5-Heptadecylresorcinol, a Biomarker for Whole Grain Rye Consumption, Ameliorates Cognitive Impairments and Neuroinflammation in APP/PS1 Transgenic Mice
Abstract
Scope
5-heptadecylresorcinol (AR-C17) is a biomarker for whole grain rye consumption, which is also an important active component with potential health benefits. The aim of this study is to investigate the protective effect of AR-C17 on cognitive deficits in amyloid precursor protein (APP)/PS1 transgenic mice.
Methods and results
Cognitive function is evaluated using Morris water maze test. The result shows that oral administration of AR-C17 (150 mg kg−1 day−1) for 5 months can ameliorate APP/PS1 transgenic mice memory impairment and improve learning ability. Moreover, AR-C17 treatment can notably reduce β-amyloid plaques accumulation and tau hyperphosphorylation while enhancing a disintegrin and metalloprotease 10 (ADAM10), postsynaptic density protein-95 (PSD-95), and synaptophysin protein expression in APP/PS1 transgenic mice. Furthermore, AR-C17 treatment reduces neuroinflammation by inhibiting microglial activation and astrogliosis as well as decreasing NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome-mediated IL-1β production and activating the sirtuin 3 (SIRT3)/superoxide dismutase 2 (SOD2) signaling pathway. Additionally, AR-C17 consumption significantly modulated gut dysbiosis in APP/PS1 transgenic mice through improving the relative abundance of Akkermansia and Lactobacillus while reducing the abundance of Clostridium and Desulfovibr according to16S rRNA analysis.
Conclusion
AR-C17 can be applied as a potential functional food ingredient to ameliorate cognitive impairments and prevent Alzheimer's disease.
1 Introduction
Alzheimer's disease (AD) is the most common cause of dementia with characteristics of a massive neuronal death resulting in memory loss, cognitive impairment, and behavioral alteration.[1] β-amyloid (Aβ) plaques excessive accumulation should be responsible for AD-associated cognitive impairment. Aβ formation are the result of the cleavage of amyloid precursor protein (APP) by β- and γ -secretases.[2] Therefore, inhibition of these secretases shows an effective strategy for AD therapy. On the other hand, activated microglia can increase the expression of some proinflammatory cytokines, which induce neuroinflammation, neuronal apoptosis and eventually causing the behavioral and psychological symptoms of AD.[3] Thus, neuroinflammation is progressively becoming a critical process in AD, and inhibition of neuroinflammation could be an efficient way to prevent the progress of AD. However, clinical trials showed there was no efficient drug for AD treatment due to its limited action and side effects.
Nowadays, epidemiological studies indicated dietary pattern and nutrients play an important role on the prevention of cognitive disorder.[4] For example, Mediterranean diets, mainly being composed of fruits, vegetables, and food rich in omega-3 fatty acids represented a valuable and mild anti-AD agents. Other reports show that higher intake of red and processed meat, fried food, and lower consumption of whole grains (WGs) has been evidenced to accelerate cognitive decline at older ages.[3] Whole grains are the entire seed of a plant, the consumption of which has been proven to decrease the risk of obesity, diabetes, heart disease, and certain cancers.[5] However, the protective effects of whole grains against neurological disorders such as Alzheimer's disease have not been fully elucidated and reported. Dietary fiber (DF) has been reported to be responsible for the health effects of WGs consumption. However, recent studies indicated that the nutritional benefits of WGs may partly be attributed to some phytochemicals other than fiber because of their strong antioxidant and anti-inflammatory effects, such as avenanthramides (AVAs) in oats and alkylresorcinols (ARs) in wheat and rye.[6, 7]
5-heptadecylresorcinol (AR-C17) is a major homologue of rye ARs which is also the biomarker of whole grain rye consumption.[8, 9] Increasing interest has recently been drawn to ARs and its homologues due to their health-promoting ability. For example, epidemiological study by Biskup et al. investigated the relationship between whole-grains consumption and the risk of developing diabetes. The result indicated that a higher ratio of plasma AR-C17 was associated with a lower risk of diabetes.[10] In our previous study, AR-C17 has been evidenced owing stronger anti-inflammatory and anti-oxidative bioactivity than other ARs homologues.[11] However, there is insufficient evidence to support the neuroprotective effects of AR-C17 on AD, and its neuroprotection mechanism has not been reported.
