
Synovial hyperplasia associated with local infiltration of leukocytes, which has been described as the major pathological feature in RA and other inflammatory arthritis,[1] could be as a result of alterations in cell death.[2] Interestingly, autophagy was initially identified as a survival mechanism in response to stress,[4] but can also play a role in cell death, known as autophagy cell death, or type II programmed cell death.[25] Besides, recent studies have connected autophagy to the regulation of proinflammatory cytokines.[24] Several studies suggest that pharmacological intensification of autophagic flux could possess disease-modifying activity in the treatment of RA acting at different levels[13, 26]; conversely, enhancement of the autophagic pathway has been implicated in RA.[9, 11] Clearly, more studies are required to elucidate the role of autophagy in RA.
We have recently reported a decreased disease severity in an acute AIA model by dietary oral administration of resveratrol, a polyphenol known for its effect on a large number of chronic diseases and its high number of therapeutic benefits.[14, 16] The reduced arthritis severity was accompanied by significant down-regulation of synovial hyperplasia, measured specifically with proliferating cell nuclear antigen, as well as by a reduction of local massive infiltration of immune and inflammatory cells. Besides, a potent decrease of cytokine-mediated inflammation and oxidative damage was described.[16] Other research has also demonstrated decreased severity in other animal models of arthritis by using resveratrol.[18-20, 27] However, whether resveratrol can regulate the abnormal synovial proliferation by regulating cell death pathways, inducing autophagic flux in an in vivo arthritis model, remained unknown. Several studies described that resveratrol induces cell death through autophagy in cancer cell lines, which suggested its use as an effective treatment in apoptosis-resistant cells.[23] The dose of resveratrol used in our model (12.5 mg kg−1 day−1) was equivalent to 2.0 mg kg−1 day−1 in humans (≈114 mg resveratrol in a 70 kg adult) according to the human equivalent formula. Data from clinical trials indicate that daily doses of resveratrol between 20 mg and 2 g are safe and well tolerated.[28]
To the best of our knowledge, this is the first study to show that prophylactic oral administration of resveratrol can have a protective effect in RA by enhancing autophagic flux and, in consequence, modulating the cross-talk existence with inflammation. To assess autophagy, we analyzed the expression of three major actors of the autophagy pathway: LC3, p62, and beclin-1. In agreement with previous studies, in which it has been reported that enhanced autophagy in synovial tissues of patients with RA is correlated with disease severity,[11] our results showed a higher expression of LC3, together with p62 and beclin-1. Interestingly, the LC3 signal continued to grow, but the level of p62 dropped down after treatment with resveratrol. In this sense, accumulation of p62 in response to metabolic stress is a feature of defective autophagy in tumors cells.[6] In fact, RA synovial tissue also manifests elevated endoplasmic reticulum stress, in part due to the synthesis of inflammatory mediators.[7] Noteworthy, the combination of decreased p62 and increased LC3 suggest that resveratrol upregulates autophagic flux. Similarly, resveratrol-enhanced autophagic flux, through an increase in LC3 expression and a decrease in p62, showed cardio- and neuroprotective effects.[29] Also, local intra-articular injection of resveratrol delays cartilage degeneration in an experimental model of osteoarthritis by inducing autophagy.[27] Besides, when the autophagy regulator beclin-1 was evaluated, resveratrol did not increase its signal, suggesting that resveratrol could support the noncanonical (beclin-1 independent) autophagy pathway in our model, similarly to data obtained in human breast cancer cells, in which resveratrol can use the activation of the noncanonical autophagy as a cell death mechanism. Likewise, noncanonical autophagy inhibits the auto-inflammatory response to dying cells, and also its defects may contribute to the pathogenesis of systemic lupus erythematosus (SLE).[30] Related to synovial tissue, our results are consistent with previous studies where rapamycin, an inductor of autophagy with mTOR inhibitory effects, also reduced the severity of synovitis, as well as local bone erosions and cartilage loss in different inflammatory arthritis model and in SLE patients.[31-34]
It is widely accepted that the relationship between autophagy and apoptosis is intertwined and clearly context-dependent, where autophagy in general cancels the induction of apoptosis and vice versa.[35] AIA synovial samples increased significantly the presence of the apoptotic cell death markers, caspase-3, and PARP; however, this process gives insufficient results to reduce the hyperplastic rheumatoid synovium. Note that, our study demonstrated that resveratrol inhibits apoptosis in synovial AIA tissue, since caspase-3 and PARP significantly decreased in the resveratrol-treated group. Similarly, resveratrol reduced the apoptosis rate of chondrocytes in vitro and in vivo[36] and the decrease of apoptosis levels by resveratrol improves insulin resistance and exhibits neuroprotective effects.[29, 37] Conversely, it has also been shown that resveratrol induces in vitro apoptosis in human RA synovial cells.[21, 22] The differences observed between the different studies can be explained by differences in dosage (usually greater when apoptosis is observed), or in the cell type and duration of treatment or stage of disease. The results of our study suggest that resveratrol treatment shifts the balance between autophagy and apoptosis toward autophagy.
