Low vitamin D status throughout life results in an inflammatory prone status but does not alter bone mineral or strength in healthy 3-month-old CD-1 male mice
Abstract
Scope
The aim of this study was to assess if exposure to different levels of dietary vitamin D pre- and postweaning impacts the intestinal-bone axis.
Methods and results
Female CD1 mice were exposed to high (5000 IU vitamin D3/kg diet, H) or low (25 IU vitamin D3/kg diet, L) vitamin D diet (modified AIN-93G) during pregnancy and lactation. At weaning (postnatal day 21), a subset of the male offspring was sacrificed and another subset was assigned to receive their dams’ respective diet (HH and LL) or the other diet (HL and LH) until sacrifice at 3 months of age. Lower level of vitamin D resulted in reduced vitamin D receptor and increased expression of pro-inflammatory genes in the colon at 3 months, lower numbers of colonic Bacteroides/Prevotella at postnatal day 21 and higher serum LPS concentration at adulthood. There was a programming effect of vitamin D on LPS levels. Mineral content, density, and strength of femurs and vertebrae were not affected.
Conclusion
Our findings suggest that low vitamin D exposure results in an inflammatory-prone status that may contribute to or be a risk factor for several diseases including inflammatory bowel disease, obesity, diabetes, and cardiovascular diseases.
Abbreviations
-
- BMC
-
- bone mineral content
-
- BMD
-
- bone mineral density
-
- IBD
-
- inflammatory bowel disease
-
- LV
-
- lumbar vertebra
-
- PND
-
- postnatal day
-
- VDR
-
- vitamin D receptor
1 Introduction
The nutritional environment to which the fetus is exposed during pregnancy and early life can have a profound effect on later health outcomes. Vitamin D is a critical factor for the prevention and cure of rickets, but also has noncalciotropic effects 1, 2. Indeed, maternal vitamin D status is important for bone mineral accrual and development in children 3, 4 but also for the immune development of the fetus 5-7. For example, in mice, vitamin D deficiency in early life results in an epigenetic reduction in number of invariant natural killer T cells in the offspring that cannot be corrected by later intervention with vitamin D 8. Low vitamin D status in utero has been implicated in increased risk of developing immune and inflammatory disorders such as type 1 diabetes 9-11 and obesity 12 and its effects may extend epigenetically to the next two generations 13. Environmental factors that are modified by the season, such as serum 25-hydroxycholecalciferol (25(OH)D), may program an individual's risk of developing inflammatory bowel disease (IBD) 14-16, but early life programming effects of vitamin D on the gastrointestinal tract, one of the most immunologically active mammalian organs, have not been thoroughly studied. 17, 18.
Mediated by its receptor, vitamin D regulates about 3% of the human and mouse genomes 19 and many genes integral to the intestinal barrier, such as mucins, tight junctions, and antimicrobial peptides, contain VDR elements 20. The vitamin D receptor (VDR) is a nuclear hormone transcription factor expressed very early in fetal life and found almost ubiquitously in all nucleated cells including immune and colonic epithelial cells 21. In this respect, VDR is a possible mediator of vitamin D effects in fetal life. In intestinal inflammation, VDR prevents colonic injury and maintains intestinal integrity 22, 23. VDR ablation in mice results in chronic and low-grade inflammation of the intestine with local increased expression of inflammatory cytokines and higher IL-6 concentration in the serum 23, 24. In line with this, serum 25(OH) D and tumor necrosis factor α concentrations are inversely associated in healthy volunteers 25.
Vitamin D effects on the host may also indirectly impact the gut microbiota, which is another important constituent of the intestinal barrier. For example, vitamin D deficient mice display a 50-fold increase in the colonic bacteria load, likely because of reduced expression of angiogenin 4 antimicrobial peptide 26. In addition, both commensal gut microbiota and pathogenic bacteria regulate the expression and localization of colonic VDR in vivo 23. However, the impact of vitamin D on the maturation of the gut microbiota in early life and its composition in adulthood has not been determined. Intriguingly, the gut microbiota was recently shown to directly regulate bone metabolism since germfree mice exhibited higher bone mass, by virtue of having reduced number of precursor osteoclasts, which was normalized upon colonization of these mice with a normal gut microbial community 27. Thus, it is possible that vitamin D may indirectly regulate bone metabolism via the gut microbiota. A possible underlying mechanism could be through gut bacteria-derived LPS, since LPS impacts osteoclastogenesis 28. It is not known if vitamin D modulates circulating LPS concentration but serum 25(OH)D concentrations below 10 ng/mL were associated with increased risk of developing hospital-acquired bloodstream infections 29.