In the present study, APP/PS1 transgenic mouse models are used to evaluate the efficacy of AR-C17 treatments, which have been widely applied to investigate the initial etiology of AD. The effects of AR-C17 administration on behavior/cognition, microglial activation, neuroinflammatory response as well as potential gut microbiota modulation were investigated in APP/PS1 transgenic mice. The current study will provide a new horizon for comprehensive utilization of AR-C17 in neuron degenerative disorders prevention and management, and may offer supports for the statement of cognitive health benefit of WGs.
2 Result
2.1 AR-C17 Prevents Cognitive Deficits in APP/PS1 Transgenic Mice
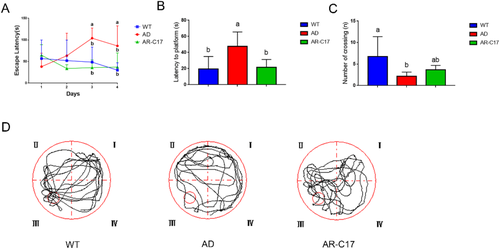
According to the result of 4 consecutive days navigation trial, no differences were found among WT, AD, and AR-C17 groups in the first 2 days. However, AD group exhibited a higher escape latency than the WT group from the third day, whereas the escape latency was notably reduced in AR-C17 group in comparison with AD group (p < 0.05, Figure 1A). In the probe trial test at day 5, all the mice from different groups were allowed to a probe trial for memory retention evaluation after the platform was removed. Compared with WT group, the latency to platform in AD group was markedly increased (p < 0.05, Figure 1B), and the frequency of crossing the target platform (third quadrant) was decreased significantly (p < 0.05, Figure 1C), demonstrating that the learning and memory ability of APP/PS1 transgenic mice were declined. In contrast, a reduced latency and increased number of crossing the platform were observed in AR-C17 group in comparison to that in AD group (Figure 1B,C). The swimming traces of mice in each group on the 5th day were recorded automatically by the camera as shown in Figure 1D. The results demonstrated that AR-C17 treatment could ameliorate cognitive deficits in APP/PS1 transgenic mice.
2.2 AR-C17 Reduces Aβ Plaques Deposition and Tau Hyperphosphorylation in APP/PS1 Mice
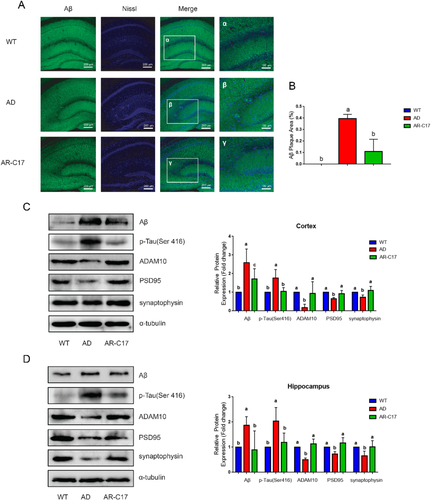
To further confirm that AR-C17 could reduce β-amyloid accumulation, extracellular senile plaques and neurofibrillary tangles, we analyzed Aβ plaques deposition in the hippocampus of mice in each group using Aβ/Nissl double staining immuno-histochemistry. The result demonstrated a higher presence ratio of Aβ deposition in the AD group, while AR-C17 treatment significantly decreased the area of Aβ plaques deposition in hippocampus (p < 0.05, Figure 2A,B). Meanwhile, the level of Aβ, hyperphosphorylated tau (p-tau; Ser 416) and A disintegrin and metalloprotease 10 (ADAM10) in mice cortex and hippocampus of each group was analyzed using western blot assay (Figure 2C,D). In comparison with WT group, the expression levels of Aβ and p-Tau (Ser 416) were significantly elevated, while the level of ADAM10 was significantly decreased in AD group (p < 0.05, Figure 2C,D). After AR-C17 treatment, the protein expressions of Aβ and p-Tau (Ser 416) were substantially suppressed, while the expression of ADAM10 was notably improved in comparation with that in AD group (p < 0.05, Figure 2C,D).
