Wheat gliadins modified by deamidation are more efficient than native gliadins in inducing a Th2 response in Balb/c mice experimentally sensitized to wheat allergens
Abstract
Scope: Wheat gluten proteins such as gliadins constitute major food allergens. Gluten can be modified industrially by deamidation which increases its solubility and enhances its use as a food ingredient. Sensitization to deamidated gluten has been reported to cause severe allergic reactions with anaphylaxis. The aim of this study was therefore to compare the sensitization and elicitation potentials of native (NG) and deamidated (DG) gliadins. The reactivity pattern of mice IgE was also compared with that of DG-allergic patients.
Methods and results: The ability of DG to sensitize Balb/c mice using intra-peritoneal administration with aluminium hydroxide as an adjuvant, and to elicit an allergic response after a challenge, was tested in comparison with NG. Mice sensitized with DG secreted higher levels of total IgE, IL-4, gliadin-specific IgE and IgG1 than mice sensitized with NG. By contrast, mice sensitized with NG produced higher levels of gliadin-specific IgG2a and INFγ. After a challenge, histamine levels were higher in mice sensitised with DG.
Conclusions: DG can sensitize mice much more efficiently than NG. Moreover, this mouse model of allergy to DG revealed an IgE reactivity pattern against purified gliadins which was very similar to that of DG-allergic patients.
Abbreviations
-
- AEDS
-
- atopic eczema/dermatitis syndrome
-
- Alum
-
- aluminium hydroxide
-
- DABA
-
- diaminobutyric acid
-
- DBPCFC
-
- double-blind placebo-controlled food challenge
-
- DG
-
- deamidated gliadins
-
- EIA
-
- exercise-induced anaphylaxis
-
- F-ELISA
-
- fluorimetric enzyme-linked immunosorbent assay
-
- Gln
-
- glutamine
-
- Glu
-
- glutamic acid
-
- IP
-
- intra-peritoneal
-
- NG
-
- native gliadins
-
- PIP
-
- prick-in-prick
-
- RBL
-
- rat basophilic leukaemia
-
- Urt
-
- urticaria
1 Introduction
Wheat is a major component of the human diet in Western countries, and yet many of the proteins in this food grain are implicated in respiratory, food or contact allergies. These include some gluten proteins that are important allergens, notably in the context of food allergy 1, 2.
Gluten proteins play a key role in determining the unique baking properties of wheat by providing the cohesiveness and viscoelasticity required for dough formation 3. They are characterized by containing high levels of glutamine (Gln) and proline, and were called prolamins to reflect this original composition. Proteins in this fraction are classified into three groups: sulphur-rich, sulphur-poor and high-molecular-weight prolamins 4. Gliadins belong to the first two groups and account for about half of gluten proteins; they are monomeric and soluble in an alcohol/water mixture. Many types of gliadins are found in each group: ω-gliadins in the sulphur-poor group and α-β-, γ-gliadins in the sulphur-rich group, each of them being highly polymorphic.
The water insolubility of gluten is a major limitation to its extensive use in food processing. The chemical deamidation of gluten, i.e. the conversion of Gln into glutamic acid (Glu) residues 5, increases its solubility and enables its utilisation as an emulsifier and stabilizer 6-8 in foods (cooked meats, clarification of red wine, breadcrumbs, pasta), hair conditioner 6 and cosmetics. The deamidation of gliadins has also been studied and has been reported to improve their solubility and their emulsifying and foaming properties 5, 9. Most of the time, the industrial deamidation process is performed with hydrochloric acid under heating conditions. Enzymatic deamidation of gluten proteins also occurs, catalysed by transglutaminases 10.
Ingestion of wheat proteins may trigger different pathologies: food allergy to wheat that has to be differentiated from coeliac disease. Indeed, these two pathologies display distinct immunological mechanisms and effectors: mostly IgE-mediated for food allergy versus T-cell mediated in celiac disease 11. In children or adults, food allergy to wheat induces a variety of symptoms: atopic eczema dermatitis syndrome (AEDS), chronic urticaria (Urt), anaphylactic shock or exercise-induced anaphylaxis (EIA) 12-14. Allergic reactions such as allergic contact dermatitis and severe anaphylaxis to diverse forms of modified gluten (hydrolysates, deamidated gluten, deamidated wheat proteins) have been reported 6, 12, 15-18. These pathologies differ from other forms of immediate hypersensitivity to wheat proteins because they are more severe and the patients can tolerate native wheat proteins.