To understand whether vitamin D may modulate intestine-bone axis, we used a healthy CD-1 mouse model to investigate the effects of dietary vitamin D supplementation beginning at the pre-conception period on intestinal and bone parameters in the offspring. We hypothesized that both intestine and bone would be amenable to nutritional programming by vitamin D, and that exposure to low vitamin D would create an inflammation-prone status and compromise bone mineral density (BMD) and bone strength at young adulthood.
2 Materials and methods
2.1 Animals and diets
All experimental procedures were in accordance with the Canadian Council on Animal Care 30 and were approved by the local animal ethics committee at the University of Toronto (ID: 20008375). Twenty-eight female and ten male CD-1 mice were purchased at 21 days of age from Charles River Laboratories (St. Constant, QC, Canada), housed under the conventional 12 h: 12 h light-dark cycle and maintained in a room with UVB-free incandescent lighting since arrival and throughout the entire study. Upon arrival, female mice were randomized to have ad libitum access to modified AIN93G diets containing either 5000 IU vitamin D3/kg diet (Diet# 119290, Dyets Inc., Bethlehem, PA, USA) or 25 IU vitamin D3/kg diet (Diet# 119289, Dyets Inc.) to generate serum 25(OH)D concentrations of ≥50 nmol/L and <30 nmol/L 31. Diet vitamin D levels were confirmed independently by Maxxam Analytics (Mississauga, ON, Canada). Both diets contained 5 g calcium/kg diet, the standard level in the AIN93G diet. Pregnant dams continued to receive the intervention diets throughout pregnancy and lactation. At weaning (D21), the dams and 1–2 male pups per litter were sacrificed, resulting in two intervention groups at this time point: high vitamin D (H-D21) and low vitamin D (L-D21) weanlings. The females were used for a complementary study. The remaining males were housed four per cage and randomized to one of two diets until sacrifice at 3 months of age, resulting in four vitamin D intervention groups at this time point. The groups are referred to as HH, HL, LH, and LL to represent maternal diet consumed during pregnancy and lactation followed by postweaning diet consumed by male offspring (H or L for High or Low Vitamin D containing diet). Freshly passed fecal pellets were collected prior to sacrifice and stored at −20°C. Blood samples were obtained via cardiac puncture in LPS-free Pyrotubes (Associates of Cape Code, Falmouth, MA, USA). The large intestine was immediately resected on ice and cut in two halves. Contents from the proximal colon were flushed with ice-cold sterile saline and stored at −20°C. A 0.5 cm section was fixed in formalin, embedded in paraffin and cut at a thickness of 5μm. The remaining intestinal tissue samples were stored at −80°C. The right femur and the second and fourth lumbar vertebrae (LV) from 3-month-old offspring were cleaned of soft tissue, wrapped in saline-soaked gauze, and stored at −80°C.
2.2 Serum 25(OH)D concentration
Serum 25(OH)D concentration was assessed via automated IDS-iSYS 25(OH)D chemiluminescence immunoassay (Immunodiagnostic Systems Inc., Fountain Hills, AZ, USA).
2.3 Immunohistochemistry of VDR expression
Colonic VDR was assessed semiquantitatively by incubation of deparaffinized and rehydrated sections with rabbit anti-mouse VDR polyclonal antibody (Abcam, Cambridge, MA, USA) and then with biotinylated goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antigens were visualized with streptavidin-horseradish peroxidase. Five fields per section (transverse and longitudinal) for a total of 15 fields per mouse were assessed blindly using the Allred scoring method 32.