2.3 AR-C17 Alleviates Synaptic Dysfunction in APP/PS1 Mice
Synapse protein markers such as postsynaptic density protein-95 (PSD-95) and synaptophysin were monitored by western blot to analyze the protective effects of AR-C17 treatment on synaptic dysfunction. The expression level of PSD-95 and synaptophysin in cortex and hippocampus were decreased in AD group compared with that in WT group, while the expression of both proteins was significantly increased in AR-C17 group (p < 0.05, Figure 2C,D).
2.4 AR-C17 Reduces Microglia Activation and Astrogliosis in APP/PS1 Mice
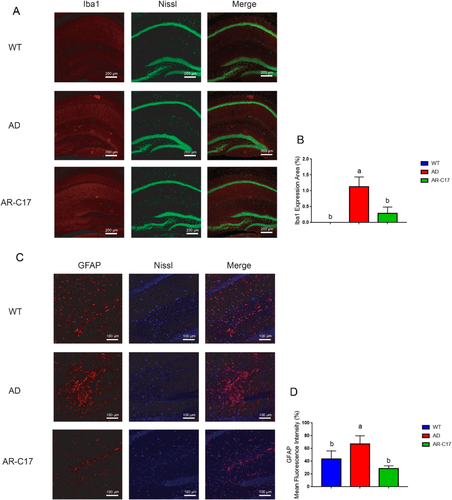
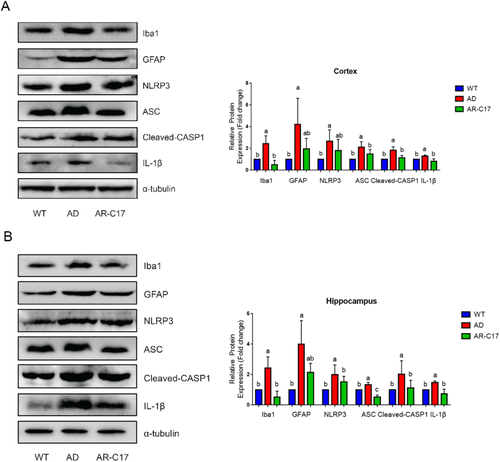
The result of immunofluorescence analysis demonstrated that there was a much more presence of activated microglia (Iba-1-positive) in the area surrounding neurons in AD group, while AR-C17 intervention significantly decreased the area of activated microglia in hippocampus (p < 0.05, Figure 3A,B). Glial changes in the hippocampus of APP/PS1 mice was analyzed with glial fibrillary acidic protein (GFAP)/Nissl double staining immunohistochemistry. The results suggested an increased expression of astrocytes (GFAP-positive) in the area surrounding neurons in AD group, while AR-C17 treatment markedly reduced astrogliosis in hippocampus (p < 0.05, Figure 3C,D). Moreover, western blot analysis indicated that Iba-1and GFAP expression in hippocampus and cortex were notably elevated in AD group compared to that in WT group (p < 0.05, Figure 4A,B). Whereas, AR-C17 treatment reversed the tendency, suggesting AR-C17 could inhibit microglial activation and astrogliosis in APP/PS1 transgenic mice.
2.5 AR-C17 Decreases Activation of the NOD-Like Receptor Family, Pyrin Domain-Containing 3 Inflammasome in APP/PS1 Mice
The protein expression of NOD-like receptor family, pyrin domain-containing 3 (NLRP3) in AD group was significantly increased in cortex and hippocampus compared with that in WT group, while AR-C17 treatment decreased NLRP3 expression (Figure 4A,B). Moreover, the protein expression of apoptosis-associated speck-like protein containing a C-terminal caspase-recruitment domain (ASC), cleaved-CASP1 and IL-1β were significantly elevated in cortex and hippocampus of AD group, while AR-C17 treatment significantly reduced the upregulated expression of these protein compared with that in AD group (p < 0.05, Figure 4A,B).
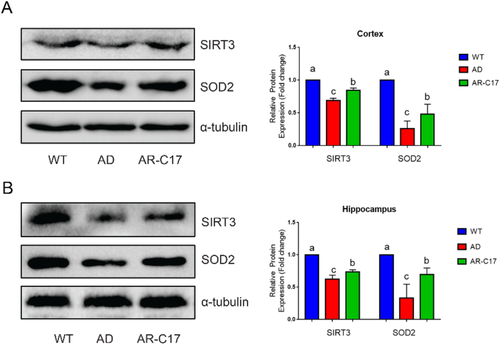
2.6 AR-C17 Activates Sirtuin 3/Superoxide Dismutase 2 Signaling Pathway
The protein expression of sirtuin 3 (SIRT3) and superoxide dismutase 2 (SOD2) in AD group was significantly reduced in cortex and hippocampus compared with that in WT group, while the tendency was reversed by AR-C17 intervention (Figure 5, p < 0.05). These results indicated that AR-C17 ameliorated cognitive dysfunction and neuroinflammation in APP/PS1 mice which might be through the activation of SIRT3/SOD2 signaling pathway.
