High glycemic index and glycemic load are associated with moderately increased cancer risk
Abstract
Scope
To obtain an up-to-date quantification of the association between dietary glycemic index (GI) and glycemic load (GL) and the risk of cancer.
Methods and results
We conducted a systematic review and meta-analysis of observational studies updated to January 2015. Summary relative risks (RRs) were derived using random effects models. Seventy-five reports were evaluated in the systematic review (147 090 cases), and 72 were included in the meta-analyses by cancer site. Considering hormone-related cancers, summary RRs comparing the highest versus the lowest GI and GL intake were, respectively, 1.05 and 1.07 for breast, 1.13 and 1.17 for endometrial, 1.11 and 1.19 for ovarian, and 1.06 and 1.04 for prostate cancers. Considering digestive-tract cancers, summary RRs for GI and GL were, respectively, 1.46 and 1.25 for esophageal (squamous cell carcinoma), 1.17 and 1.10 for stomach, 1.16 (significant) and 1.10 for colorectal, 1.11 and 1.14 for liver, and 1.10 and 1.01 for pancreatic cancers. In most of these meta-analyses, significant heterogeneity among studies was observed. In subgroup analyses, case–control studies and studies from Europe tended to estimate higher RRs.
Conclusion
High-GI and high-GL diets are related to moderately increased risk of cancer at several common sites.
Abbreviations
-
- CI
-
- confidence interval
-
- FFQs
-
- food frequency questionnaires
-
- GI
-
- glycemic index
-
- GL
-
- glycemic load
-
- IGFs
-
- insulin-like growth factors
-
- RR
-
- relative risk
1 Introduction
Carbohydrate foods increase postprandial glycemia and insulin secretion at different rates, depending on the types of the carbohydrates, the amount and nature of fiber contained, the processing method, and the presence of other nutrients. These differences are captured by the glycemic index (GI), a ranking system for carbohydrate foods based on their ability to raise blood glucose concentrations 1. Glycemic load (GL), the product of a food's GI and total available carbohydrate content, was introduced to incorporate the effect of the total amount of carbohydrate consumed. Therefore, the overall GI reflects the quality of carbohydrates consumed, whereas the total dietary GL reflects both the quantity and quality of carbohydrates 2.
Factors influencing the metabolism of glucose may have a relevant role in the development of chronic diseases, including not only diabetes and cardiovascular diseases, but also cancer at selected sites 3-8. In particular, chronically elevated insulin concentrations are thought to represent the underlying mechanism for the GI/GL-related increased risks of several cancers. Insulin acts as a growth factor and increases the bioavailability and bioactivity of insulin-like growth factors (IGFs), such as IGF-1, by enhancing their synthesis and by decreasing their binding proteins. IGF-1 can promote tumor development by inhibiting apoptosis, stimulating cell proliferation and sex-steroid synthesis 6, 9, 10. IGF-1 has also angiogenic proprieties. Moreover, other conditions related to glucose metabolism, including insulin resistance, hyperglycemia, obesity, and diabetes may influence the risk of coronary heart disease and cancer 6, 7, 11-13.
In 2008, a meta-analysis reviewed results from 39 observational studies considering the relation between dietary GI/GL and the risk of cancer, and reported significantly increased risks for the highest versus the lowest categories of intake for colorectal (summary relative risk, RR = 1.26 for GI, and RR = 1.18 for GL) and endometrial cancers (RR = 1.36 for GI, and RR = 1.22 for GL). No significant association was observed for pancreatic and breast cancer 14. A number of studies were published subsequently on the issue, and their results have been considered in some reviews and meta-analyses focused on a single type of cancer 15-23. In 2012, a meta-analysis of cohort studies summarized the evidence on GI and GL and diabetes-related cancers (i.e. bladder, breast, colorectum, endometrium, liver, and pancreas), and suggested, overall, a modestly increased risk 24. Analyses on site-specific cancers revealed significant positive associations between GI and breast (RR for the highest versus the lowest category = 1.06; 95% confidence interval, CI: 1.02, 1.11) and colorectal cancer (RR = 1.08; 95% CI: 1.00–1.17), and between GL and endometrial cancer (RR = 1.21; 95% CI: 1.07–1.37).
