The realm of vitamin K dependent proteins: Shifting from coagulation toward calcification
Abstract
In the past few decades vitamin K has emerged from a single-function “haemostasis vitamin” to a “multi-function vitamin.” The use of vitamin K antagonists (VKA) inevitably showed that the inhibition was not restricted to vitamin K dependent coagulation factors but also synthesis of functional extrahepatic vitamin K dependent proteins (VKDPs), thereby eliciting undesired side effects. Vascular calcification is one of the recently revealed detrimental effects of VKA. The discovery that VKDPs are involved in vascular calcification has propelled our mechanistic understanding of this process and has opened novel avenues for diagnosis and treatment. This review addresses mechanisms of VKDPs and their significance for physiological and pathological calcification.
Abbreviations
-
- ALP
-
- alkaline phosphatase
-
- BMP-2
-
- bone morphogenetic protein-2
-
- CKD
-
- chronic kidney disease
-
- FMC
-
- fetuin mineral complex
-
- GRP
-
- Gla-rich protein
-
- HA
-
- hydroxyapatite
-
- MGP
-
- matrix Gla protein
-
- VKAs
-
- vitamin K antagonists
-
- VKDPs
-
- vitamin K dependent proteins
-
- VKOR
-
- vitamin K epoxide reductase
-
- VSMCs
-
- vascular smooth muscle cells
1 Introduction
Vitamin K is arguably the least known vitamin and a member of the fat-soluble vitamins. The function of vitamin K remained unresolved until 1974, when Stenflo and colleagues described the presence of a modified glutamate (Glu) residue in prothrombin, a protein involved in blood coagulation 1. Shortly thereafter it was discovered that vitamin K is an essential cofactor in the posttranslational modification of glutamate residues known as γ-glutamylcarboxylation 2. Today, these modified, protein-bound glutamate residues are known as γ-carboxyglutamate (Gla) residues. The conversion of Glu into Gla turned out to be essential for biological activity of prothrombin by granting its calcium binding activity 1.
Since the discovery of prothrombin, at least 14 more proteins have been identified that contain Gla residues. These proteins together build the so-called vitamin K dependent protein (VKDP) family. VKDPs can be categorized into two groups; hepatic and extrahepatic VKDPs. Hepatic VKDPs are mainly involved in balancing blood coagulation by bridging their Gla residues through calcium with negatively charged phospholipids 3, 4. Extrahepatic VKDPs have distinct functions, yet their Gla residues also have high affinity for calcium. Osteocalcin was the first extrahepatic VKDP identified and is involved in regulation of calcification of bone matrix 5. Some 10 years later, another extrahepatic VKDP associated with calcification was isolated from demineralized bone 6. This VKDP was termed matrix Gla protein (MGP) and is involved in the inhibition of ectopic calcification. A more recently identified VKDP, Gla-rich protein (GRP), is also isolated from bone and cartilage. GRP is also known as upper zone of growth plate and cartilage matrix associated protein (Ucma) 7, 8. Similar to MGP, GRP has been purified from calcified cartilage 9. In this review, we discuss the functions of VKDPs beyond coagulation and describe their role in physiological and pathological calcification.
2 Vitamin K
Vitamin K was identified in the early 1930s by the Danish researcher Hendrik Dam 10. He observed that chickens fed a low fat diet displayed an increased bleeding tendency. When substituting the diet with cholesterol, the bleeding phenotype did not disappear. It turned out that the lipid fraction contained an antihemorrhagic factor that was designated “Koagulations vitamin,” nowadays abbreviated as vitamin K 10.
All forms of vitamin K share a common 2-methyl-1,4-naphthoquinone ring structure called menadione (vitamin K3) and an isoprenoid side chain at the 3-position. Menadione is a synthetic chemical compound (Fig. 1A) 11. Depending on length and saturation of the side chain, vitamin K is classified as phylloquinone (vitamin K1) or as one of the menaquinones (vitamin K2) (Fig. 1A). Phylloquinone contains a phytyl side chain and is found in green as well as nongreen leafy vegetables 12. Menaquinones consist of a side chain with repeating isoprene residues containing an unsaturated bond. Depending on the number of repeats, menaquinones are subcategorized as MK-n with n being the number of isoprenoid residues. The longer the side chain, the more lipophilic menaquinones are. Menaquinones are found in bacterially fermented food and in liver. In addition to nutritional uptake, intestinal bacteria also synthesize menaquinones. In human intestines, microorganisms of the genera Bacteroides and Lactococcus synthesize menaquinones 13, 14. However, Suttie and colleagues postulated that the contribution of bacteria-derived menaquinones to human vitamin K status is limited due to the limited absorption of fat-soluble vitamins in the colon 15. In a typical western diet, phylloquinone presents the major fraction of vitamin K intake 16.