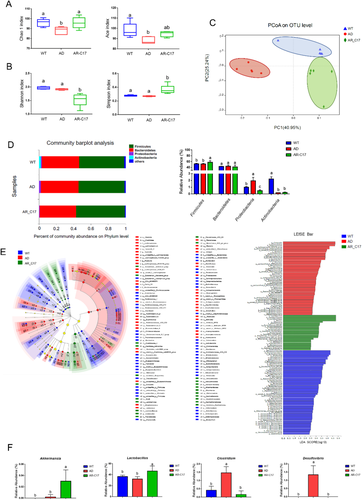
2.7 AR-C17 Modulates the Gut Microbiota Composition in APP/PS1 Mice
To evaluate the effect of AR-C17 on gut microbiota composition in APP/PS1 mice, 16S rRNA sequencing was performed. Compared with WT group, AD group displayed a lower abundance of fecal bacteria (Chao1 and Ace indices), while AR-C17 treatment reversed the tendency significantly (p < 0.05; Figure 6A). However, the alpha diversity (Shannon and Simpson indices) of the WT and AD group were higher than that in AR-C17 group, suggesting that the microbial heterogeneity was reduced significantly in AR-C17 treated APP/PS1 transgenic mice (p < 0.05, Figure 6B). Principle coordinates analysis (PCoA) was conducted to investigate the difference in bacterial taxa composition of each group (Figure 6C). The result provided the visualization of differences between AD group and AR-C17 group, suggesting AR-C17 intervention could modulate the bacterial community composition of APP/PS1 mice. Moreover, differential abundance at the phylum and genus level of each group was analyzed. At phylum level, there were no significant changes in Firmicutes and Bacteroidetes between WT and AD group, while AR-C17 treatment could significantly improve the abundance of Firmicutes (p < 0.05, Figure 6D). Meanwhile, AR-C17 treatment significantly decreased the abundance of Proteobacteria, which was markedly increased in AD group (p < 0.05, Figure 6D). No significant change was observed in the abundance of Actinobacteria between AR-C17 group and AD group (Figure 6D). At the genus level, Muribaculaceae, Lactobacillaceae, Lachnospiraceae, Ruminococcaceae, Bacteroidaceae, and Rikenellaceae were the dominant bacteria in all three groups (Figure S1, Supporting Information). In addition, the characteristic bacteria for each group at genus level was identified by using linear discriminant analysis effect size (LEfSe) method (Figure 6E). According to the comparison of gut microbiota from AD group, AR-C17 intervention could notably increase the abundance of Akkermansia and Lactobacillus, while decrease the abundance of Clostridium and Desulfovibrio (p < 0.05, Figure 6F).
3 Discussion
Despite tremendous efforts made in the treatment of Alzheimer's disease, it still lacks efficient way to prevent the progression of this disease, making the reconsideration of novel therapeutic strategy. Nowadays, dietary pattern and nutrition intervention maybe an effective attempt to reduce the risk and progression of AD. It is the first time to reveal that AR-C17 consumption is a potential strategy for improving cognitive deficits and reducing the amyloid plaque deposition and tau hyperphosphorylation both in the cortex and hippocampus in APP/PS1 mice. Accumulation and aggregation of amyloid-β (Aβ) and generation of neurofibrillary p-tau tangle play an important role in the pathogenesis of AD.[12] Aβ is produced via the proteolytic processing of APP by β- and γ-secretases.[13] ADAM10 is the main α-secretase that cleaves APP in non-amyloidogenic pathway, inhibiting the generation of β-amyloid peptide. Thus, increased activity of ADAM10 has been proven to be an effective way in treating neurodegenerative diseases and inhibiting β-amyloid deposition in brain.[14] The results in this study indicated that AR-C17 could significantly improve ADAM10 protein expression and reduce β-amyloid deposition. It is a well-documented notion that synaptic failure induced by Aβ toxicity, tau hyperphosphorylation, and mitochondrial dysfunction are closely associated with cognitive deficits.[15] Synaptophysin and PSD95 are considered as the biomarkers of synaptic plasticity, which are decreased in brain of AD patient. Synaptophysin is a presynaptic vesicle associated protein, participating in the release of neurotransmitters, and PSD 95 is a postsynaptic scaffolding protein which is required for the stabilization at synapses.[16] In this study, AR-C17 could upregulate the proteins expression of Synaptophysin and PSD95, indicating its potential role in restoring synaptic deficits in AD.