Murine models constitute good tools to improve our understanding of allergic sensitization mechanisms and symptom elicitation. Their immunology is well documented. Indeed, IgG1 production in mice and rats is induced by IL-4- and IL-5-secreting Th2 cells and is followed by IL-4-induced IgE production. The Th2 response is down-regulated by Th1 cell activation that causes INF-γ secretion, inducing IgG2a production. In addition, various symptoms may be observed in mice after a challenge. Murine models of sensitization to wheat allergens have also been developed 19-21, one of them by our laboratory: a Balb/c model of sensitization to native gliadins by intra-peritoneal (IP) injections 21.
The aim of the present study was thus to compare the sensitization and elicitation potentials of native and deamidated gliadins using this Balb/c model. The reactivity of mouse IgE directed against the different gliadin classes, native or deamidated, was also compared with the IgE-specificity pattern of patients allergic to deamidated gluten.
2 Materials and methods
2.1 Purification of gliadin fractions
Crude gliadins were extracted from wheat flour (Hardi cultivar) using the sequential procedure developed by Osborne et al. as adapted by Nicolas et al. 22, 23. Briefly, the albumin-globulin fraction of the flour was removed by several washings with saline buffer; the flour was then suspended in 70% ethanol for 1 h at room temperature to solubilize the gliadins. After centrifugation (20 000×g for 20 min at 4°C), the supernatant, i.e. the whole native gliadin extract (NG), was collected and freeze-dried. αβ-, γ, ω1,2- and ω5-gliadins were further purified by ion-exchange chromatography and reverse-phase HPLC according to the technique described by Popineau et al. 24.
2.2 Deamidation procedure
Deamidation was carried out by dispersing 20 mg of NG in 1 mL of 0.1 N hydrochloric acid. The temperature was increased to 90°C and maintained at this level for 1 h. The reaction was stopped by neutralizing with 0.1 N NaOH. The resulting mixture was dialyzed for 5 days against water (changed every day) and centrifuged to recover the supernatant. This soluble material was then freeze-dried. The same procedure was used for the deamidation of purified gliadin fractions.
2.3 Electrophoresis
Native (NG) and deamidated (DG) total gliadin extracts were analyzed using acid-PAGE in a gel of 10% acrylamide 25. They were dissolved at 1 mg/mL in a 0.025 M acetic acid buffer containing 20% v/v glycerol and 6 M urea; 20 μL of each extract were then loaded onto the acid-PAGE and migration was performed towards the cathode. The gel was then stained with Coomassie blue.
2.4 Determination of the degree of deamidation
The deamidation level of each fraction was evaluated by determining the Gln content according to the method described by Kuhn et al. 26. The precipitates were dissolved in water containing norleucine (2.5 mM) as an internal standard. The samples were hydrolysed for 24 h at 110°C in HCl 6 N. They were then solubilized with 10 μL of a drying solution (40% ethanol, 40% water, 20% triethylamine) and evaporated. They were mixed with 20 μL of derivation solution (70% ethanol, 10% water, 10% triethylamine, 10% phenyl isothiocyanate), dried and dissolved in 200 μL buffer containing 95% Na2HPO4 2 mM (pH 7.4) and 5% CH3CN. The amino acid composition was determined by HPLC (Picotag Column, Waters, C18 3 μm, 15 cm×4.6 mm).
Percentage deamidation was calculated from Glu residues and diaminobutyric acid (DABA) titration. The level of Gln residues was evaluated from the DABA/norleucine ratio, while the level of Glu was determined from the glutamic acid (not converted in DABA)/norleucine ratio. Percentage deamidation was calculated as Glu/(Gln+Glu)*100.