Chemical structures of vitamin K and the vitamin K cycle. Panel A displays the three basic forms of vitamin K. The upper structure represents vitamin K1, also known as phylloquinone. The middle structure represents vitamin K2, also known as menaquinones with n representing the length of the isoprenoid side chain thereby indicating the respective menaquinone. The bottom structure represents vitamin K3, also known as menadione. Vitamin K3 is a 2-methyl-1,4-naphthoquinone ring structure that forms the basis for all K-vitamins. Panel B represents the carboxylation cycle in which glutamate (Glu) residues are converted into γ-carboxyglutamate (Gla) residues through conversion of reduced vitamin K (KH2) to vitamin K epoxide (KO). KO is recycled by vitamin K epoxide reductase (VKOR) such that it can be reused. Illustration credit: Nutrition.eu.
Vitamin K exerts its biological function as a cofactor for posttranslational modification of specific Glu residues in VKDPs. Glu residues are modified by an enzymatic reaction known as the vitamin K cycle (Fig. 1B). In this reaction both γ-glutamyl carboxylation and vitamin K recycling take place. Gamma-glutamyl carboxylase (GGCX) converts reduced vitamin K hydroquinone (KH2) into vitamin K epoxide (KO) thereby facilitating the addition of a carboxylic acid to a Glu residue. This results in formation of a Gla residue in the VKDP. Next, KO is recycled by vitamin K epoxide reductase (VKOR). VKOR is a multimer that efficiently reduces KO so it can be reused several hundred times to fuel the carboxylation reaction 12, 17.
2.1 Vitamin K antagonists
Around the same time vitamin K was discovered, a VKOR inhibitor was identified. Cattle fed spoiled clover showed increased bleeding tendency when being dehorned resulting in lethal hemorrhages. The spoiled clover contained a compound, later termed dicoumarol, responsible for the observed hemorrhages 18. Because of the potency to cause severe bleedings, warfarin, a coumarin derivative, was synthesized and used as a rodenticide. After a failed suicide attempt with warfarin by a US military soldier, it became clear that warfarin could be used as a drug to treat coagulation-related problems. Coumarins act as vitamin K antagonists (VKAs). They neither interfere with absorption and transport of vitamin K, nor with carboxylation. They inhibit vitamin K recycling by blocking VKOR activity 19. As a consequence, depletion of tissue vitamin K reserves occurs, resulting in undercarboxylation of VKDPs.
As VKA treatment is not liver specific, extrahepatic VKDPs are also affected. This side effect of VKA has only recently been recognized. One such consequence of VKA treatment is increased calcification of the vasculature 20-22. Calcification of the vasculature induced by warfarin was first observed by Price and colleagues 23. They injected rats with daily doses of warfarin combined with vitamin K1 to prevent lethal hemorrhages. Administration of the combination of warfarin and vitamin K resulted in depletion of vitamin K1 in extrahepatic tissue but not in liver because of the biodistribution kinetics of vitamin K1. After 2 weeks of treatment, aortic calcifications occurred that progressively increased at 3, 4, and 5 weeks of treatment. Calcification was ascribed to the inhibition of γ-carboxylation of MGP. This was confirmed by two other experiments in which mice were fed a warfarin-enriched diet. In addition to calcifications, histochemical analysis revealed the presence of chondrocyte-like cells in the vessel wall associated with calcifications 20, 22.
3 Calcification
Calcification is a physiological process required to create mineralized tissue such as teeth and bones. Mineralized tissues perform various functions ranging from internal support to protecting vital organs 24. However, calcification can also be detrimental. Ectopic calcification of soft tissues is pathological and presents a risk factor for increased mortality 25, 26. Below, we will address the involvement of VKDP in both physiological and pathological calcification.
3.1 Physiological calcification
Physiological calcification is a multifactorial, complex process, which is tightly regulated 27. The main site of physiological calcification is the bone matrix of the skeleton 28. Both bone formation and remodeling are performed by bone cells: osteoblasts, osteoclasts, and osteocytes 29-31. Osteoblasts originate from mesenchymal cells. They synthesize an extracellular matrix, which then becomes mineralized by precipitation of insoluble calcium salts from the extracellular fluids following matrix maturation 30. Osteoclasts are specialized hematopoietic cells that resorb bone by solubilizing calcium phosphate crystal deposits and breaking down the matrix structure 31, 32. Osteocytes are derived from osteoblasts and are the most abundant cells in the mature bone matrix. They are involved in regulation of both systemic and local mineralization, bone formation, and bone resorption 33. Chondrocytes, constituting cartilage, are a special type of bone-associated cells with a crucial role in skeletal development and growth.
Bone formation starts from a cartilage template. This template is replaced by bone in a process called endochondral ossification. Intramembrous ossification is another essential process for skeletal development independent of a cartilage template. Prior to calcification of the cartilage template, chondrocytes become hypertrophic (Fig. 2). Hypertrophy induces expression of typical bone markers such as runt-related transcription factor 2/ core-binding factor subunit alpha-1 (Runx2/Cbfa1), collagen type X, and alkaline phosphatase (ALP), a hydrolytic enzyme that inactivates pyrophosphate, a potent calcification inhibitor. ALP is secreted by chondrocytes in matrix vesicles, the nidus for calcification of the cartilage template 34. Inside these vesicles, hydroxyapatite (HA) crystals composed of calcium and phosphate are formed 35. Finally, calcified cartilage is invaded by blood vessels, osteoclasts, and osteoblast precursor cells. Osteoclasts remove the cartilage extracellular matrix and osteoblasts deposit bone onto the remnants. After calcification, a cartilage growth plate, responsible for future longitudinal growth remains. In the growth plate, chondrocytes maturate and calcify until bone growth stops (Fig. 2) 36.