Moreover, Aβ plaques deposition induces activation of microglia, which could cause neuroinflammation and eventually lead to neuronal death. Activated microglia can produce cytokines and chemokines that induce astrogliosis, which is a hallmark of neuroinflammation.[17] Consequently, the proinflammatory mediators could induce a vicious inflammatory cycle, causing neuronal cell dysfunction and death in the brain constantly.[18, 19] Meanwhile, in the central nervous system (CNS), inflammation occurring and progressing can be mediated via activation of the NLRP3 inflammasome, which is a multiprotein complex including NLRP3, ASC, and CASP1. Assembly and activation of the NLRP3 inflammasome could result in the generation of activated caspase-1, and then initiate maturation and secretion of IL-1β.[17] Evidence suggests that deficiency of NLRP3 and ASC could decrease Aβ plaque deposition and reduce microglial inflammatory activation both in vitro and in vivo.[20] Therefore, targeting the NLRP3 inflammasome associated neuroinflammation is an efficient way for neuroprotection. Many plant-derived natural compounds including epigallocatechin gallate (EGCG), curcumin and resveratrol have been studied for exerting neuroprotective activity though regulating NLRP3 inflammasome-mediated IL-1β production.[21] In consistence with previous reports, our results suggested that β-amyloid deposition along with microgliosis and astrogliosis were reduced in AR-C17-treated APP/PS1 mice. Moreover, the decreased expressions of NLRP3 inflammasome and its down-stream expression of proinflammatory cytokine suggested that AR-C17 played a neuroprotective role in AD, at least in part, associated with its anti-inflammatory effects.
To further study the mechanism of AR-C17 on ameliorating cognitive impairments and neuroinflammation in APP/PS1 transgenic mice, SIRT3/SOD2 signaling pathway and gut microbiota modulation were analyzed. SIRT3 has been well documented in participating in the regulation of mitochondrial function and in the prevention of oxidative stress.[22] Previous study indicated that SIRT3 activation could lower Aβ and Tau accumulation in the brain of AD mice, playing a neuroprotective role in reducing oxidative neurotoxicity.[23, 24] Activation of SIRT3 could mediate hippocampal synaptic adaptations and ameliorate cognitive deficits in APP mutant mice.[25] Moreover, SIRT3 deficiency could induce brain mitochondrial dysfunction, microgliosis and elevate NLRP3 inflammasomes and IL-1β expression, which exacerbated cognitive decline in calorie-rich western diet induced mice.[26] Meanwhile, SIRT3-mediated deacetylation of mitochondrial SOD2 was necessary to prolong mitochondrial NLRP3 inflammasome assembly, decreasing NLRP3 super complex formation and activation.[27] Our present study showed that AR-C17 could significantly increase the expression of SIRT3 and SOD2, indicating AR-C17 played a neuroprotective role in AD through modulating SIRT3/SOD2 signaling pathway.