2.5 Analysis of patient IgE reactivity against native and deamidated gliadins
Sera from different patients (seven allergic to deamidated wheat proteins and five allergic to wheat) were obtained from the Allergology Department (A. D. Moneret-Vautrin, University Nancy I, Epinal Hospital Centre). These patients displayed different symptoms: AEDS, Urt, or EIA.
Allergy to native or deamidated wheat proteins was established by prick-in-prick (PIP) tests to natural or deamidated wheat flour, and confirmed one month later by a positive double-blind placebo-controlled food challenge (DBPCFC) after 3 wk of a wheat-free diet or by an evident positive effect of an avoidance diet when DBPCFC could not be performed for ethical reasons. The DBPCFC procedure has been described elsewhere 27. Blood collection, PIP, and DBPCFC were performed with informed consent of the patients or their parents.
Fluorimetric Enzyme-Linked Immunosorbent Assay (F-ELISA) was performed to quantify human gliadin-specific IgE on total gliadin extracts and native and deamidated purified gliadin fractions, as described by Bodinier et al. 27.
2.6 Sensitization of mice
Six-wk-old Balb/cJ female mice from the Centre d'Elevage René Janvier (France) were fed an irradiated semi-synthetic diet devoid of plant proteins. The mice were housed in filtered cages under standard specific pathogen-free husbandry conditions and were acclimatised for 3–4 wk prior to immunisation. All experiments were performed according to the European Community rules on animal care (laboratory accreditation number: C44502; investigator accreditation number: 4474).
Before use, freeze-dried native (NG) and deamidated (DG) gliadins were rendered soluble in 70% ethanol at 5 mg/mL and then slowly diluted at 0.1 mg/mL in sterile phosphate-buffered saline (PBS). Three groups of mice (n=8–10 per group) were established: the first (control) group was sensitised with aluminium hydroxide (Alum) diluted in PBS, the second group was sensitized with 10 μg NG (according to the technique described by Bodinier et al. 21) adsorbed on Alum and the third group was sensitized with 10 μg DG adsorbed on Alum. Intra-peritoneal (IP) sensitizations were performed at days 0, 10, 20 and 30.
2.7 Characterization of the immune response of mice
2.7.1 Immunoglobulin assay
The efficiency of mouse sensitisation was evaluated by measuring the concentrations of both Th2 (total IgE, NG-specific and DG-specific IgE, NG-specific and DG-specific IgG1) and Th1 (NG-specific and DG-specific IgG2a) immunoglobulins. Determinations were performed in individual serum samples collected from the retro-orbital venous plexus on day 37 (after four IP injections). Ig reactivity was determined by F-ELISA, as previously described 21, 28.
2.7.2 Rat basophilic leukaemia (RBL) test
RBL cells were plated at a rate of 105 cells/well in a 96-well plate and cultured for 24 h. On day 2, the supernatant was discarded and replaced by medium containing diluted pooled sera (1:100), and the cells were cultured for 24 h. On day 3, after two washes, IgE-sensitized cells were stimulated in 100 μL Tyrode buffer containing 50% deuterium oxide with either 0.1 μg/mL NG, 0.1 μg/mL DG or 0.1 μg/mL rat anti-mouse IgE, for 45 min at 37°C. Percentage degranulation was calculated from the percentage of β-hexosaminidase activity, measured as previously described 27, 29, 30.
2.7.3 Elicitation of the allergic reaction
On day 38, the mice were challenged with an IP injection of either 1mg native gliadins (NG-sensitized mice+half control group) or 1mg deamidated gliadins (DG-sensitized mice+half control group) with aluminium hydroxide. Symptom scoring was performed according to Li et al. 31, and 1 h after the challenge, the mice were bled to determine plasma histamine levels. Histamine was assayed using the Histamine Research ELISA kit (LDN 10-2100 from Labor Diagnostika Nord), following manufacturer's recommendations.