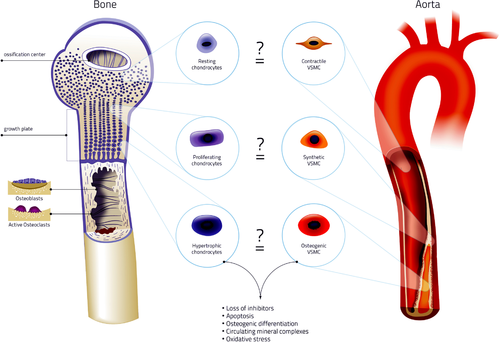
Similarities of physiological and pathological calcification. In the cartilage growth plate chondrocytes mature. Resting chondrocytes proliferate and become hypertrophic. The onset of hypertrophy is accompanied with loss of calcification inhibitors. This causes HA crystal formation in matrix vesicles, which serve as a nidus for calcification. Eventually, hypertrophic chondrocytes become apoptotic and these apoptotic bodies serve as calcification nidus. Calcification is further accelerated by activation of osteogenic genes such as Runx2/Cbfa1 and osteocalcin. Similar mechanisms are involved in vascular calcification. Stress signals such as increased calcium and phosphate induce calcification through VSMC phenotypic switching: they lose calcification inhibitors and contractility markers. This is accompanied by upregulation of osteoregulatory genes. Additionally, VSMCs start secreting matrix vesicles that calcify in the extracellular matrix of the vessel wall. Phagocytosis of calcium crystals by neighboring VSMCs induces apoptosis, which further accelerates calcification. Finally, bone mineralization and vascular calcification are intertwined by bone remodeling 126. Remodeling is performed by bone multicellular units and causes release of mineral complexes from the bone into the blood. These mineral complexes can lodge in soft tissues to form a nidus for calcification. Thus, physiological and pathological calcification share striking similarities. Resting chondrocytes share many similarities with contractile VSMCs. Both cell types are resistant to calcification. Proliferating chondrocytes share similarities to synthetic VSMCs, which possess a limited capacity to calcify. Finally, hypertrophic chondrocytes share similarities with osteogenic VSMCs. Both lose calcification inhibitors, shed matrix vesicles, and have expression of genes associated with osteogenesis. Illustration credit: Nutrition.eu.
Bone is continuously remodeled to preserve skeletal health. This is performed by bone multicellular units composed of osteoblasts and osteoclasts (Fig. 2) 24. Alterations in the balance between bone formation and degradation change the bone mass and density. This results in compromised bone health, which is seen in osteoporosis and osteopetrosis 37, 38. Thus control of mineralization is crucial for normal functioning of the body and is partially regulated by extrahepatic VKDPs.
3.2 VKDPs in physiological calcification
VKDPs have high affinity for calcium-based matrices through their Gla residues 39. Osteocalcin and MGP were, until recently the only VKDPs known to be involved in physiological calcification. With the recent discovery of GRP, a new kid on the block has appeared 9.
3.2.1 Osteocalcin
Osteocalcin is the most abundant, noncollagenous protein in bone. It is expressed by mature osteoblasts and early hypertrophic chondrocytes 40, 41. Two different roles of osteocalcin in calcification have been proposed: (i) regulation of bone mineralization and (ii) regulation of osteoblast and osteoclast activity 42, 43. Osteocalcin-deficient mice have increased bone mass and increased ALP levels indicating that osteocalcin is involved in regulation of bone mineralization 44. Using Fourier transform infrared microspectroscopy, it was shown that bones from osteocalcin-deficient mice had less matured and smaller HA crystals as compared to wild-type mice 45. Osteocalcin has, in the carboxylated conformation, a high affinity for calcium. It has been reported that osteocalcin modulates HA crystal morphology and growth 42, 46. Hunter et al. demonstrated that osteocalcin interferes with early stages of calcification through delaying HA nucleation without influencing the final amount of HA crystals formed 46. However, the carboxylation status of osteocalcin in these experiments was not reported. Additionally, osteocalcin is involved in regulation of osteoblast and osteoclast activity. The increased bone mass seen in osteocalcin-deficient mice suggests osteoblast activity is increased in the absence of osteocalcin 44. Contradictory, it has been shown that osteocalcin stimulates osteoblast activity and increases bone formation by enhancing the initial adherence of osteoblasts in vitro 47. Additionally, mice with a HA/collagen composite implant had increased bone healing and active osteoblasts in the presence of osteocalcin 48. It has been suggested that osteocalcin performs this effect through a G-protein coupled receptor 49. Taking advantage of the effects of osteocalcin on bone, mouse models have been developed to study osteogenic processes and skeletal repair in vivo 50. However, once again the carboxylation status of the osteocalcin used in these studies was not reported.
Osteocalcin affects osteoclasts and consequently, bone remodeling, which is dependent on the carboxylation status. It serves as a chemotactic signal for osteoclasts, stimulates osteoclast differentiation, maturation, and activity thereby increasing bone turnover rates 51-55. These effects are moderated by calcium release from stores into the cytosol and were shown to be independent of vitamin K dependent carboxylation 53.