There is no doubt that dysregulated autophagy could modulate inflammasome activity and be a key driver of multiple autoinflammatory and autoimmune diseases.[24, 38] The inflammasome has been established as a molecular platform for caspase-1 activation and subsequently IL-1β production, which is significantly elevated in RA. Indeed, IL-1β blockade is a biological RA therapy that targets IL-1 response. We show here that IL-1β is a key target for resveratrol to suppress inflammation in RA. In addition, the concentration of other related clinical-pathological parameters such as CRP and PGE2 were also lower in RA animals with resveratrol supplementation. These findings are consistent with those obtained in vitro and in vivo, where resveratrol suppressed inflammatory response in both human and animal arthritis studies.[16, 18, 19, 39-41] Also, treatment with resveratrol prevented the IL-1β-mediated induction of PGE2 in several cell lines, even synoviocytes or chondrocytes.[36, 39, 40] Importantly, autophagy suppresses IL-1β signaling by activation of p62 degradation via lysosomal and proteasomal pathways.[42] Besides, IL-1β induces autophagosome formation, suggesting that IL-1β may limit its own secretion through a feedback mechanism.[10] Thus, enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced IL-1β expression.[43] Consistent with this, our study showed that the expression levels of p62 directly correlated with the serum levels of IL-1β, CRP, and PGE2. Also, the bone and cartilage loss protection by the autophagy inductor rapamycin was associated with a decrease in IL-1β expression in synovium.[31]
Moreover, impaired autophagy could activate not only inflammasome, but also might prevent degradation of p65-NF-κB exacerbating the NF-κB pathway, considered a key regulator of tissue inflammation, angiogenesis, and proliferation. Our data show that resveratrol down-regulated the levels of p65-NF-κB protein expression in AIA animals similar to other studies, which demonstrated that resveratrol suppresses inflammation by down-regulating NF-κB activation.[40, 44] Remarkably, our data described a strong positive correlation between p62 and p65-NF-κB. In this sense, it has been described how NF-κB induces expression of p62 and as defective autophagy results in accumulation of p62, which can activate NF-κB.[45] To the best of our knowledge, this is the first study to show that pharmacological intensification of autophagic flux by resveratrol in an AIA model limits the cross-talk existence with inflammation.
New blood vessel formation plays an indispensable role in the rheumatoid pannus growth and RA progression. Previously, we have described how the serum concentration of CINC-1, a murine homologue of human IL-8 chemokine, was reduced in arthritic animals supplemented with resveratrol.[16] IL-8 can directly affect RA angiogenesis. For all this, we would like to study in greater depth whether resveratrol can contribute to modulate the abnormal synovial proliferation by regulating angiogenesis. We studied two critical mediators of angiogenesis in inflammatory arthritis, VEGF and Ang-1, because they are acting in initial and later stages of angiogenesis, respectively.[46] Both were increased in synovial tissues of the AIA group and were considerably decreased after resveratrol treatment, although only Ang-1 reached significant values. These results are consistent with the observed reduction of serum IL-1β and synovial expression p65-NF-κB levels by resveratrol supplementation, since VEGF and Ang-1 can be induced by IL-1β via NF-κB.[46] Moreover, it has been described how resveratrol alleviates RA by suppressing vascular density in the cartilage matrix,[19] and unpublished observations of our group show that resveratrol clearly prevented angiogenic response induced by mitochondrial dysfunction. Our data suggest that the reduction of synovial hyperplasia after resveratrol supplementation may be also related to decreases of VEGF and Ang-1.