2.7.4 Gliadin-specific activation of spleen cells and assay of cytokines
Bled mice were sacrificed by vertebral dislocation and spleen cells were harvested from each group. After the lysis of red blood cells (180 mM NH4Cl and 17 mM Na2EDTA) and several washes, pooled splenocytes were resuspended in supplemented RPMI medium (10% heat-inactived foetal calf serum, 1% l-glutamine, 1% penicillin/streptomycin). The cells were incubated in 96-well culture plates (106 cells/well) in the presence of either PBS as a negative control, Concanavalin A (1 μg/mL) as a positive control, NG (20 μg/mL), or DG (20 μg/mL) for 60 h at 37°C (under 5% CO2). The supernatants were then collected and stored at −80°C until further assay. IL-4 and INF-γ were assayed using the mouse IL-4 and INF-γ CytoSet™ kit (CMC0043 and CMC4033 from Invitrogen), following manufacturer's recommendations.
2.8 Statistical analysis
Values were expressed as the mean±SEM. GraphPad Prism version 5.02 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com) was used to carry out statistical analyses. The Mann–Whitney and Kruskal–Wallis rank non-parametric tests were used for comparisons between the groups of mice with respect to IgG1, IgE, IgG2a, histamine assay and symptom scoring. When the sera were pooled for each group, Student's t test and the ANOVA parametric test performed to make comparisons between the three groups in terms of cytokine assays and the RBL mast cell test. P values of <0.05 were considered to be statistically significant.
3 Results
3.1 Characterisation of deamidated gliadins
Purified gliadin fractions chemically deamidated under acidic conditions displayed deamidation levels as follows: 52% (±1) for α/β-gliadins, 32% (±6) for γ-gliadins, 36% (±12) for ω1.2-gliadins and 51% (±15) for ω5-gliadins. The deamidation level of the total gliadin extract, which included α/β-, γ- and ω-gliadins, reached 53% (±9) which was within the range obtained for separate gliadin types.
The migration of native and deamidated total gliadins in an acid-PAGE gel revealed two main differences (Fig. 1), the first relative to migration distances and the second to the appearance of the bands. Native gliadins were characterised by at least five clear bands at the top of the gel, while when deamidated they were located in the middle part of the gel as a slightly stained vertical drag. The difference in migration reflected the increase in net charges of deamidated gliadins, and the fuzzy aspect of the zone revealed the broad heterogeneity of deamidated gliadins that results from chemical modification.

Pattern of IgE reactivity in allergic patients (Table 1)
IgE from patients allergic to native wheat proteins reacted against different NG types with patterns that varied as a function of the patient. IgE from these patients reacted less to each deamidated fraction. IgE from patients allergic to deamidated wheat proteins were much more reactive against deamidated gliadin fractions. Interestingly, these patient sera displayed high concentrations of IgE that were reactive against two particular gliadins: native and deamidated γ and ω1.2 gliadins. Glutenin subunits were also IgE-immunoreactive for some patients allergic to native wheat proteins but it was hardly not the case for IgE from patients allergic to deamidated wheat proteins; their deamidation was thus not considered (data not shown).
| Clinical characteristics | Specific IgE concentrations (ng/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum no. | Sex | Age | Type of allergy | Symptoms | α NG | α DG | γ NG | γ DG | ω1,2 NG | ω1,2 DG | ω5 NG | ω5 DG | Tot NG | Tot DG |
| 30 | F | 24 | Deamidated wheat proteins | URT | 66 | 44 | 185 | 57 | 193 | 31 | + | 94 | ||
| 34 | F | 18 | EIA | 2 | 1 | 42 | 17 | 30 | 2 | 21 | ||||
| 78 | F | Ad | EIA | + | 15 | 13 | 71 | 57 | 55 | 13 | 3 | 73 | ||
| 88 | F | 28 | EIA | 6 | 13 | 9 | 10 | 8 | 7 | 10 | ||||
| 352 | M | 12 | EIA | + | 0.19 | 9 | Sat | 17 | 158 | 144 | + | 145 | ||
| 390 | M | 10 | URT | 2 | 7 | 54 | 30 | 40 | + | 38 | ||||
| 497 | F | 24 | URT | 7 | 21 | 10 | 19 | 19 | ||||||
| 9 | M | 1.5 | Native wheat flour | AEDS | 15 | 9 | 6 | 4 | 13 | 8 | ||||
| 32 | M | 1 | AEDS | 6 | 6 | 10 | 8 | 15 | 14 | 9 | 7 | 10 | 7 | |
| 302 | M | 1 | URT | 44 | 23 | 20 | 19 | 6 | 4 | 3 | + | 53 | 31 | |
| 324 | M | 11 | URT | 3 | + | |||||||||
| 834 | M | 37 | EIA | + | + | 4 | + | 54 | 24 | 8 | ||||
- Patients allergic to deamidated wheat proteins (white), or wheat (grey). The data expressed correspond to the IgE concentration (ng/mL). Age: “ad”, Adult. Values: “+”, detected but not quantified, “sat”, concentration higher than standards in the scale. Symptoms: AEDS, Atopic Eczema/Dermatitis Syndrome; URT, Urticaria; EIA, exercise-induced anaphylaxis.