2.4 Microbiota composition analysis
DNA was extracted from the fecal and colonic contents samples using the E.Z.N.A.TM Stool DNA Isolation Kit (Omega Bio-Tek, Doraville, GA, USA) modified to include a lysozyme digestion step at 37°C for 30 minutes. DNA concentration and purity were assessed spectrophotometrically (OD260/280, 260/230) (Nanodrop Technologies, Wilmington, DE, USA). Counts of Bacteroides/Prevotella, Clostridium coccoides group and Clostridium leptum group were determined by quantitative real-time PCR as we previously described 33 using 10 ng of DNA. Data are expressed as 1og10 colony forming units per gram of feces or colonic contents. Clostridium coccoides was not detected in an initial screening of fecal samples from 4 to 5 mice per age and dietary treatment (data not shown).
2.5 RNA extraction and quality assessment
Total RNA was extracted from whole-thickness proximal colons using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and stored at −80°C. RNA concentration, purity and quality were assessed spectrophotometrically (OD260/280, 260/230) and with an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA, USA).
2.6 Microarray analysis of gene expression in the proximal colon
Two hundred nanograms of RNA (RNA Integrity Number ≥7) from 3 mice per group (H-D21, L-D21, HH-3Mo, and LL-3Mo) were labeled using Illumina TotalPrep-96 RNA Amplification kit (Ambion, Wilmington, DE, USA) and 1.5 ng of the generated cRNA were hybridized onto the Mouse WG-6 V2 Beadchips containing 45 281 probes covering approximately 19 000 unique, curated genes in the NCBI RefSeq database (Build 36, Release 22) and representing the mouse whole genome (Mouse Ref-8 v2.0). Microarrays were hybridized, washed, and scanned according to the manufacturer's protocols. All samples passed Illumina's sample dependent and independent quality control metrics. Data were extracted using GenomeStudio Version 2010.2 (Illumina Inc., San Diego, CA, USA), imported in Genespring v11.5.1 (Agilent Technologies, USA) and normalized using a standard quantile based normalization followed by a “per probe” median centered normalization. All data analysis and visualization were performed on log2 transformed data. A total of 36 065 probes that were in the upper 80th percentile of the distribution of intensities in 100% of any of the 1 of 4 groups were retained. Data were analyzed by ANOVA using false discovery rate Benjamini and Hochberg multiple testing correction threshold of p < 0.05 34. An unsupervised clustering was applied using a Pearson centered correlation as a distance metric with average linkage rules. Microarray experiments and data analysis were performed at the Ontario Cancer Institute Genomics Centre. Genes were annotated to gene ontologies and classified into protein classes and pathways using Panther 35.
2.7 Serum LPS determination
Serum LPS concentration was determined using an enzyme linked immunoassay (LPS ELISA Kit, Cusabio Biotech, Barksdale, DE, USA).
2.8 Bone mineral content (BMC), bone mineral density (BMD), and peak load of femurs and LV
BMC, BMD and peak load of femurs and LV were measured in offspring at 3 months of age. As previously described 36, BMC and BMD of whole femurs, the proximal 1/3 of the femur and LV4 were determined using dual-energy X-ray bone densitometry with the following parameters: speed = 2 mm/minute; resolution = 0.01 × 0.01 mm (Orthometrix, White Plains, NY) and a specialized software program (Host Software version 3.9.4; Scanner Software version 1.2.0). Peak load of femur midpoint, femur neck and LV2 was measured by placing each individual bone in one of three specially designed jigs for the Materials Testing System (Model 4442, Instron Corp., Norwood, MA, USA; Bluehill 2) and a crosshead speed of 2 mm/min was applied until fracture occurred 36. For the femur midpoint testing, a span width of 6 mm was used. Peak load of LV2 was determined as the first peak of the load-deformation curve.
2.9 Statistical analyses
All statistical analyses were performed using PASW Statistics 18.0 software (SPSS Inc., Somers, NY, USA). Litter characteristics and dams’ fecal microbiota data were analyzed by unpaired Student's t-test. All other data were analyzed by two-way ANOVA with “dietary vitamin D and age” or “mother's diet and postweaning diet” as the main effects. Tukey's Honest Significant Difference post hoc tests were used to assess statistically significant (p < 0.05) interaction effects. Outliers were detected using Grubb's test and removed from the datasets.