There is no doubt that autophagy is currently a focus of biomedical research because of its role in aging and in the development of human pathologies, among them numerous rheumatic diseases including RA. We should not forget that autophagy has different roles in normal and pathological cells and that enhanced autophagic flux may be crucial for the induction of autophagic cell death.[25] Thus, in RA synoviocytes, inhibition of autophagy reduces the cell death.[47] The results obtained support the therapeutic target of autophagy in RA as well as the consideration of diet in the management of RA. In this regard, it is widely recognized that a favorable lifestyle, which includes a healthy diet pattern, is considered mandatory in the management of many common pathologies, such as cardiovascular diseases, but not in other inflammatory diseases such as RA. Also, it is clear that obesity is associated with more severe symptoms among RA patients.[48] Resveratrol has been highlighted too by its anti-obesity properties.[49] Future studies should evaluate if resveratrol could be an effective co-adjuvant medication in the management of RA patients, also in RA patients with obesity.[41] Preventive diet strategies with resveratrol and other nutritional agents could open a potential therapeutic window to modulate the risk of RA or influence its course.[50-52]
4 Experimental Section
Animals and Study Groups
All animal care and experimental protocols for this study were conducted in accordance with the Guiding Principles in the Care and Use of Animals of the European and regional normative and were approved by the Local Ethical Committee of Animal Experimentation (process number 2016/R04). Eighteen female Lewis rats (7–8 weeks old, average weight 146 g) were purchased from Harlan Iberic Interfaune (Barcelona, Spain). The animals were randomly divided into three groups of six rats each one: healthy group, AIA group, and AIA rats treated with resveratrol (resveratrol group).
Dosage Information
To evaluate the preventive effect of resveratrol on acute AIA model, resveratrol group rats received daily doses (12.5 mg kg−1 day−1) of resveratrol by oral gavage from 2 months before AIA induction and until the sacrifice day. Resveratrol (Sigma-Aldrich, St Louis, USA) was prepared by dissolving it in a total volume of 100 μL of water. After weighing the resveratrol, 1 mL of bidistilled water was added and mixed well in a vortex, obtaining a whitish suspension, which was supplied freshly prepared.[53] Animals in the Healthy and AIA groups received an equal volume of water without added resveratrol, also by oral gavage. The optimized dose of fresh resveratrol used in the model was based on the group´s previously published studies and was the equivalent to 2.0 mg kg−1 day−1 in humans (≈114 mg resveratrol in a 70-kg adult) according to the human equivalent formula.[16]
Antigen-Induced Arthritis
AIA was induced after immunization with methylated bovine serum albumin (mBSA; Sigma-Aldrich). The rats were immunized by multiple s.c. injections on the dorsum, on days 21 and 14 before the induction of AIA, with 1 mL of 500 μg mBSA dissolved in sterile saline and emulsified with 500 μL of Freund´s complete adjuvant (Sigma-Aldrich). To induce AIA (day 0), 500 μg mBSA in 50 μL sterile saline was injected into the intra-articular cavity of both knee joints of the AIA and resveratrol rats. The knee joints of the healthy rats were injected with 50 μL of sterile saline. Two days after induction of AIA, coinciding with the severe acute phase of arthritis, all experimental animals were euthanized.[16]
Histological Analysis
Knee articulations of all groups were obtained, immediately fixed during 24 h in 4% paraformaldehyde, decalcified by 96 h with 1:1 concentration of 20% citric acid and 40% formic acid, and embedded in paraffin. Midsagittal sections (4 μm thick) were cut and stained with hematoxylin and eosin for morphological examination. According to the semi-quantitative modified Osteoarthritis Research Society International score, the grade of the synovial lesion was scored from 0 to 11 by evaluating the proliferation of lining and sublining tissue, infiltration of inflammatory cells, pannus formation, edema, and necrosis.[54]
Immunohistochemistry