It is widely accepted that gut microbiota dysbiosis was correlated with the development and progress of AD.[28] The microbial diversity was decreased in AD participants, including reduced ACE, Chao1, and Shannon index.[18] AR-C17 treatment could modulate the abundance of gut microbiota. However, AR-C17 treatment significantly decreased microbial heterogeneity, which might be partially due to the antibacterial effect of AR-C17. Previous study showed that the level of Firmicutes was reduced, while the level of Proteobacteria was increased in APP/PS1 mice.[2] However, the tendency was reversed by AR-C17 treatment. Meanwhile, AR-C17 treatment could significantly increase the abundance of Akkermansia and Lactobacillus while decrease the abundance of Clostridium and Desulfovibrio at genus level. According to reported literature, Akkermansia was closely associated with glucose and lipid metabolism.[29] Yang et. al. suggested that oral treatment of Akkermansia could modulate gut permeability, reduce neuroinflammation, and restore neuronal function, thus ameliorating cognitive deficits in HFD-fed mice.[28] Meanwhile, Lactobacillus consumption could improve physiological function and cognitive ability of aged mice.[30] Moreover, Clostridium are positively associated with obesity, insulin resistance, and AD development. Desulfovibrio could decrease the integrity of intestinal endothelium and drive the maturation of microglia by decreasing the level of short-chain fatty acids (SCFAs).[12] The inhibitory effect of AR-C17 on Desulfovibrio and Clostridium would be beneficial for the treatment of AD. Thus, AR-C17 could alleviate cognitive impairment and neuroinflammation through regulating gut microbiota profile, which may be ascribed to the crosstalk between AR-C17 and gut microbiota. However, further study is needed to illustrate their underlying mechanisms.
4 Concluding Remarks
In summary, AR-C17 consumption could reduce Aβ plaques accumulation and tau hyperphosphorylation as well as enhance ADAM10, PSD-95, and synaptophysin expression. Meanwhile, AR-C17 treatment reduced neuroinflammation by inhibiting the activation of microglial, astrogliosis, and reducing NLRP3 inflammasomes. The modulation of AR-C17 on SIRT3/SOD2 signaling pathway and gut microbiota might play an important role in neuroprotection.
5 Experimental Section
Materials
AR-C17 (CAS: 41442-57-3) was purchased from Mingxu (Shanghai, China). Antibodies against-Aβ (A16265), ADAM10 (A10438), GFAP (A14673), ionized calcium binding adapter molecule (Iba-1; A12391), synaptophysin (A6344), NLRP3 (A5652), SIRT3 (A5718), caspase-1(A0964), and IL-1β (A16288) were obtained from Abclonal (Wuhan, China). Antibodies against-Drp1 (ab184247), PSD95 (ab76115), TMS1/ASC (ab10403), SOD2/MnSOD (ab13533), anti-alpha tubulin antibody (ab7291), and Goat anti-Rabbit IgG H&L (HRP) (ab205718) were obtained from Abcam (Cambridge, UK). Antibody against-phospho-Tau (Ser416) (#15013) was obtained from Cell Signaling Technology (MA, USA).
Animals and Treatment
24 APP/PS1 transgenic mice (3 months, B6C3-Tg/APPswe, PSEN1dE9 /85Dbo/JNju) and 12 of their wild-type mice as the control group were obtained from GemPharmatech (Nanjing, China) and supplemented with AIN-93G diet for a total of 5 months. After 1 week adaptation, mice were raised in cages alone under a 12/12 h light/dark cycle at 23 °C with humidity of 55% and provided with food and water ad libitum. All animal experiments were authorized by the Animal Ethics Committee of Peking University Health Science Center (approval no. LA2018143). Transgenic mice were randomly assigned into two groups including AD group (APP/PS1, n = 12) and AR-C17 group (APP/PS1, n = 12). The control group was composed of wild-type mice (WT, n = 12). The mice in AR-C17 group were oral administrated with 150 mg kg−1 AR-C17, while AD and WT groups were oral administrated with 0.5% CMC-Na aqueous solution instead of AR-C17.
Dosage Information
It has been reported that the maximum amount of ARs in rye was 3.2 mg g−1, among which AR-C17 was the major homologues.[31, 32] Based on the dietary guidelines for Chinese residents in 2016, the daily dosage of grain was 250–400 g per 60 kg of adult weight. The human maximum equivalent dose of ARs was calculated to be 13.3–21.3 mg kg−1 bodyweight for a 60 kg human, respectively. The dosage for mice was calculated as 121–194 mg kg–1 bodyweight.[33] In this study, the final intragastric average dosage was set to be 150 mg kg−1.