Serum osteocalcin levels are currently considered to be one of the most sensitive markers of bone formation 56. This makes osteocalcin an attractive target to study pharmaceutical and nutraceutical effects on bone 57. Indeed, serum levels of uncarboxylated osteocalcin have been reported to be a measure for vitamin K status in bone 58. However, clinical trials with vitamin K supplementation did not find a consistent protective effect on bone loss, even though the carboxylation status of osteocalcin was improved 59-61. This observation is in accordance with part of the in vitro work, where some of the effects of osteocalcin were found to be independent of its carboxylation status.
Recently, the skeleton was put forward as an endocrine tissue with a hormonal function for osteocalcin regulating male fertility and energy metabolism 62, 63. A detailed review on this topic has been provided by Karsenty and Ferron 64.
3.2.2 Matrix Gla protein
MGP is widely expressed and accumulates in cartilage and calcified tissues 65. In cartilage, MGP is expressed by resting, proliferative and late hypertrophic chondrocytes 66. In contrast to osteocalcin, MGP expression is absent in early hypertrophic chondrocytes and osteoblasts. Furthermore, MGP levels are elevated early on during bone formation 67. MGP is present in matrix vesicles in cartilage, suggesting its function as a regulator of cartilage calcification 68. It was demonstrated that both cartilage and bone-derived MGP was carboxylated. The role of MGP in cartilage and bone calcification was elucidated in MGP-deficient mice 69. MGP-deficient mice have normal size and weight at birth, however as they age, growth is retarded compared to wild-type animals. Age-matched MGP-deficient animals are significantly smaller than wild-type mice and histological analysis shows unregulated calcification of growth plate cartilage. Consequently, the growth plate becomes disorganized. Newman and colleagues demonstrated an anti-apoptotic effect of MGP in proliferative chondrocytes and suggested that increased apoptosis is the cause of growth plate disorganization 70. This effect appears to be independent of MGP carboxylation, as warfarin treatment of proliferative chondrocytes of chickens had no effect on chondrocyte survival 71. Warfarin treatment to inhibit vitamin K dependent carboxylation did however induce calcification in hypertrophic chondrocyte cultures 71. Calcification could be counteracted by co-treatment with vitamin K. Additionally, MGP overexpression in resting and hypertrophic chondrocytes delayed chondrocyte maturation, inhibited cartilage calcification, and blocked endochondral ossification.
The calcification inhibitory effect of MGP is partly based on its direct HA-binding ability through Gla residues 72. Keutel disease is caused by mutations in the MGP gene resulting in nonfunctional protein 73. In Keutel disease patients, increased cartilage calcification is observed, similar to MGP-deficient mice. MGP overexpression in teeth of mice caused severe hypomineralization 74. Moreover, inhibition of calcification was shown to be dependent on Gla residues: transgenic mice with an MGP mutant that cannot be carboxylated acquired a phenotype similar to MGP-deficient mice 75. The lack of carboxylated MGP has also been associated with pathological calcification in osteoarthritic cartilage 76.
MGP affects osteogenesis, chondrocyte maturation, and consequently inhibits calcification through interaction with bone morphogenetic protein-2 (BMP-2) 77, 78. BMP-2 is a potent inducer of bone formation in both skeletal and soft tissues 79, 80. MGP regulates BMP-2 activity via binding to BMP-2 with its Gla residues 81. This emphasizes the need of carboxylation of MGP in bone and cartilage formation.
3.2.3 Gla-rich protein
GRP is a recently discovered VKDP with a high number of Gla residues. The first report in 2008 identified GRP as novel transcript in a human fetal growth plate cartilage cDNA library 8. It was hypothesized that GRP is a secretory marker for resting chondrocytes. This was confirmed by Stock and colleagues who showed that GRP is cartilage specific 7. More recently, GRP was shown to be widely expressed by both skeleton-associated tissues and soft tissues 9.
In vitro experiments showed that GRP is involved in regulation of osteogenesis by delaying osteoblast maturation 7. Subsequently, GRP was knocked down in zebrafish, which caused severe growth retardation and perturbance of skeletal development 82. Additionally, collagen type II and aggrecan content in cartilage was reduced. This phenotype could be reproduced by treating zebrafish with VKA. The effect was ascribed to inhibition of GRP carboxylation since other VKDPs are not expressed during that phase of zebrafish development 82. Surprisingly, GRP-deficient mice did not display a clear phenotype during normal development 83. Cartilage development was normal indicating that GRP is not essential for normal bone formation. Current studies aim to investigate the involvement of GRP in skeletal homeostasis and in mechanical properties of the skeleton during disease.
3.3 Pathological calcification
3.3.1 Background
Under physiological conditions soft tissues such as skin, kidneys, and blood vessels do not calcify 84. Vascular calcification is a disease that was described as being present already over 5000 years ago 85. Additionally, the presence of calcification in atherosclerosis in 4000-year-old mummies was reported 86. Already more than 100 years ago, pathologists reported the presence of bone-like structures in extraskeletal tissues 87. Pathological triggers cause soft tissues to become prone to calcify 88. This is pathological or ectopic calcification. Here we focus on the role of vascular smooth muscle cells (VSMCs) in vascular calcification.