Meta-analyses on GI, GL, and cancer risk have been recently made available on selected cancer sites, but most of them considered only cohort studies, and very recently published data have not been reviewed yet. Among them there are the National Enhanced Cancer Surveillance study 25, with more than 20 000 cases of a number of different cancers, the Australian Cancer Study on esophageal cancer 26, the Australian Ovarian Cancer Study 27, results on endometrial cancer from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) study 28 and from the Shanghai Endometrial Cancer study 29, results on breast and liver cancer from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, with about 200 liver cancer cases 30 and more than 11 000 breast cancer cases 31, results on colorectal cancer from the Italian segment of the EPIC cohort 32 and from a US case–control study with more than 1000 cases 33, and results on liver cancer from the Shanghai Men's and Women's Health study 34. Further, to our knowledge, no meta-analysis is currently available for esophageal or liver cancer.
To obtain an up-to-date quantification of the association between GI and GL and the risk of cancer from case–control and cohort studies and investigate between-study heterogeneity, we conducted a comprehensive systematic review and meta-analysis updated to January 2015, including all cancer sites for which adequate data were available.
2 Materials and methods
2.1 Search strategy
The meta-analysis was conducted following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines for reporting 35. Published reports providing results for the association of dietary GI and/or GL with the risk of cancer at various sites were identified from the Medline database through PubMed, by using the string “((cancer) OR (neoplasm) OR (carcinoma)) AND ((glycemic index) OR (glycemic load) OR (glycaemic index) OR (glycaemic load)).” No restriction was applied to the research. The literature search was updated to January 2015. Additional pertinent reports were extracted from checking the reference lists of the retrieved articles and some reviews on the topic 14-21, 24.
2.2 Study selection
Studies were included if they (i) had a case–control or cohort design, (ii) included occurrence of any type of cancer among the outcomes, (iii) examined the association with GI and/or GL, and (iv) provided the RR estimate (i.e. odds ratios or RRs, or hazard ratios collectively referred to as RRs) and the corresponding 95% CI for (at least) the comparison between the highest versus the lowest categories of GI/GL intake, or sufficient information for their calculation. That is, the study reported RR estimates and standard errors (SEs), variances, CIs, or p values of the significance of the estimates, or crude data 36. Two authors (C.G. and S.G.) screened the search results independently against the inclusion criteria to identify eligible papers, and discrepancies were discussed and resolved. When multiple reports were published on the same study population 31, 37-44, we included in the systematic review only the most informative or the most updated one 31, 38, 40, 42. Five reports were therefore excluded 37, 39, 41, 43, 44. Further, one report was excluded because providing dose–response risk estimates but no information on the range of exposure 45, and another one because proving risk estimates only for cancer overall 46. One report was identified from the reference lists of the articles retrieved 47. For some reports 22, 27, 48, 49, we obtained missing information directly from the authors. The flow chart for the selection procedure is reported in Supporting Information Fig. 1.
2.3 Data extraction
All data were extracted independently in a standard format and cross-checked by three reviewers (C.G., S.G., and F.T). Disagreement was resolved by discussion. The following data were extracted from each study: last name of the first author, publication year, sex of the participants, study design, country, tumor site, number of subjects (cases and controls/noncases/cohort size), period of enrolment (for case–control studies) or follow-up (for cohort studies), dietary assessment methods (type, number of food items, and whether it had been validated), reference food for GI/GL (white bread or glucose), comparison levels (i.e. values used to define the upper category and the lowest category taken as reference) of GI and/or GL, the estimates of the RR (i.e. odds ratio/hazard ratio/RR) for the comparison between the highest versus the lowest category of GI and GL intake, and adjustment for confounding factors. Since confounding is a crucial issue in observational studies, we extracted from each study RR estimates adjusted for the largest number of confounding factors. When they were not given, we calculated RR estimates from tabular data. When available, we used RR estimates stratified for sex, subsite (for colorectal cancer), or menopausal status for female cancers.
2.4 Statistical analysis
We conducted meta-analyses of GI and GL in relation to the risk of cancers of the breast, endometrium, ovary and prostate (i.e. hormone-related cancers), and of squamous cell carcinoma of the esophagus (ESCC), cancer of the stomach, colorectum, liver, and pancreas (i.e. digestive-tract cancers); that is, cancer sites for which at least four studies were available.