3.3 Induction of an allergic response to deamidated gliadins in mice/comparison with native gliadins
3.3.1 Effect on the Th2 response
Gliadin (NG or DG)-specific IgG1 were determined as early markers of Th2 response (Fig. 2A). Both NG-sensitized and DG-sensitized mice secreted significantly higher levels of gliadin-specific IgG1 (p<0.005) than control mice. DG-sensitized mice produced significantly higher levels of gliadin-specific IgG1 than NG-sensitized mice (p<0.005) against both native and deamidated gliadins. However, in both groups (DG and NG), the concentrations in NG-specific IgG1 and DG-specific IgG1 were equivalent.

Both NG-sensitized and DG-sensitized mice secreted significantly higher levels of total and gliadin-specific IgE (p<0.005) than the control mice (Fig. 2B and C). DG-sensitized mice produced larger quantities of total IgE and gliadin-specific IgE than NG-sensitized mice (p<0.01). NG-specific IgE concentrations were higher in NG-sensitized mice than those of DG-specific IgE (p<0.01). Surprisingly, no significant difference in the recognition of native or modified gliadins was observed in DG-sensitized mice.
The ability of spleen cells from mice in all three groups to secrete IL-4 in response to specific activation with native or deamidated gliadins was tested. As shown in Fig. 2D, spleen cells from both NG-sensitized and DG-sensitized mice secreted significantly higher levels of IL-4 than control mice (p<0.005) whatever the activation conditions (even with PBS): thus activation with both NG and DG had the same impact on IL-4 production.
An RBL cell degranulation test was performed to evaluate the functionality of gliadin-specific IgE produced by NG and DG sensitized mice (Fig. 2E). With or without gliadin modification, we observed that the specific IgE produced by DG and NG sensitized mice were able to induce RBL cell degranulation. Higher percentages of degranulation were observed when RBL cells were pre-incubated with IgE from DG-sensitized mice and were then placed in contact with either DG or anti-IgE (in correlation with the assay of total IgE).
3.3.2 Effect on the Th1 response (IgG2a/INFγ)
In order to compare the Th1 response in the two sensitized groups, the concentrations of gliadin-specific IgG2a were determined first of all (Fig. 3A). Both NG- and DG-sensitized mice secreted gliadin-specific IgG2a when compared to the control group (p<0.005). NG-sensitized mice displayed significantly higher concentrations of gliadin-specific IgG2a than DG-sensitized mice (p<0.005). No significant difference between native and deamidated gliadin-specific IgG2a levels was observed within each sensitized group.

As shown in Fig. 3B, activation with native gliadins resulted in a significant secretion of INFγ by spleen cells in both NG and DG-sensitized mice (p<0.01); the production of INFγ was higher in NG-sensitized mice than in DG-sensitized mice (p<0.05), whereas activation with DG have caused no significant secretion of INFγ.
3.3.3 Effect on the elicitation phase
Clinical symptoms were observed in mice IP challenged with an IP injection of either native gliadins (NG-sensitized mice and half of control mice) or deamidated gliadins (DG-sensitized mice and the other half of control mice). Severe symptoms were observed in both NG-sensitized and DG-sensitized mice, with equivalent average scores (3/5: characterized by wheezing and laboured breathing, Fig. 4A). Control mice challenged with NG displayed no symptoms (score level 0/5), while some of the control mice challenged with DG presented with mild symptoms (score 1/5, characterized by scratching and rubbing around the nose and head).