Calcification of the vascular system is mainly present in the arterial vessel wall, and results in reduced arterial elasticity and subsequent altered hemodynamics. This contributes to development of aortic stenosis, cardiac hypertrophy, hypertension, heart failure, and myocardial ischemia 89, 90. Since vascular calcification has an impact on cardiovascular disease development, coronary artery calcium content is now used as a measure for atherosclerotic plaque burden in asymptomatic individuals 91, 92.
Vascular calcification can manifest as intimal or medial calcification (Fig. 3). It was long considered to be a passive bystander, not amendable for intervention. However, recent evidence clearly put forward that vascular calcification is a regulated process sharing similarities with bone formation 93.
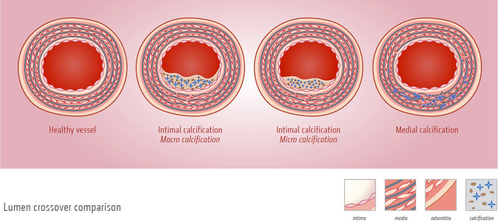
Different manifestations of vascular calcification. Different types of vascular calcification can occur in the vasculature. (A) Healthy blood vessel in the absence of calcification. (B) Blood vessel containing an atherosclerotic plaque in which macrocalcification is present. (C) Blood vessel with an atherosclerotic plaque in which microcalcifications are present. Microcalcifications are responsible for destabilizing the plaque, causing an increased risk of plaque rupture. (D) Blood vessel with medial calcification with calcium deposits present along the elastic laminae of the tunica media. Illustration credit; Nutrition.eu.
3.3.2 Intimal calcification
Atherosclerosis is the leading cause of death and disability in western societies and often associated with calcification (Fig. 3) 94. Intimal or atherosclerotic calcification is often caused by a combination of single-risk factors, such as hypertension, inflammation, or dyslipidemia 95. Recently, inflammation was linked to microcalcifications in atherosclerotic plaques 96. Atherosclerosis is known to be a chronic inflammatory disease 97. Vascular calcification contributes to this inflammatory state as macrophages phagocytose HA crystals subsequently activating inflammatory cytokine expression 98. Intimal calcification can either manifest as spotty microcalcifications or as diffuse mineral deposits (Fig. 3) 20, 99. The effect of calcification on atherosclerotic plaque stability is still a matter of debate. It was shown in postmortem coronary arteries that massive calcification is not related to plaque stability 100. However, the presence of microcalcifications has been shown to be detrimental for plaque stability 101, 102. Of the microcalcifications present in coronary arteries, only the calcification spots in the fibrous cap seem to have an adverse effect on biomechanical plaque stability. The presence of microcalcifications in lipid pools or necrotic cores has little influence on biomechanical stability 103.
3.3.3 Medial calcification
In medial calcification, also known as Mönckeberg sclerosis, amorphous mineral deposits are formed along the elastin fibers of the tunica media (Fig. 3) 104. Medial calcification has been found throughout the entire vascular tree and is highly prevalent in the aging population, in chronic kidney disease (CKD) patients, and people with diabetes mellitus 105, 106. VSMCs have been shown to play a key role in regulating medial calcification.
3.4 Molecular mechanisms of vascular calcification
Based on literature, at least four different processes involved in development of vascular calcification have been identified including (i) loss of calcification inhibitors, (ii) circulating mineral complexes, (iii) apoptosis, and (iv) osteochondrogenic differentiation of VSMCs. In all of these processes, an important role for VKDPs has been put forward.
3.4.1 Loss of calcification inhibitors
Human body fluids are supersaturated with regard to both calcium and phosphate 107. However, spontaneous precipitation of calcium phosphate crystals does not occur. This is due to tight control of calcium precipitation by calcification inhibitors. Fetuin-A and MGP are both proteins with strong anticalcification properties 108, 109. Fetuin-A is a circulatory protein that inhibits calcium phosphate crystal precipitation through formation of a fetuin-MGP mineral complex 110. In fetuin-A deficient mice, spontaneous soft tissue mineralization is present 111. MGP prevents calcification of the tunica media by preventing precipitation of calcium phosphate crystals onto elastin fibers 73, 112. The ability of MGP to bind calcium phosphate crystals is dependent on its Gla residues and consequently, vitamin K status 23, 113.
3.4.2 Circulating mineral complexes
Bone remodeling and vascular calcification are connected. By prevention of vascular calcification, the low bone mineral density phenotype in MGP-deficient mice can be restored 114. A circulating mineral complex, called the fetuin mineral complex (FMC), was detected in serum of rats treated with etidronate to prevent bone calcification 115, 116. The FMC originates from bone and contains MGP 117. Vitamin K dependent γ-carboxylation of MGP is essential for the presence of MGP in the FMC 118. Upon warfarin treatment of the rats, MGP can no longer be detected in the FMC. Additionally, this study also showed that MGP serum levels rise dramatically upon prevention of bone calcification. Virtually all serum MGP seems associated with the FMC. Additionally, upon inhibition of HA precipitation FMC is formed 110.