The summary RR was estimated by pooling the study-specific RR estimates comparing the highest versus the lowest categories of GI/GL intakes using the random effects models, which consider both within- and between-study variation 50. Heterogeneity among studies was assessed using the χ² test (because of its limited power, we considered heterogeneity to be significant at p < 0.10) and the I² statistic, i.e. the proportion of total variation contributed by between-study variation 51, 52.
We also examined changes in results after exclusion of each study in turn to evaluate the robustness of the summary estimates. Subgroup analyses were carried out to explore between-study heterogeneity 51. Heterogeneity was investigated by looking at the possible factors that could influence the estimates. In particular, when, for a cancer site, data were available from at least four studies, we carried out subgroup analyses according to study design, area, sex (when appropriate), BMI, cancer site (only for colorectal cancer), and menopausal status (for female cancers). Some reports providing only continuous RR estimates in stratified analyses were not considered in our subgroup analyses 28, 53, 54. For the analyses by BMI, we considered two different strata: BMI < 25 kg/m2 and BMI ≥ 25 kg/m2. For some studies we pooled the RRs of GI/GL in subjects with BMI of 25–30 kg/m2 and BMI ≥ 30 kg/m2 to obtain the RR in subjects with BMI ≥ 25 kg/m2, using fixed-effects models 21, 55-58. One study showing RRs for GL for BMI < 30 kg/m2 and BMI ≥ 30 kg/m2 was not considered in this stratified analysis 33.
Presence of publication bias was assessed by examination of funnel plots 59 and by applying the tests proposed by Begg and Mazumdar and by Egger 60. All the statistical analyses were performed using the STATA software (version 10; StataCorp, College Station, TX, USA).
3 Results
3.1 Study characteristics
Seventy-five reports published between 1997 and 2015 were evaluated in the systematic review, for a total of 147 090 cases of cancer (Supporting Information Table 1). Thirty-two reports gave results from case–control studies and 43 gave results on 20 different cohorts (including a cohort based on data from the PLCO study). Among the case–control studies, 17 were from Europe, six from North America, four from Asia, and five from other countries (i.e. one from Uruguay, one from Mexico, and three from Australia). Among the 20 cohorts, six were from Europe, 12 from North America, and two from Asia.
Almost all studies used food frequency questionnaires (FFQs) for assessment of dietary habits, with variable number of items (from 29 to 200 items). Nearly all FFQs were validated. A similar number of reports used white bread or glucose as reference food for GI/GL calculation. Most reports adjusted for major confounding factors, with 70 out of 75 (93%) providing RR estimates adjusted for energy intake, and a similar proportion of reports estimates adjusted for BMI or physical activity (n = 69). Indicators of social class, such as education, race/ethnicity, or income, were accounted for in about 70% of reports; RRs were adjusted for smoking in about 2/3 of the reports.
Seventy-two reports were included in the meta-analyses by cancer site. We did not consider three reports 61-63 investigating cancer sites for which less than four studies were available (i.e. thyroid, kidney, lung).
3.2 GI, GL, and cancer risk
The study-specific and summary RRs of hormone-related cancers according to the highest versus the lowest category of GI and GL are shown in Fig. 1 (panels A and B, respectively). The summary RRs were all above unity but nonsignificant, ranging from 1.05 (breast) to 1.13 (endometrium) for GI, and from 1.04 (prostate) to 1.19 (ovary) for GL.
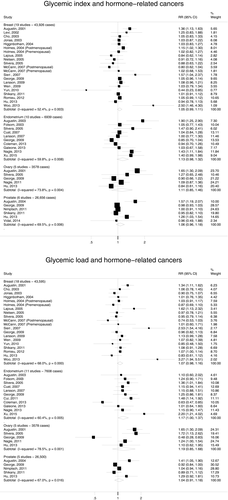
Figure 2 showed results for digestive-tract cancers. GI significantly increased colorectal cancer risk (summary RR = 1.16, 95% CI: 1.07–1.25). All other summary RRs were above unity, but not significant; they ranged from 1.10 (pancreas) to 1.46 (ESCC) for GI, and from 1.01 (pancreas) to 1.25 (ESCC) for GL.