3 Results
3.1 Litter characteristics
There was no significant difference in the litter size and sex ratio between the low and high vitamin D dams. At postnatal day (PND) 1 and 14, average pup litter weights did not differ between dams fed a low versus high vitamin D diet, but at PND 21, pups from high vitamin D dams weighed less than their low vitamin D counterparts (p < 0.023; Table 1). There was no difference in food intake between 4 and 14 weeks of age (data not shown) and neither dam nor pup diet had an effect on body weight (Supporting Information Fig. 1).
| Dam Dietb | p-Value | ||
|---|---|---|---|
| Low | High | ||
| Litter size | 11.0 ± 0.4 | 12.0 ± 0.4 | NS |
| Gender ratio | |||
| Males | 6.0 ± 0.5 | 6.0 ± 0.4 | NS |
| Females | 5.0 ± 0.3 | 6.0 ± 0.3 | NS |
| PND body weightc | |||
| PND 1 | 1.96 ± 0.04 | 1.88 ± 0.03 | NS |
| PND 14 | 9.43 ± 0.19 | 9.06 ± 0.19 | NS |
| PND 21 | |||
| Males | 15.79 ± 0.32 | 14.79 ± 0.29 | 0.023 |
- a Values are expressed as mean ± SEM.
- b Vitamin D levels: 25 IU/kg diet (Low; n = 14); 5,000 IU/kg diet (High; n = 15).
- c PND: postnatal day. At PND 1 and 14 genders were combined; gender was determined at PND 21.
- NS, nonsignificant, p > 0.05.
3.2 Serum 25(OH)D concentration
Postweaning diet had a significant (p < 0.001) effect on circulating 25(OH)D with the mice that were weaned to a low vitamin D diet (HL: 14.20 ± 0.45 nmol/L and LL: 13.80 ± 0.78 nmol/L; n = 3/group) having markedly lower 25(OH)D concentration in comparison to their counterparts that received a high vitamin D diet (LH: 112.3 ± 3.62 nmol/L and HH: 120.1 ± 3.09 nmol/L; n = 3/group) at weaning through to 3 months of age. Dam's diet during pregnancy and lactation did not affect vitamin D status of the offspring at 3 months of age (p = 0.174).
3.3 Vitamin D deficiency results in lower colonic VDR levels
At PND 21, the weanlings exposed to high vitamin D had 40% greater VDR expression than the weanlings exposed to low vitamin D (p < 0.05; Fig. 1A). Similarly, in the adult offspring, VDR expression was 50% higher (p < 0.001) in the offspring reared by the high vitamin D dams than their low vitamin D counter parts, while the effect of postweaning diet was not significant (Fig. 1B). VDR mRNA expression was not different among groups at weaning or 3 months of age (qPCR; data not shown).
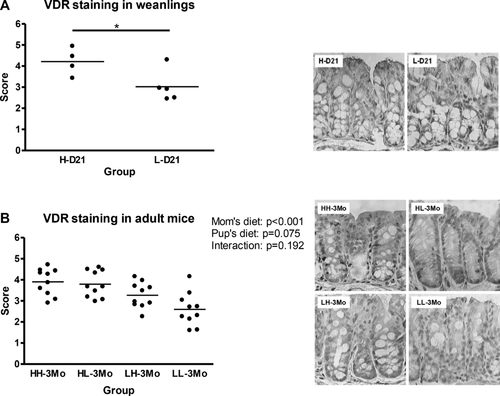
3.4 Intestinal microbiota composition varies with age and dietary levels of vitamin D
In the proximal colon, there was a significant but biologically modest increase in the bacterial load from weaning to 3 months of age independent of vitamin D status. The offspring that underwent a diet switch at weaning had a moderately lower bacteria count than the mice that received the high vitamin D diet (p = 0.0053 for both; Fig. 2A and B). At PND 21, low vitamin D mice had an importantly lower colonic Bacteroides/Prevotella counts than their high vitamin D weanlings (p = 0.0021). This difference disappeared at adulthood because there was a significant increase in the Bacteroides/Prevotella counts from weaning to 3 months of age in the low, but not in the high vitamin D mice (p = 0.0002; Fig. 2C). This effect was confirmed for % Bacteroides/Prevotella calculated versus total counts (data not shown). The HL and LH groups had lower Bacteroides/Prevotella counts than the HH group (p = 0.0045 and p = 0.0024) and the LL group (p = 0.0103 and p = 0.0035; Fig. 2D). There was no change in C. leptum counts (Fig. 2E and F).