3.4.3 Apoptosis
Apoptosis is a common feature in various tissues, including the vasculature where it regulates cell number. However, sometimes apoptosis may be upregulated or phagocytosis may be impaired. Apoptosis in atherosclerosis is associated with plaque instability 119. Additionally, the observed paucity of VSMCs in ruptured plaques when compared to stable plaques suggests VSMC apoptosis might play a role in a phase prior to rupture. Apoptotic VSMC generate negatively charged membrane particles, which—if not phagocytozed properly—promote initiation of calcification by serving as the initial nidus for calcium phosphate crystal precipitation 120-122. Calcification inhibitory proteins such as MGP tend to block the HA nidus on these membrane structures or form stabilized crystal forms. In 1967, Anderson first used the name “matrix vesicles” in cartilage development and calcification 123. Tanimura and co-workers were the first to reported an association between matrix vesicles and vascular calcification 124. Vesicular structures have been found in both the tunica intima and media and were likely derived from VSMCs.
3.4.4 Osteogenic differentiation of VSMC
As described above, VSMCs display a high degree of phenotypic plasticity that is triggered by both extra- and intracellular signals (Fig. 4) 125, 126. VKDPs are involved in controlling this phenotypical plasticity. In the development of vascular calcification, VSMCs play an important role as contractile, synthetic, and osteogenic VSMCs 19. In normal vasculature, contractile VSMCs in the tunica media regulate vessel tone and diameter to maintain hemodynamic balance 126. Phenotypic switching of VSMCs is necessary to deal with varying conditions of vascular tissue 127. VSMCs can transdifferentiate toward a synthetic or osteogenic phenotype that can calcify the vascular wall (Fig. 4) 128. Osteogenic differentiation of VSMCs can be initiated by exposure to elevated phosphate concentrations and by BMP-2 129.
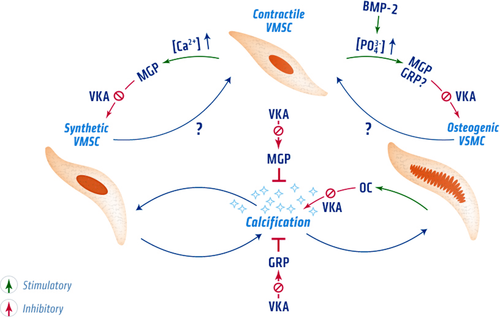
Control of the VSMC phenotypic switch by VKDPs. Normally, VSMCs are in the contractile phenotype. However upon exposure to stress signals such as elevated calcium and phosphate levels, transdifferentiation toward a synthetic or osteochondrogenic phenotype occurs. Whether VSMCs can directly switch toward to an osteogenic phenotype or first go through the synthetic state remains to be determined. VKDPs are involved in regulating these phenotypical switches. Carboxylated MGP is involved in maintaining the contractile phenotype of VSMC and supports vascular elasticity. It further assists in maintaining the contractile phenotype by inhibiting BMP-2 through its Gla residues. VKA treatment accelerates VSMC phenotypical switching by preventing carboxylation of VKDPs. Uncarboxylated MGP was shown to be incapable of preventing vascular calcification. In VSMCs transdifferentiated toward an osteogenic phenotype, osteogenic genes become activated such as osteocalcin that can modulate vascular calcification by binding HA crystals through its Gla residues. However, osteocalcin has also been shown to stimulate osteogenic differentiation, which promotes vascular calcification. Both synthetic and osteochondrogenic differentiation result in calcification of the vascular extracellular matrix. GRP, a recently discovered VKDP, is thought to be involved in regulating phenotypic switching of VSMCs. In osteoblast cells, GRP was found to inhibit osteogenic differentiation. However, it still remains to be determined whether GRP is also involved in osteogenic differentiation of VSMC and whether this effect is affected by VKA treatment. Illustration credit: Nutrition.eu.
A crucial step in osteogenic transdifferentiation is activation of cyclic adenosine monophosphate (AMP) 130. cAMP causes phosphorylation of extracellular signal regulated kinase 1/2 (Erk 1/2) via the MAPK pathway. Phosphorylated Erk 1/2 upregulates expression of Runx2/Cbfa1, a gene essential for osteoblast differentiation and bone development 131, 132. Once Runx2/Cbfa1 expression is upregulated, other osteoregulatory genes such as ALP and osteocalcin that promote calcification of the vascular matrix are activated 90.
3.5 VKDPs in vascular calcification
3.5.1 Osteocalcin
Osteocalcin is specific to bone and teeth and is not expressed by VSMCs under physiological conditions. However, in the atherosclerotic vessel wall osteocalcin is abundantly present, produced by osteoblast-like VSMCs 133-135. Recently, it was shown that also platelets contain and secrete osteocalcin in atherosclerotic plaques 136. Osteocalcin serum levels are elevated in rats fed a warfarin diet, which correlated with the observed medial calcification 137. Clinically, osteocalcin might serve as a predictor of vascular calcification 138-140. However, to introduce undercarboxylated osteocalcin as a biomarker for vascular calcification, more research is required as it is essential to take bone metabolism into account.