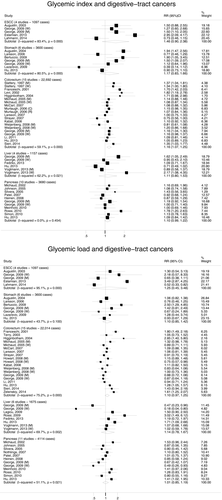
Study-specific and summary relative risks (RRs) of digestive-tract cancers for the highest versus the lowest category of glycemic index (panel A) or glycemic load intake (panel B).
ESCC, esophageal squamous cell carcinoma.
3.3 Sensitivity analyses
The sensitivity analyses produced consistent results. In particular, the exclusion of each study in turn did not appreciably modify the pooled RR of colorectal cancer according to GI, which remained statistically significant. Moreover, in the meta-analysis of GI and breast cancer, the exclusion of the study by Yun et al. 54, i.e. the only study reporting a significantly decreased risk, led to a summary RR of 1.05 (95% CI: 1.00–1.13), very similar to that found in the main analysis. Accordingly, the exclusion of the study by Woo et al. 64, i.e. the study providing the highest RR, led to a summary RR of 1.03 (95% CI: 0.99–1.10). In the meta-analysis of GL and pancreatic cancer, the exclusion of the study by George et al. 40, reporting a significant decreased risk in women, resulted in a summary RR of 1.08 (95% CI: 0.93–1.26), in line with findings from the main analysis. Conversely, when we omitted the study by Coleman et al. 28 from the meta-analysis of GL and endometrial cancer, the summary RR increased from 1.17 (borderline) to 1.24 (95% CI: 1.12–1.36), with no longer heterogeneity among studies (I2 = 0.0%, p = 0.563). Further, when the study by George et al. 40 was excluded from the meta-analyses of GL and ovarian cancer as well as liver cancer, the summary RRs increased to 1.43 (95% CI: 1.18–1.74) and 1.41 (95% CI: 1.07–1.85), respectively, and the heterogeneity largely decreased (I2 = 34.3, p = 0.207 for ovarian and I2 = 40.5, p = 0.135 for liver cancer).
3.4 Subgroup analyses
Supporting Information Tables 1 and 2 show results for GI and GL and the risk of either hormone-related or digestive-tract cancers, in subgroup. In general, case–control studies and studies from Europe tended to report higher RRs.
High GL significantly increased stomach cancer risk in men only; similarly, high GI and GL significantly increased colorectal cancer risk in men, whereas estimates for women were of lower magnitude. High GI was significantly associated to pancreatic cancer in women only.
A positive significant association of GL was found with endometrial or pancreatic cancer risks only among subjects with BMI ≥ 25 kg/m2. The RR estimates among overweight subjects were 1.34 (95% CI: 1.13–1.59) for endometrial and 1.44 (95% CI: 1.02–2.03) for pancreatic cancer, with low heterogeneity (I2 = 0.0 and 3.4, respectively). For breast cancer, no meaningful difference emerged according to menopausal status. Similar summary RRs were found for colon and rectal cancer for both GI and GL.
There was no evidence of publication bias as tested by Egger's and Begg and Mazumdar's tests for all cancer sites considered in the meta-analyses (data not shown).
4 Discussion
This comprehensive and updated meta-analysis of observational studies suggests that high-GI and high-GL diets are related to modest increased risks of cancer for most sites. The estimated summary RRs were all above unity and ranged from 1.05 (breast) to 1.46 (ESCC) for dietary GI, and from 1.01 (pancreas) to 1.25 (ESCC) for dietary GL. These associations, however, were not significant, with the exception of that between dietary GI and colorectal cancer.
Inference is therefore difficult due to nonsignificant results. Still, some effects might be of some relevance at the population level because of the high incidence of several of these neoplasms, in particular breast and colorectal cancers 65. If real, the associations observed can be related to the unfavorable impact of GI/GL on blood glucose levels, insulin, and IGFs, which may promote tumor growth 66.
Significant associations of high GL intake were found with either endometrial or pancreatic cancer risks only among overweight individuals, suggesting that BMI represents an effect modificator of the associations of GL with these two forms of cancer. Overweight strongly influences insulin resistance and hyperinsulinemia 67. It is thus likely that overweight individuals have a greater insulin response to their diet for their physiological condition compared to lean individuals. Similar effects have been reported for GL, which appears to increase the risk of coronary heart disease only in overweight women 5.