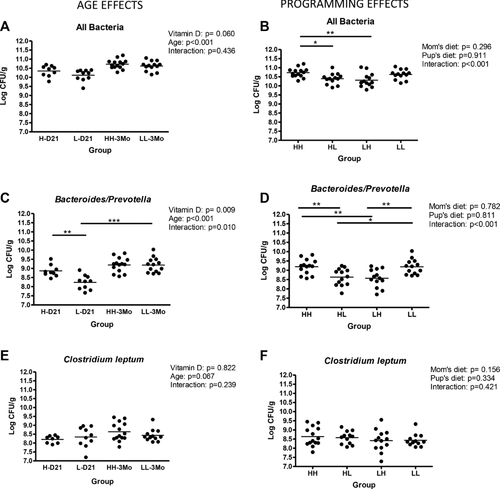
In the feces, total bacterial counts were slightly lower at 3 months of age compared to PND 21 and in the diet switch groups compared to the HH and LL groups (Fig. 3A and B). Bacteroides/Prevotella counts were not altered by age, but were slightly lower in the HL and LH groups compared to HH (p = 0.0009 and p = 0082) and to LL (p = < 0.0001 and p = 0.0011) groups (Fig. 3C and D). Clostridium leptum counts were not affected by age or vitamin D but there was a significant interaction effect of dam's diet and postweaning diet (p < 0.028; Fig. 3F).
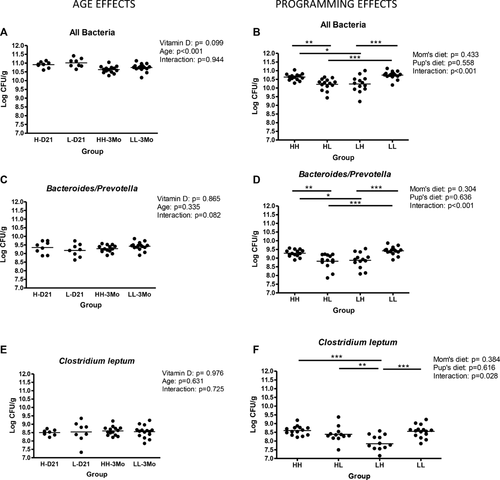
3.5 Vitamin D affects age-dependent gene expression in the colon
One hundred fourteen probes representing 104 genes were differentially expressed among groups (Supporting Information Table 1), 14 of which have a VDR elements 20. Hierarchical clustering analysis using the 114 probes showed that samples first cluster based on the age and then based on their vitamin D status (Supporting Information Fig. 2). Fifty genes were upregulated and 54 genes were downregulated from weaning to 3 months of age. At PND 21, 46 genes were upregulated in the high vitamin D mice and 58 genes were upregulated in the low vitamin D mice. Genes upregulated with an absolute fold ≥1.5 (values for each gene are given in parentheses) in the high vitamin D mice included iron transporter (Slc11a2; fold change 2.3, average of two probes), cationic amino acid transporter member 8 (Slc7a8; fold change 1.5), delta-like 1 homolog (1.6), insulin-like growth factor 2 mRNA-binding protein 3 (1.6, average of two probes), lipoprotein lipase (1.5), S100 calcium binding protein G (2.4), transforming growth factor β 3 (1.6), UDP galactosyltransferase 8A (1.5), and the undefined gene E130106K10Rik (1.5), while those upregulated in the low vitamin D group included elastin (1.6). At 3 months of age, 57 genes were upregulated in the high vitamin D mice and 47 genes were upregulated in the low vitamin D mice. Genes upregulated with an absolute fold ≥1.5 in the high vitamin D mice included aldehyde dehydrogenase family 1 subfamily A1 (1.6), cytochrome P450 family 4 subfamily b polypeptide 1 (1.5), proteasome 26S subunit non-ATPase 8 (1.6), and the Riken cDNA C230078M08 gene (1.6), while those upregulated in the low vitamin D group included inter-alpha trypsin inhibitor heavy chain 2 (1.6), several Ig genes (Igk-C (5.6), Igh-VJ558 (4.0), LOC100047628 (5.6), LOC637227 (3.1), LOC669053 (3.6), LOC100047162 (3.6), RIKEN cDNA 2010001M09 (2.4), and the undefined genes LOC232065 (1.9) and LOC381774 (11). The classification of the differentially expressed genes into gene ontologies including biological processes, molecular function and cellular component, as well as Panther protein classes and pathways is given in supporting Table S1. Briefly, 51, 46, and 41% of the genes annotated to biological processes were classified into the metabolic process, cellular process and cell communication categories, respectively, and 18% of the genes annotated to cellular components were classified as extracellular matrix genes (Supporting Information Table 1). The majority of the genes fell into distinct pathways, except for a group of collagen genes and laminin that were all annotated to the integrin-signaling pathway (Supporting Information Table 1).