Many studies show both Runx2/Cbfa1 and osteocalcin upregulation by VSMCs when exposed to elevated extracellular phosphate concentrations. This has put osteocalcin forward as marker of osteochondrogenic VSMCs. Osteocalcin-deficient mice did not display increased vascular calcification. However, high levels of osteocalcin corroborate with increased calcification of VSMCs in vitro 141. Additionally overexpressing osteocalcin in the VSMC cell line MOVAS causes upregulation of markers of osteogenic differentiation 139. This was ascribed to stabilizing HIF-1α thereby altering glucose metabolism. Moreover, knockdown of osteocalcin in MOVAS cells reduced osteogenic differentiation 139. These findings indicate that osteocalcin is not simply a passive bystander of calcification but acts as an active regulator in osteochondrogenic differentiation of VSMCs 142.
Osteocalcin positive endothelial progenitor cells have been isolated from patients with coronary atherosclerosis or type 2 diabetes 140, 143. These cells bear calcification potential, and were associated with severity of aortic calcification 144. The calcification potential was further demonstrated in cell culture where they formed calcified nodules and expression of osteogenic markers in response to inflammatory signals 145.
3.5.2 Matrix Gla protein
MGP was first linked to vascular calcification by Shanahan and co-workers in the early 1990s. They found upregulated MGP expression in late passage, dedifferentiated VSMCs as compared to freshly transplanted VSMCs. Moreover, they observed high MGP expression in human atherosclerotic plaques 146, 147. These reports caused a paradigm shift. Until that time calcification of soft tissues was considered to be a passive process. Nowadays, evidence is present that vascular calcification is a highly regulated cell-mediated process 93.
Different groups demonstrated in vitro that MGP mRNA levels are upregulated in VSMCs in response to high calcium levels 148, 149. High extracellular calcium is a key signal regulating expression of MGP via a G-protein coupled mechanism similar to the calcium-sensing receptor (CaR) 150. However, the function of MGP only became clear in MGP-deficient mice, which died within 2 months after birth as a consequence of massive hemorrhages due to blood vessel rupture caused by arterial calcification 69. The same group showed that VSMC-specific MGP knock-in rescued the calcification phenotype of MGP-deficient mice 75. Additionally, elastin haploinsufficiency in MGP-deficient mice reduced vascular calcification 128. The necessity of carboxylation for MGPs proper functioning was also demonstrated using in vivo mutagenesis experiments 75. Here glutamic acid residues were replaced by aspartic acid residues, which cannot be carboxylated. The GlaMGP-deficient mice died due to massive calcification of the vascular system, even though uncarboxylated MGP was present.
Although MGP is present in the circulation, increasing circulating levels did not prevent calcification of the vascular matrix suggesting MGP inhibits calcification through a local rather than a systemic effect 75. Therefore it is assumed that plasma MGP levels reflect local synthesis and thus might serve as predictor of vascular calcification 151-153.
The significance of vitamin K dependent carboxylation in regulating vascular calcification became clear in rats fed a warfarin enriched diet. These animals developed rapid calcification of the elastic lamellae and heart valves 23. Vascular calcification induced by warfarin was similar to that seen in MGP-deficient mice. In accordance with in vitro assays, warfarin treatment in rats caused an increase in MGP mRNA and protein levels in the vasculature. The effect of warfarin on MGP was confirmed by in vitro calcification experiments 154, 155. This prompted researchers to investigate the effect of VKA in patients. Since the first report, this detrimental effect of VKA has been demonstrated in multiple experiments 20, 156, 157. However, some trials in which VKA was administered found no association with increased coronary artery calcification 158, 159. More research is needed to reveal the precise mechanism by which VKA exert this clinical manifestation.
In addition to MGP's affinity for HA crystals, the presence of chondrocyte-like cells in the vascular wall of MGP-deficient mice implicated MGP is involved in controlling VSMC transdifferentiation (Fig. 4) 160. Indeed, MGP has been shown to inhibit osteogenic transdifferentiation by interacting with BMP-2 78, 161. BMP-2 is a potent inducer of calcification via osteogenic transdifferentiation of VSMCs 162. VSMCs exposed to BMP-2 increase activin-like kinase receptor 1 (ALK1) expression 163. Consequently, ALK1 activation upregulates MGP expression, which in turn inhibits BMP-2 activity thereby reducing osteogenic transdifferentiation of VSMCs. Inactivation of BMP-2 required the carboxylation of MGP 164. CKD patients suffer from increased circulating calcium and phosphate concentrations and subsequently high mortality due to vascular calcification. Osteogenic transdifferentiation of VSMCs is further accelerated by VKA and is associated with up- and downregulation of a number of genes including MGP 165, 166. The bisphosphonate etidronate, a drug used to inhibit bone loss, has been shown to upregulate MGP expression in the vessel wall and reduce vascular calcification 167.
3.5.3 Gla-rich protein
GRP is present in the normal vasculature and expression is increased when calcification occurs 168. GRP co-localized with mineral deposits in the vascular media of CKD and diabetes mellitus patients. GRP accumulation might be due to binding of HA crystals through Gla residues, similar to the function of MGP. Whether GRP fulfills a backup mechanism for MGP to inhibit vascular calcification, has to be investigated.