For antigen unmasking, the sections for beclin-1, SQSTM1/p62, PARP, and vascular endothelial growth factor (VEGF) were heated in a pressure cooker, while sections for Ang-1 and NF-κB p65 subunit were treated with 10% formic acid for 12 min and those for caspase-3 were treated with proteinase K (Dako, Glostrup, Denmark) for 6 min. Endogenous peroxidase was blocked using peroxidase-blocking solution (Dako). After washing in PBS (Dako) with 0.2% Tween20 (PBST 0.2%), non-specific binding sites were blocked with 0.2% IgG-free bovine serum albumin (Sigma-Aldrich) in PBST for 30 min. Then, sections were incubated with their specific primary antibody: anti-beclin-1 (1:150, Cell Signaling Technology, Danvers, MA, USA), anti-p62 (1:62, R&D Systems, Minneapolis, MN, USA), anti-PARP (1:100, BD Biosciences Pharmigen, San Diego, CA, USA), anti-VEGF (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Ang-1 (1:100, Abcam, Cambridge, UK), anti-p65 subunit (1:550, Santa Cruz Biotechnology), and anti-active caspase-3 (1:100, Abcam). After overnight incubation at 4 °C, sections were washed in PBST followed by horseradish peroxidase rabbit/mouse secondary antibody (Dako) incubation. Slides were then washed again and bound antibodies were detected with diaminobenzidine substrate (Dako) incubation. Finally, sections were counterstained with Gill III hematoxylin (Merck, Madrid, Spain) and mounted with DePeX (Sigma-Aldrich). Negative controls were performed with first antibody diluent instead of the primary antibody. When indicated, specimens were not counterstained to obtain a clearer detail of positive signal. Multiple images of each slide were taken using an Olympus BX61 microscope, and positive signal of the whole synovial tissue was measured using the processing image software Image J (version 1.50e; National Institutes of Health, WA, USA).
Immunofluorescence
For autophagy marker LC3B analysis, slides were incubated with 10% formic acid for 12 min and then washed in PBS (Dako) with 0.2% Tween (PBST). Nonspecific bindings were blocked using 0.2% IgG-free bovine serum albumin in PBST previous to the incubation with LC3B primary antibody (1:300, Cell Signaling Technology) overnight at 4 °C. After rinse, secondary antibody, Alexa Fluor 568 donkey anti-rabbit (Life Technologies, Eugene, OR, USA; red fluorescence signal) was incubated for 1 h at room temperature. Finally, sections were counterstained with nuclear marker 4′,6-diamidino-2-phenylindole (Sigma-Aldrich; blue fluorescence signal), and mounted with Glicergel (Dako). Multiple images of each slide were taken using a confocal microscope (Confocal Laser Scanning Microscopy, LEICA-SP2), and positive signal of the whole synovial tissue was measure using the processing image software Image J.
IL-1β, PGE2 and CRP Serum Concentrations
Serum was obtained from the blood samples, and after previous centrifugation, samples were stored at −80 °C until assay. IL-1β level was determined in serum samples using a Rat Cytokine Magnetic Bead Panel (Milliplex, Merck Millipore, Madrid, Spain). Specific enzyme-linked immunosorbent assay kits were used to measure PGE2 (Arbor Assays, Ann Arbor, MI, USA) and CRP (Alpha Diagnostic International, San Antonio, TX, USA) according to the manufacturer´s instructions. All experiments were conducted in duplicate.
Statistical Analysis
All statistical calculations were performed using the GraphPad PRISM version 5 statistical software (GraphPad Software, La Jolla, USA). The data were presented as the mean ± SEM or as representative results, as indicated. Data were analyzed using the Mann–Whitney test. Where multiple comparisons were performed, the ANOVA test was used. Correlations were analyzed by Spearman´s test. The differences were considered significant when p ≤ 0.05.
Acknowledgements
J.A.F.-R. and M.A.-B. contributed equally to this study. The authors would like to thank to Epidemiology Service and Experimental Surgery Unit of INIBIC-CHUAC for statistical advice and help with animal model, respectively. The authors’ work was supported by Fondo de Investigación Sanitaria (grants RETIC-RIER RD16/0012/0002 and PI12/02771) and AGRUP2015/05 and AGRUP2018/03 (CICA-INIBIC) integrated in the National Plan for Scientific Program, Development and Technological Innovation 2013–2016 and funded by the ISCIII-General Subdirection of Assessment and Promotion of Research–European Regional Development Fund (FEDER) “A way of making Europe.” JA.F.-R. is recipient of a grant from Diputación A Coruña 2018 and O.R.-G. was supported by Contrato predoctoral ISCIII (PI18/01803).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