Morris Water Maze Test
To evaluate the memory and spatial learning ability of mice, Morris water maze (MWM) test was performed according to a published method with slight modifications.[3] Briefly, 1 week before sacrifice, the mice were placed in a 120 cm diameter × 50 cm height round tank filled with water (22 ± 1 °C), in which a transparent escape platform (diameter 10 cm) was fixed in the third quadrant and submerged 0.5 cm below the surface of water. 4 days prior to the final probe test, each mouse was allowed to search for the hidden platform freely within 120 s. If the mouse failed to find the hidden platform within 120 s, manual operation was applied to make the mouse remember the spatial location. On the 5th day, the final probe test was performed, the hidden platform was removed, and each mouse was allowed to navigate for 120 s. All the data was collected and analyzed with the software of Morris water maze video analysis system (ZS-001, Beijing, China).
Animal Dissection and Tissue Collection
2 days after behavioral test, four mice (n = 4) were anesthetized and transcranial perfused with 0.9% normal saline followed by 4% paraformaldehyde solution. After fixing for 10 min, the whole brain was separated carefully and then immersed in 25% sucrose solution for 2 h. Subsequently, the brains slices (40 µm) were prepared and stored at −20 °C before immunofluorescence assay. The rest mice (n = 8) in each group were sacrificed after fasting overnight. Then, the cerebral cortex and hippocampus were quickly dissected, weighed, and stored at −80 °C prior to western-blotting analysis.
Immunofluorescence Double Labeling
For immunofluorescence analysis, frozen brain tissue sections were blocked with the sealing fluid (3% donkey serum and 10% Triton dissolved in 0.1 m PBS) for 30 min, followed by incubating with primary antibody for 12 h at 4 °C. After PBS rinsing, the brain slices were incubated with the targeted secondary antibodies (Alexa Fluor 488, 1:500, for Aβ; DyLight 594, 1:500, for Iba-1, DyLight 594, 1:500, for GFAP) at room temperature for 1 h. After PBS washing, DAPI (1:1000, Invitrogen) and Nissl (1:1000, Invitrogen) were applied to localize nuclei and neuron of brain cell for 1.5 h at RT in the dark. The slices were subsequently examined using Olympus FV1200 confocal.
Western-Blotting Assay
Western blotting analysis was performed according to the authors' previous study.[29] After fractionated by 12% SDS-PAGE, the gel was transferred to a PVDF membrane and incubated with specific antibodies, followed by analyzing with a Bio-Rad imaging system (Bio-Rad Laboratories, Hercules, USA).
16S rRNA Gene Sequencing Analysis
16S rRNA gene sequencing analysis was performed according to the authors' previous study.[29] Briefly, total DNA were extracted from the fecal samples using DNA Mini Kit (Qiagen, Valencia, CA, USA) and stored at −80 °C for further analysis. The concentration of isolated DNA was detected by Nano-Drop 1000 spectrophotometer (Thermo Fisher, MA, USA). Universal primers were used to amplify the V3-V4 hypervariable regions of the bacterial 16S rRNA gene according to previous study.[29] The Illumina MiSeq platform (Illumina, San Diego, USA) was used for paired-end sequence of the purified amplicons.
Bioinformatics Analysis
The 16S rRNA genes analysis of gut microbiota was performed with online platform (www.i-sanger.com) according to the authors' previous study.[29] Differences in alpha diversity of gut microbiota were based on the Chao1, Ace, Shannon and Simpson diversity indices analysis, and differences in beta diversity were performed with PCoA. Analysis of statistically significant differences in relative abundance of genera among each group were performed according to LEfSe based on the following criteria: the alpha value used for factorial Kruskal–Wallis test was set as less than 0.05 and the threshold on the logarithmic linear discriminant analysis (LDA) score was set as higher than 2.0.
Statistical Analysis
The results were presented as mean ± SD. Comparative analysis of differences between groups was performed using one-way ANOVA followed by Duncan's multiple comparisons. All results were considered statistically significant at p < 0.05. The graph was created on GraphPad Prism (San Diego, CA, USA).
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant 31701640), Beijing Excellent Talents Funding for Youth Scientist Innovation Team (2016000026833TD01), Support Project of High-Level Teachers in Beijing Municipal Universities (IDHT20180506), and School Level Cultivation Fund of Beijing Technology and Business University for Distinguished and Excellent Young Scholars.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
J.L.: conceptualization, methodology, writing-original draft preparation; Y.W.: data curation and analysis, software, visualization; Z.W.: writing-reviewing and editing; W.B.: data curation and visualization; Y.H.: software and investigation; Z.W.: data curation; J.W.: supervision and project administration.