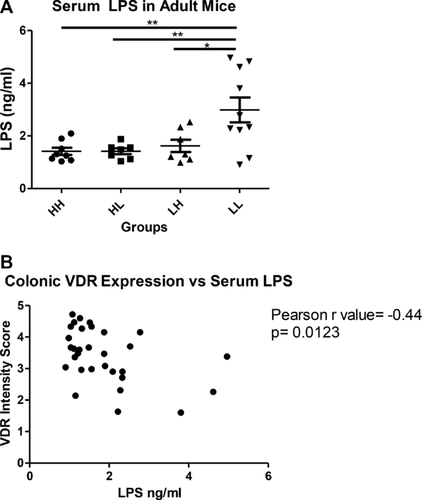
3.6 Vitamin D and serum LPS
Serum LPS concentration was markedly higher in the 3 month old LL mice than any of the other three intervention groups (p = 0.0023; Fig. 4A). Serum LPS concentration was significantly and negatively correlated with colonic VDR expression (Fig. 4B).
3.7 Vitamin D and bone mineral and strength
BMC and BMD of whole femurs, proximal 1/3 femur and LV4 at 3 months of age did not differ among groups. Moreover, yield load and peak load at the femur midpoint did not differ among groups. Exposure to high vitamin D in utero and throughout suckling resulted in a lower (p = 0.039) peak load at the femur neck compared to those exposed to low vitamin D during the same time period (Table 2). Peak load of LV2 did not differ among groups.
| Dam diet | Pup diet | p-Value | ||||
|---|---|---|---|---|---|---|
| Low | High | Dam diet | Pup diet | Dam diet × pup diet | ||
| Bone mineral | ||||||
| Whole femur | ||||||
| BMC, mg | Low | 39.47 ± 1.23 | 39.94 ± 1.02 | |||
| High | 39.21 ± 1.31 | 39.61 ± 1.17 | NS | NS | NS | |
| BMD, mg/cmb | Low | 84.65 ± 1.55 | 84.68 ± 1.54 | |||
| High | 83.37 ± 2.09 | 83.27 ± 1.78 | NS | NS | NS | |
| 1/3 Proximal femur | ||||||
| BMC, mg | Low | 13.70 ± 0.47 | 13.94 ± 0.47 | |||
| High | 13.81 ± 0.39 | 13.57 ± 0.42 | NS | NS | NS | |
| BMD, mg/cmb | Low | 90.42 ± 1.67 | 91.83 ± 1.66 | |||
| High | 89.36 ± 1.94 | 88.74 ± 1.84 | NS | NS | NS | |
| LV4 | ||||||
| BMC, mg | Low | 10.72 ± 0.60 | 10.75 ± 0.50 | |||
| High | 10.19 ± 0.45 | 10.67 ± 0.61 | NS | NS | NS | |
| BMD, mg/cmb | Low | 66.75 ± 1.88 | 69.43 ± 2.09 | |||
| High | 67.09 ± 1.68 | 67.08 ± 3.09 | NS | NS | NS | |
| Biomechanical strength | ||||||
| Femoral midpoint | ||||||
| Yield load, N | Low | 15.83 ± 0.76 | 16.80 ± 0.93 | |||
| High | 16.64 ± 0.67 | 16.23 ± 0.79 | NS | NS | NS | |
| Peak load, N | Low | 37.48 ± 1.39 | 36.84 ± 1.92 | |||
| High | 36.29 ± 1.45 | 34.59 ± 1.64 | NS | NS | NS | |
| Femoral neck | ||||||
| Peak load, N | Low | 23.29 ± 1.16 | 21.44 ± 0.89 | |||
| High | 20.21 ± 0.90 | 20.47 ± 0.78 | 0.039 | NS | NS | |
| LV2 | ||||||
| Peak load, N | Low | 76.95 ± 4.21 | 76.14 ± 2.55 | |||
| High | 74.93 ± 3.05 | 69.76 ± 4.31 | NS | NS | NS | |
- a Values are expressed as mean ± SEM.