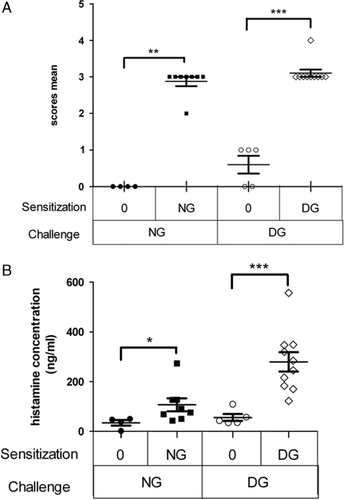
Plasma histamine levels were measured after the IP challenge with allergen in order to evaluate the intensity of the elicitation phase of the allergic response (Fig. 4B). Both NG-sensitized and DG-sensitized mice secreted significantly high levels of histamine (p<0.05 in NG-sensitized and p<0.005 in DG-sensitized compared to the controls). However, histamine release was notably higher in DG-sensitized mice than in NG-sensitized mice (p<0.01).
3.4 Reactivity profiles of mouse IgE against the different gliadin classes
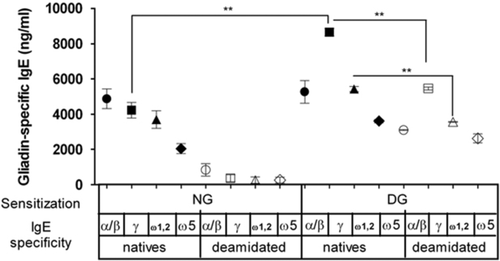
The reactivity of mouse IgE against the different gliadin classes (native and deamidated α/β-, γ-, ω1.2- and ω5-gliadins) was analyzed using pooled sera (Fig. 5). NG-sensitized mice displayed elevated concentrations of IgE specific to the different classes of native gliadins (i.e. α/β-, γ-, ω1.2- and ω5-gliadins) and which were roughly related to the proportion of each gliadin class in wheat flour, whereas the recognition of deamidated gliadin components was very weak. Mice sensitized with DG displayed a totally different profile: they displayed higher concentrations of IgE specific to γ- and ω1.2-gliadins (in their native or deamidated state) than to other gliadin classes.
4 Discussion
Because of recent reports concerning several cases of allergic reactions to modified glutens 16, the aim of our study was to investigate the allergenic potential of a gliadin fraction modified by deamidation, compared with the native gliadin fraction. In order to examine the impact of gliadin deamidation on sensitization and on the elicitation phases of an allergic response, we used an IP-sensitized Balb/c mouse model in which several immunological parameters could be measured.
In human, the symptoms of patients allergic to deamidated wheat proteins (anaphylactic shock, ElA, Urt) are often more severe than symptoms of patients allergic to wheat (gut irritation, AEDS, EIA, anaphylactic shock) 1, 16, 17. The main proteins responsible for these pathologies appear to be γ and ω1.2 gliadins with respect to IgE reactivity profile of these patients (Table 1).
The results of the present study demonstrated that deamidated gliadins sensitize mice much more effectively than native gladins. Firstly, DG-sensitized mice displayed higher concentrations of Th2 response markers: total and specific IgG1 and IgE, even though the IL-4 levels were quite similar in both sensitized groups. Moreover, these IgE were functional and could elicit the degranulation of RBL cells. Secondly, mice sensitized with DG displayed significantly lower concentrations of Th1 response markers (specific IgG2a and INFγ than mice sensitized with NG). Thus sensitization to DG seemed to tip the scales in favour of a Th2 response. Finally, DG-sensitized mice displayed a more severe elicitation phase of their allergic reaction: despite equivalent symptom scores, mice sensitized with DG secreted larger quantities of histamine than mice sensitized with NG. All these results thus showed that in mice, deamidated gliadins were more effective in inducing Th2 antibody production (sensitization) and effector cell activation (elicitation) than native gliadins. We also evidenced that the higher sensitization of mice to DG (more total IgE) was not biased by the experimental procedure: both NG and DG displayed equivalent adsorption on aluminium hydroxide (data not shown). This allergenic effect must be associated with some of the physicochemical properties of DG. Indeed, these proteins are more soluble in water, so when they were injected via the IP route they may spread more easily in the blood and thus come into contact with the immune system.