High heterogeneity across studies was detected in most of the meta-analyses by cancer site. Therefore, even if we used random effects models to take heterogeneity into account, our summary estimates should be interpreted with caution. We tried to overcome the problem of heterogeneity by pooling results from more homogeneous subsets of studies (i.e. subgroup analyses). In analyses by study design, we found generally higher summary RR estimates in case–control than cohort studies. Still, a number of cohorts supported a detrimental role of high-GI/GL diets in cancer risk. In particular, positive associations for dietary GI were found in men of the National Institute of Health-AARP (NIH-AARP) cohort for stomach and liver cancer 40, in the ORDET study for breast cancer 68, and in the Shanghai Women's Health study (SWHS) for liver cancer 34. For GL, positive associations were observed in the Women's Health Study (WHS) for colorectal cancer 69, in the Hormones and Diet in the Etiology of Breast Tumors Study (ORDET) for breast cancer 68, in the EPIC cohort for breast cancer 31, in the National Breast Screening Study (NBSS) for endometrial 58 and ovarian cancers 70, and in the Nurses’ Health Study (NHS) for endometrial cancer 47. A possible explanation of the higher RR estimates in case–control than in cohort studies is that the former are more prone to problems of recall and/or selection bias. Still, most of the case–control studies included in our meta-analyses had validated FFQs administered by trained interviewers, and this offers additional control over the quality of the measurement compared to self-administered FFQ used in cohorts. Further, case–control studies could include subjects with a diet characterized by higher GI/GL than cohort studies 14. This observation derives from the fact that analyses in case–control studies were based on greater upper quantiles of GI/GL, in the absence of marked differences in the lowest quantiles. If associations of small magnitude exist, these are difficult to be detected in cohort studies on populations with more homogenous diets and a narrower range of total dietary GI/GL. Further, the small differences between case–control and cohort studies may reflect possible differences in dietary habits between European and North American populations. Indeed, 12 out of 20 cohorts were conducted in the United States, and only six in Europe; in contrast, 17 out of 32 case–control studies were conducted among Europeans and only six among North Americans. European populations tended to consume high amount of bread, refined carbohydrates, and pasta 71-73, whereas the diet of North American populations is rich in high-fat foods and therefore tends to have low GL values.
Adjustment for potential confounding factors was different across studies, and this may have resulted in residual confounding. However, most studies allowed for BMI/physical activity, energy intake, tobacco, and some indicators of social class, as showed in Supporting Information Table 1. Thus, residual confounding should be limited.
Furthermore, studies used different methods of segregations according to GI/GL (e.g. tertiles, quartiles, quintiles), and there was heterogeneity of thresholds of various levels, including the highest one used in the current meta-analysis. This may reflect different dietary habits among populations, as well as the different choice of the reference food (glucose or white bread) for GI/GL calculation. Indeed, when white bread was the reference food, the GI value against glucose was calculated simply by multiplying by 0.7 2. Since we considered quantiles of the distribution and not absolute values, the choice of white bread or glucose as reference did not materially influence the results. When conducting stratified analyses by reference food, we observed no material differences in the summary RR estimates for white bread and glucose (data not shown).
Some stratified analyses considered a limited number of studies, and their results must therefore be interpreted with caution. Further, some positive findings in stratified analyses may be attributed to the role of chance following multiple testing.
Criticisms have arisen concerning the use of GI in epidemiological studies. Indeed, GI is an indicator of quality of carbohydrates but not of the cumulative GL of the diet, and does not vary in response to the amount of food consumed 74.
The main advantages of the present meta-analysis were the inclusion of a large number of studies and cases and the consequent statistical power to detect moderate associations, and the use of a systematic meta-analytic approach to summarize our results and investigate reasons for between-study heterogeneity. Finally, results from Begg's and Egger's tests indicate that publication bias is unlikely to have appreciably influenced our results.
In conclusion, our results, derived from the most up-to-date evidence, are suggestive of modest increased risks for most cancer sites for high-GI and high-GL diets.
Acknowledgments
This work was supported by the Italian Foundation for Cancer Research (FIRC) and by the Italian Ministry of Health, General Directorate of European and International Relations. FT was supported by a fellowship from the FIRC. CG was supported by Fondazione Umberto Veronesi.
The authors have declared no conflict of interest.