- b Vitamin D levels: 25 IU/kg diet (LOW); 5000 IU/kg diet (HIGH).
- For bone mineral outcomes: n = 10 for all groups.
- For biomechanical strength outcomes (Dam diet, Pup diet): Low, Low n = 14; Low, High n = 14; High, Low n = 14; High, High n = 15.
- NS, nonsignificant, p > 0.05; BMC, bone mineral content; BMD, bone mineral density; LV, lumbar vertebrae.
4 Discussion
Low vitamin D levels parallel an inflammatory immune response in disorders such as IBD, obesity, and heart disease 37-39. We report an inflammatory status, as indicated by a significant increase in serum LPS concentrations, in mice with low serum 25(OH)D, but otherwise healthy. Effects of dietary vitamin D on immune activation and inflammation in normal physiology have not been extensively studied but our data are in line with a previous study showing that serum 25(OH)D and tumor necrosis factor α concentrations are inversely correlated in frequent tanning bed users in comparison to those with minimal UV exposure 25. Low serum 25(OH)D concentration is also a predictor of sepsis in humans 40 and vitamin D may interact with dietary fat-induced inflammation, which is at least in part mediated by LPS (reviewed in 41). Interestingly, the serum LPS concentration in the mice that were continuously exposed to the low vitamin D diet was approximately twice as high as the mice in the other groups, which is representative of a chronic and low grade inflammatory state that has been previously defined as metabolic endotoxemia 42. Furthermore, this could be prevented by exposure to supplemental vitamin D, either in early life or adulthood. LPS is a component of the cell membrane of Gram-negative bacteria that is continuously released in the gut upon their death 43. It has been proposed that the death of Gram-negative Bacteroides results in translocation of LPS to the intestinal capillaries via a Toll-like receptor4 dependent pathway, ultimately resulting in secretion of proinflammatory cytokines and inflammation 42. In our study, less counts of Bacteroides/Prevotella, such as in the HL and LH groups, were not accompanied by higher serum LPS concentration. It is likely that an increase in LPS translocation is the result of increased intestinal permeability. Indeed, VDR and Cyp27b1 knockout mice display increased permeability after treatment with dextran sodium sulfate to induce colitis 22, 44 suggesting that a continuously low vitamin D status may predispose to inflammatory conditions. In line with this, at the protein level, the colonic VDR expression was significantly higher in the vitamin D supplemented weanlings and adult offspring, while the effect of postweaning diet was not significant. These findings suggest that lack of adequate exposure to vitamin D during in utero and suckling periods may have a programming effect on the colonic VDR expression that cannot be corrected by vitamin D supplementation later in life. LPS has been shown to reduce VDR protein expression and inhibit 1,25(OH)2D3 function in human monocytic cells 45. Interestingly, we also found a significant negative correlation between serum LPS concentration and the VDR protein expression suggesting an alternative explanation for the reduction in VDR levels via LPS negative regulation. To determine the impact of vitamin D on the intestinal gene expression signature and uncover any molecular links to the observed inflammatory status in the low vitamin D mice we utilized a whole genome microarray approach. As expected, expression of classical vitamin D targets, such as calbindin D9K (S100g), whose expression is known to correlate with calcium transport activity, was higher in the vitamin D supplemented mice. This was also confirmed by qPCR (data not shown). The gene expression results indicated an inflammation-prone status in the mice with low dietary vitamin D, particularly in adulthood; specifically, we observed an increase in the expression of immune (such as Igh and IgKC), permeability and extra cellular matrix related genes (such as solute carriers, collagen, and elastin) in the colons. These results highlight some similarities between the colonic gene expression profile of vitamin D deficient animals and IBD patients since enrichment in the immune/inflammatory and structure/permeability pathways has been previously reported in both Crohn's disease and ulcerative colitis patients 46. Moreover, IBD as well as obese patients, carry a higher percentage of Ig-coated bacteria in their feces, likely as a result of an altered mucosal response contributing to inflammation 47, 48. Future studies could investigate if the higher expression of Ig genes that we observed in low vitamin D mice results in increased amounts of colonic Ig-coated bacteria.