Interestingly, the experimental observations achieved using this animal model (sensitization efficacy of deaminated gliadin extracts, elicitation phase and IgE reactivity patterns) were similar to those made in patients allergic to deamidated wheat proteins. Indeed, the sera from DG-sensitized mice displayed a particular and specific IgE composition, with higher concentrations of IgE directed against both native and deamidated γ-gliadins, than sera from NG-sensitized mice. A trend could also be observed in the case of IgE directed against native, and notably deamidated ω1.2-gliadins, even though the difference was not significant between these two groups of mice. This particular IgE reactivity pattern was similar to that of patients allergic to deamidated wheat proteins 14. It might be interesting to determine which epitopes are implicated in the IgE response of mice. Indeed, in humans, a dominant epitope (“QPQQPFP”) has been identified in the repetitive region of γ and ω1.2 gliadins 14, and it is not contained in the amino-acid sequences of either α or ω5 gliadins.
Differences can be seen between the IgE response of mice and humans. Indeed, a precise analysis of mouse IgE specificity showed that, contrary to humans, mice sensitized with DG displayed equivalent concentrations of specific IgE directed against both NG and DG. Moreover, unlike human patients, our DG-sensitized mice secreted specific IgE directed against all purified native gliadins. In humans, IgE directed against native forms of γ and ω1.2 gliadins are mainly detected, but IgE directed against other native gliadin classes are rarely observed. These observations may be related to the specific composition of each fraction tested. Gliadins are particularly rich in Gln residues 4. After the chemical deamidation performed in this study, Gln residues must still remained, probably distributed in a random manner throughout the sequence, as suggested by their behaviour under electrophoresis (Fig. 1). Taking account of the residual number of Gln, it can therefore be hypothesized that some epitopes of the native form are still present and may be responsible for the production of specific IgE directed against native gliadins.
The differences between humans and mice may also be explained by the mode of sensitization used in our mice (IP route). Indeed, in our mouse model, the efficiency of DG sensitization could be explained by the drastic conditions of sensitization (systemic injection) that enabled a direct interaction between the allergen and the immune system. In humans, the sensitization conditions are very different because an allergen is exposed to the digestive tract and may be modified before it comes into contact with gut immune system. Indeed, DG are more soluble at pH 7 than at an acidic pH. This may impact the digestion process as they may aggregate in the stomach at an acidic pH and thus be more resistant than NG to digestion by stomach enzymes (pepsin). When they reach duodenum (where pancreatic juices neutralise the pH), DG may be more soluble and they may pass more easily through the intestinal epithelial barrier than NG.
This study was based on the model of mice sensitized via the IP route to native gliadin extracts developed by Bodinier et al. 21. During our study, serum samples from NG-sensitized mice displayed IgE, IgG1 and IL-4 concentrations that were quite close to those observed by these authors 21. Thus this experimental protocol led to a reproducible model. Even though Kumagai et al. evaluated the ability of DG to induce a tolerance mechanism in NG-sensitized rats, the allergenic potential of this DG fraction was not studied 20. Thus the animal model of allergy to DG used during our study was the first ever to have been described. This IP-sensitized mouse model displayed a pattern of specific IgE that was similar to that seen in human patients, even though the allergen did not transit through the digestive tract. So despite a less physiological sensitization mode, we were able to obtain a highly relevant model of allergy to deamidated gliadins. It would however be interesting to compare these results with those obtained using an oral sensitized model in order to study the effect of digestion on both native and deamidated gliadins, and their impact on the gut immune system.
The authors would like to thank Professor D. A. Moneret-Vautrin for supplying the sera, and Jean-Louis Lescure and Sylvie Triballeau for their support and technical expertise. Pascal Gourbeyre is a recipient of a grant from the French Ministère de la Recherche et de la Technologie (MRT).
The authors have declared no conflict of interest.