In vitro, GRP delayed osteoblast maturation and downregulated both ALP activity and osteocalcin expression 7. Interestingly, BMP-2 was shown to downregulate GRP expression in chondrocytes 169. This suggests a possible role for GRP in the regulation of VSMC transdifferentiation and consequently, calcification of the vessel wall (Fig. 4). However, in contrast to MGP-deficient mice GRP-deficient mice did not develop vascular calcifications under physiological conditions 83. It remains to be investigated whether GRP-deficient mice develop vascular calcification in challenging situations such as nephrectomy.
4 Clinical consequences of vitamin K deficiency
As explained above vitamin K deficiency entails risk of soft tissue calcification. In addition, vitamin K deficiency is associated with increased bleeding tendency. The most life-threatening complication of vitamin K deficiency occurs in newborns, especially those who are breastfed. Due to the placental barrier newborns have vitamin K deficiency and a thus increased risk of bleeding, also known as vitamin K deficiency bleeding. In most countries, vitamin K is administered prophylactically at birth, which has resulted in a dramatic decrease of vitamin K deficiency bleeding demonstrating the importance of vitamin K intake 170. CKD patients are also known to have a poor vitamin K status 106, 171, 172. Vitamin K deficiency in the CKD population has been shown by decreased vitamin K levels in serum as well as with increased circulating levels of undercarboxylated VKDPs 172-175. CKD patients suffer from increased vascular calcification. Patients treated with VKA to control thrombotic tendencies have functional vitamin K deficiency and are at risk to develop vascular calcification 21, 176. Additionally, patients on long-term VKA treatment have a 30% increased risk of bone fractures as compared to controls 177. However, this observation is inconsistent since other studies showed no effect of VKA treatment on bone loss 178-181. Vitamin K deficiency also occurs as a consequence of disturbed intestinal absorption, antibiotic therapy or dietary deficiency 12. Our knowledge about vitamin K and its biological functions has revealed a number of assays and circulating biomarkers that report vitamin K status including coagulation assays, measurement of undercarboxylated VKDPs (prothrombin, MGP, osteocalcin) in serum, vitamin K intake, and serum levels and vitamin K metabolites in urine 182-184. Due to the omnipresence of vitamin K deficiency, it is of interest to look into the therapeutical administration of vitamin K. Vitamin K administration might be able to counteract or attenuate vascular or bone disease progression.
4.1 Therapeutic applications of vitamin K
Since observational studies demonstrated a relationship between vitamin K status and bone mineral density (BMD), a number of placebo-controlled trials have been conducted to investigate this relationship. In the VIKI dialysis study, vitamin K deficiency was observed in hemodialysis patients and found to be a strong predictor of vertebral fractures and vascular calcifications 185. Hip fracture risk and bone mineral loss have been reported to be decreased in people consuming high amounts of vitamin K 177, 186.
Literature is inconclusive as to whether vitamin K supplementation has an effect on bone health. Several groups report no or marginal effects of vitamin K supplementation 187, 188. In contrast, other studies found vitamin K supplementation to preserve BMD 186, 189. In addition to bone health, vascular calcification has been associated with vitamin K status 190. In postmenopausal women, receiving 1 mg vitamin K1 for 3 years increased vascular compliance, distensibility, and intima-media thickness was reported 191. Moreover, vitamin K1 was found to slow down progression of coronary artery calcification in healthy, elderly adults 192. A randomized pilot trial in which different concentrations of vitamin K2 were administered to hemodialysis patients, confirmed that vitamin K deficiency of hemodialysis patients could be reversed by vitamin K supplementation and that VKDP all showed improved carboxylation 193, 194.
Currently, several clinical trials such as the VitavasK (NCT01742273), VitaK-CAC (NCT01002157), VITAKANDOP (NCT01232647), SAFEK (NCT01533441), OVWAK VII (NCT00990158) are underway. The results of these trials will provide further insights into the therapeutic potential of vitamin K in treating both bone disorders and vascular diseases.
5 Conclusion
Although first discovered as the coagulation vitamin, vitamin K's biological functionality through γ-carboxylation extends to VKDPs involved in vascular disease. Osteocalcin, MGP, and GRP are all three VKDPs involved in regulation of both physiological mineralization of bone and pathological calcification of soft tissues. They regulate calcification at different levels including inhibition of calcium crystal formation and VSMC phenotypic switching. Soft tissue calcification is characteristic in CKD patients, patients suffering from diabetes mellitus, and in the aging population. In these populations vitamin K deficiency is common. Vitamin K supplementation trials in healthy elderly have been performed, and vitamin K supplementation in patients suffering from vascular calcification are currently underway. Although based on indirect evidence, these results suggest that the use vitamin K is potentially beneficial in attenuating the progression of vascular calcification. The outcome of these trials will further evaluate the therapeutic potential of vitamin K administration to improve outcomes for populations at risk of vascular calcification.
Potential conflict of interest statement: C. Vermeer is the CSO of VitaK BV, a spin-off company owned by Maastricht University.