We found lower counts of colonic Bacteroides/Prevotella in the low vitamin D weanlings but not at 3 months of age suggesting a retarded establishment of these bacteria in the LL mice. The developmental diversification of the gut microbes is affected by host factors. These factors include genetics as well as environmental factors such as contact with maternal microbiota during natural delivery or the hospital environment in cesarean-section delivered infant 49, 50. In order to account for the maternal transmission of microbiota to the offspring during birth, we analyzed the gut microbiota composition of the dams on low or high vitamin D diets (Supporting Information Fig. 3), but we did not find a significant difference in their microbiota composition, excluding a role for vertical transmission from the mothers in this context. Bacteroidetes, to which Bacteroides/Prevotella belong, are one of the four main phyla within the fecal microbial community and their counts increase sharply at weaning in healthy animals. A prominent member of the Bacteroidetes phylum, Bacteroides thetaiotaomicron, stimulates intestinal blood vessel development in germ-free animals 51 and impacts intestinal glycosylation affecting the establishment of other gut bacteria 52. Therefore, it is possible that vitamin D-dependent modulation of Bacteroides/Prevotella at weaning impacts postnatal intestinal maturation. Intriguingly, Bacteroides/Prevotella counts in both colon and feces were reduced in the diet-switch groups, HL and LH, at 3 months of age. These data are not completely understood at present but may be explained by the mismatch in dietary vitamin D levels between in utero and adult life and therefore be in line with the predictive-adaptive response hypothesis 53. It has been proposed that vitamin D impacts colonic microbial counts via regulation of vitamin D-dependent antimicrobial peptides secreted by the host 26. Though, these genes were not differentially expressed in our study. Furthermore, VDR and gut microbiota composition may be inter-related and impact intestinal homeostasis and inflammation via modulation of nuclear factor kappa β 22, 45, 54, 55. Metagenomics studies, also involving germfree animals, are needed to further understand the relationship between dietary vitamin D and the gut microbiota.
Despite the elevated serum LPS concentration in mice with continuously low vitamin D status and changes in microbiota among groups, BMC and BMD of LV4, whole femurs and 1/3 proximal femur, as well as bone strength at femur midpoint and LV2 was not different among groups. Thus, the relatively small but statistically significant difference in peak load at the femur neck due to dam diet may not be biologically significant. BMC, BMD, and bone strength were assessed at 3 months of age, representing an early stage of adulthood, given our previous findings that the maximal accumulation of bone mineral and bone strength is achieved by this age 56. The overall lack of differences in bone outcomes may also be due to the healthy state of the mice as they did not experience any dietary challenges, except for the different level of vitamin D. Moreover, the AIN93G diet, which was the basis for both diets, was developed to meet the nutritional needs of growing rodents 57 but it is possible that the level of calcium in the diet (5 g/kg diet) compensated for the low vitamin D status.
In summary, our study shows that dietary vitamin D has a pleiotropic effect on intestinal physiology. The intestine is able to respond to vitamin D during both early and later stages of maturation and vitamin D effects are age-dependent. In particular, low circulating levels of vitamin D result in an inflammation-prone gene expression profile in the colon, which is accompanied by decreased expression of VDR protein and an altered microbiota composition as well as higher serum LPS. In the healthy mouse model used here, these outcomes did not affect bone health; though, our findings suggest that low vitamin D levels may concur in the etiology of diseases with an inflammatory component.
Acknowledgment
This study was supported by a Natural Sciences and Engineering Research Council Discovery grant and a University of Toronto Connaught Fund grant. Wendy E. Ward holds a Canada Research Chair in Bone and Muscle Development. The authors would like to thank Reinhold Vieth for the measurement of serum 25(OH)D, Carl Virtanen at the Ontario Cancer Institute Genomics Centre for the analysis of the microarray data and Sophia Li, Natasha Singh, Ravneet Padda, Wen Su, and Andrea Glenn for technical support.
The authors have declared no conflict of interest.




